Introduction
Anaplastic carcinoma of the pancreas is rarely
observed and accounts for <10% of all types of pancreatic
carcinoma (1,2). Undifferentiated carcinoma with
osteoclast-like giant cell tumors (UC-OGC) is a variant of
anaplastic carcinoma and the incidence of this tumor has been
reported to be <1% of all malignant neoplasms of the pancreas
worldwide (3). Due to the rarity of
cases of UC-OGC, the clinicopathological features remain unclear
and the surgical outcome of UC-OGC cases is controversial (4,5). The
case of a patient with UC-OGC, who underwent an initial curative
surgical resection followed by a second resection of the remnant
pancreas, due to the detection of poorly differentiated tubular
adenocarcinoma four years following the initial surgery, is
presented in the current report. A meta-analysis of previous
reports is also provided, focusing on the clinicopathological
features of UC-OGC by comparing short-term and long-term survivors
post-surgery.
Case report
A 37-year-old female was referred to the was
referred to the Tsukuba Gastrointestinal Hospital (Tsukuba, Japan)
due to epigastralgia. The patient had no specific medical or family
history. The laboratory data demonstrated elevated levels of serum
amylase (2,483 IU/l; normal range, 37–124 IU/l), however, the
leukocyte count (4,800/μl; normal range, 4,000–9,000/μl) and
C-reactive protein level (0.4 mg/dl; normal range, ≥0.3 mg/dl) did
not indicate inflammation. Among the tumor markers examined, the
carbohydrate antigen (CA) 19-9 and elastase-1 values were increased
to 135 U/ml (normal range, 0–37 U/ml) and 8,600 ng/ml (normal
range, 100–400 ng/ml), respectively. Abdominal ultrasonography
demonstrated a tumor containing a cystic component (diameter, 4 cm)
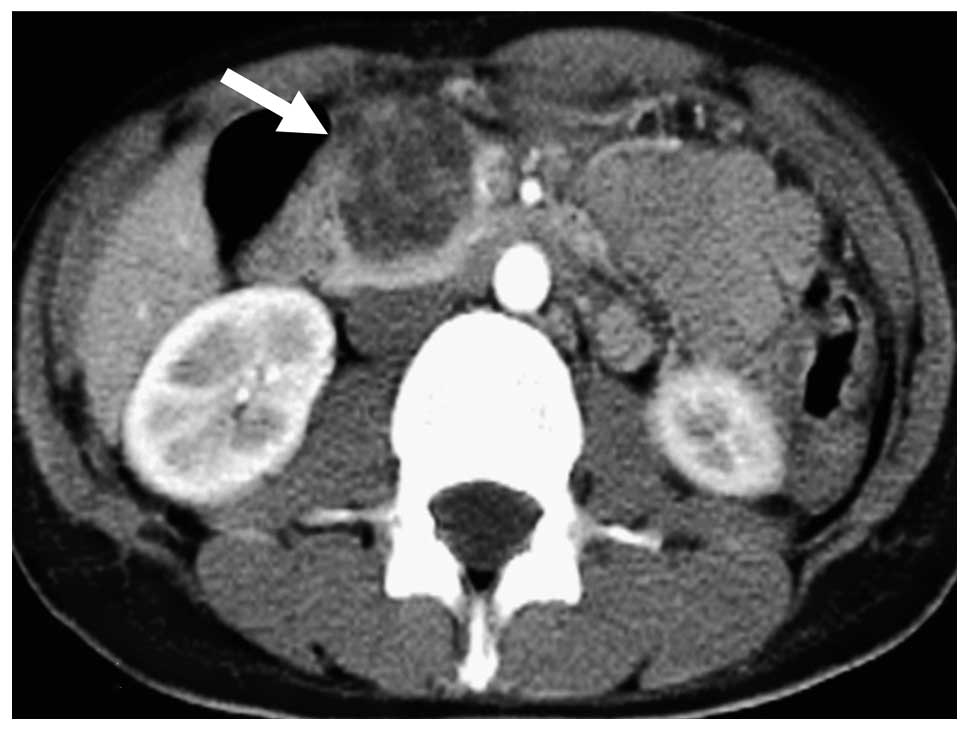
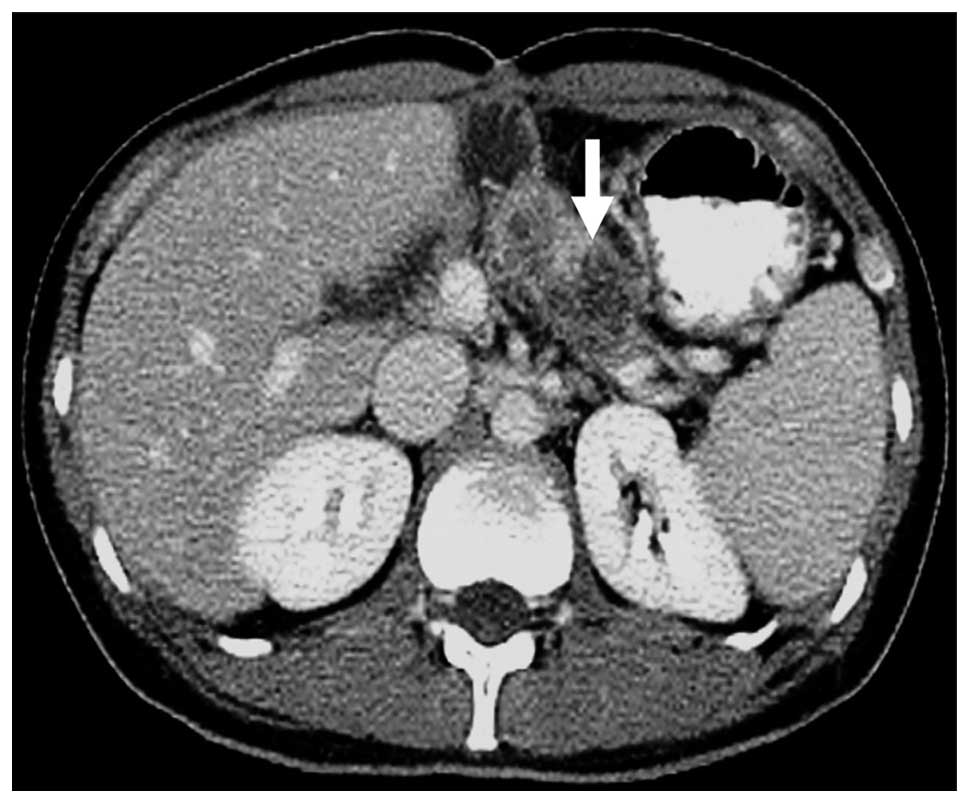
in the pancreatic head. Abdominal computed tomography (CT)
demonstrated a tumor containing a cyst-like low-density area and an
enhanced septum (Fig. 1). Lymph
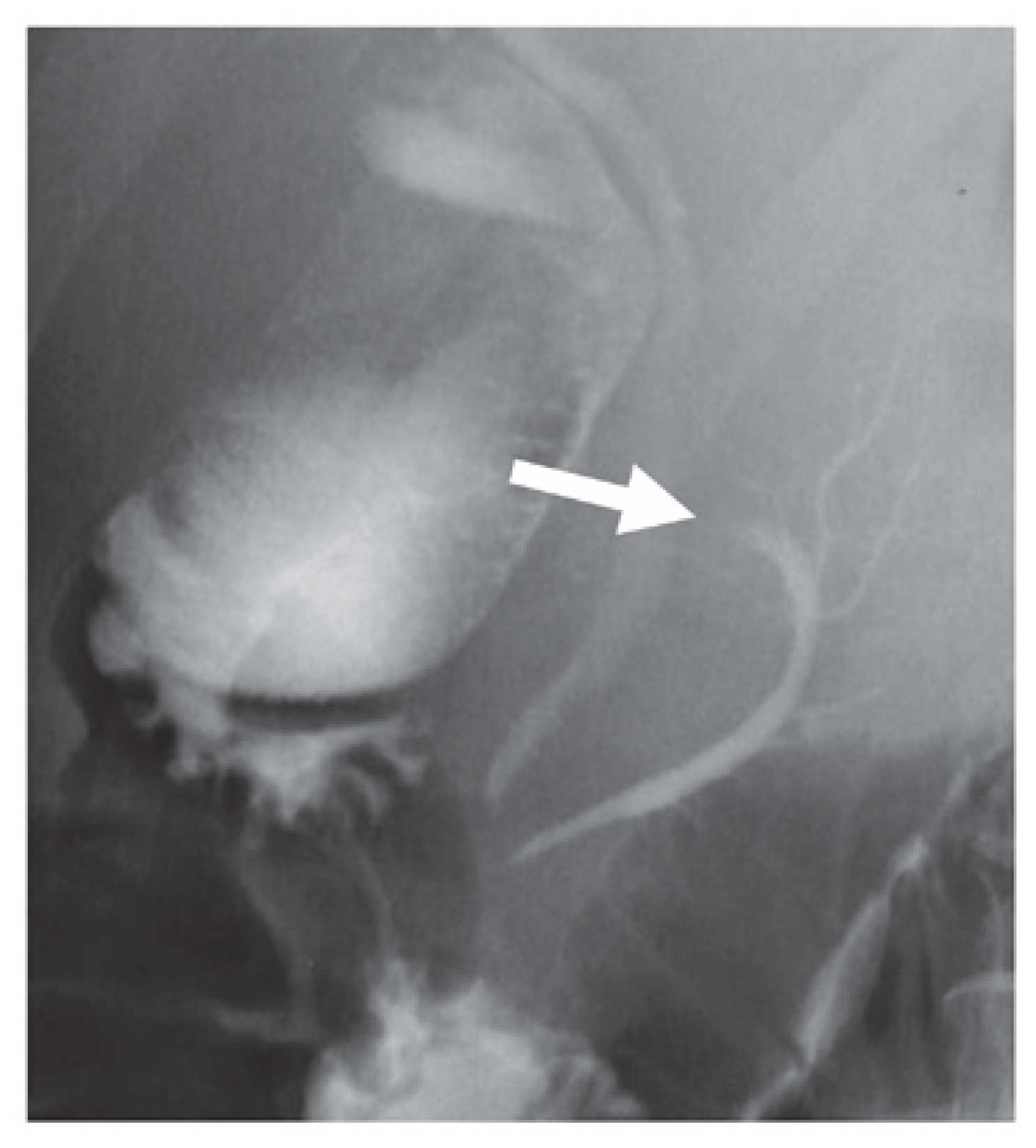
node swelling was not detected. Endoscopic retrograde
cholangiopancreatography showed an elliptical filling defect of the
main pancreatic duct at the pancreatic body (Fig. 2).
Based on these findings, the preoperative diagnosis
of the cystic pancreatic tumor was a mucinous cystadenocarcinoma
due to the interruption of the main pancreatic duct. A
pylorus-preserving pancreaticoduodenectomy was performed to resect
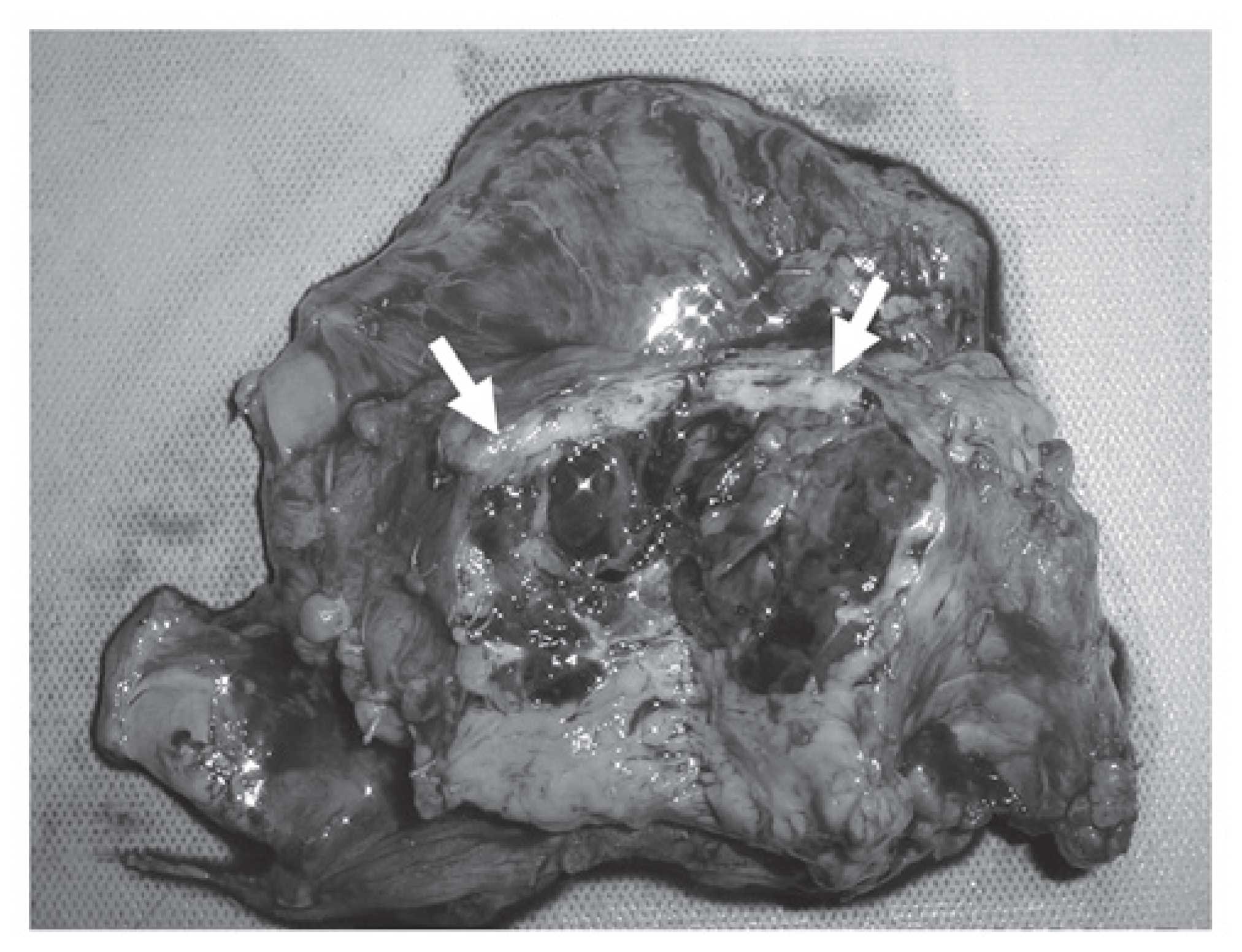
the suspected malignancy. Macroscopically, the tumor measured 4 cm
in diameter and was covered with a relatively thick capsule;
internal bleeding and necrosis on the cut surface was also observed
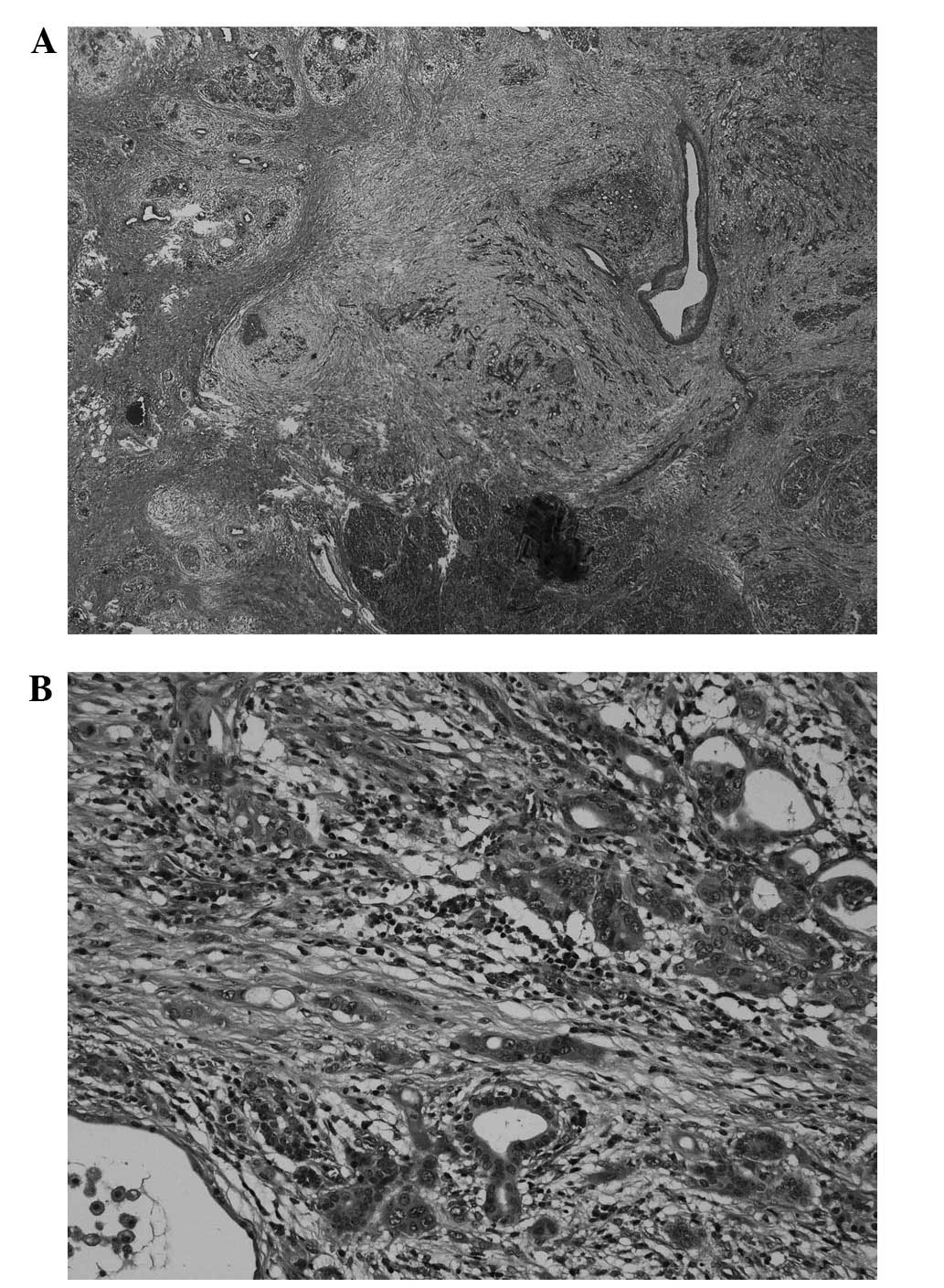
(Fig. 3). Histopathologically,
multinucleated giant cells resembling osteoclasts were observed.
The tumor consisted of slightly atypical medium-sized or small
round cells, and spindle cells. Furthermore, there was a
concomitant component of well-differentiated tubular adenocarcinoma
(Fig. 4A and B). Giant cells
resembling osteoclasts were positive for vimentin and negative for
p53, and the well-differentiated adenocarcinoma was positive for
p53. The tumor was finally diagnosed as a UC-OGC of the pancreas.
In addition, the histopathological analyses demonstrated that the
tumor was curatively resected with a negative margin. The CA19-9
value returned to the normal level (normal range, 0–37 U/ml).
Adjuvant chemotherapy with gemcitabine (1,000 mg/m2) was
administered once every four weeks, with one rest week, for the six
months following surgery, and no recurrence was observed until
three years postoperatively.
Four years following surgery, the patient’s CA19-9
level increased again to 380 U/ml. CT revealed a small lesion
(diameter, 2 cm) in the remnant pancreas (Fig. 5) and there were no additional
recurrent lesions. The patient opted to receive a resection of the
tumor in the remnant pancreas rather than undergo second-line
chemotherapy. A partial resection of the remnant pancreas was
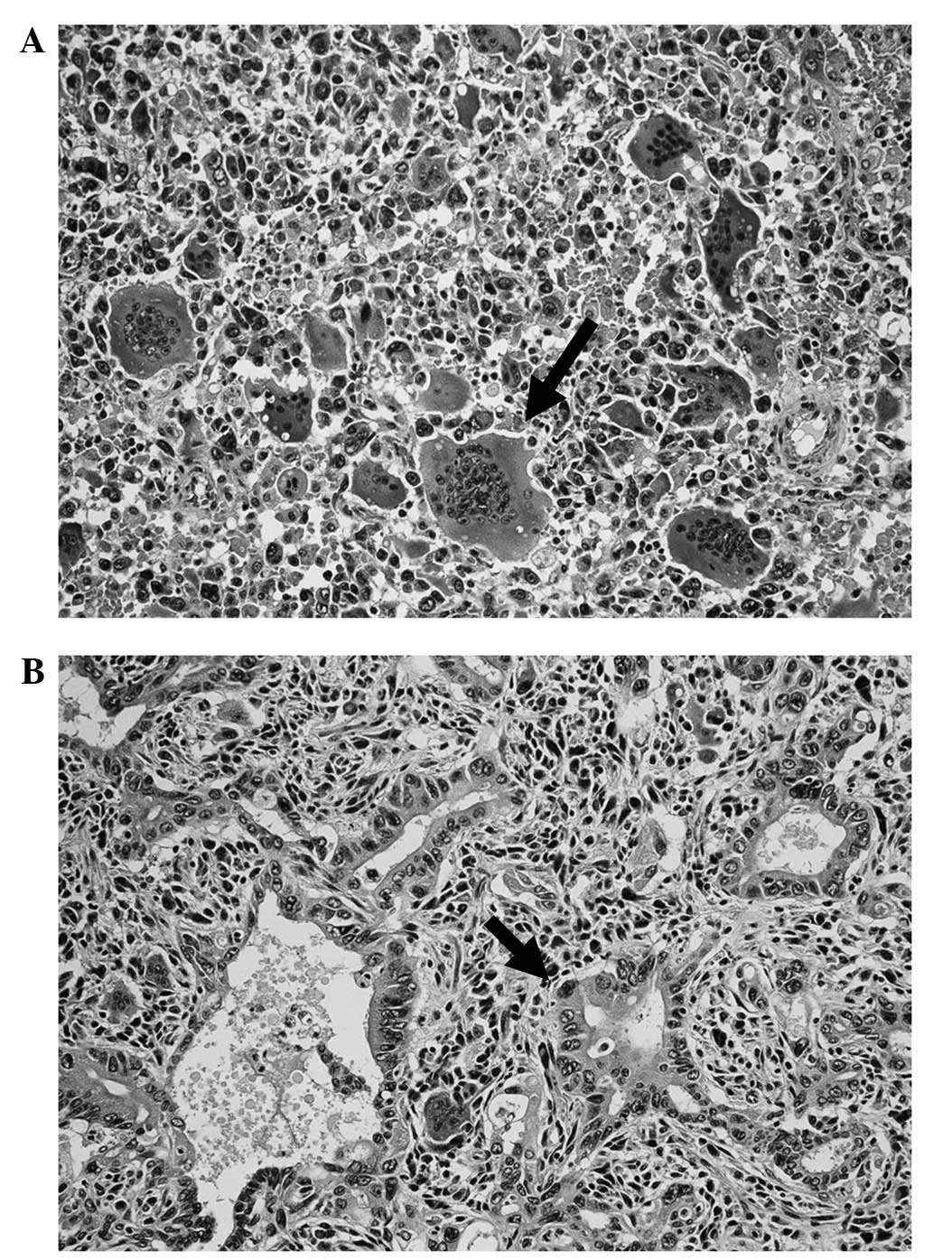
subsequently conducted as the second surgery. The histopathological
diagnosis of the tumor in the remnant pancreas was a poorly
differentiated tubular adenocarcinoma (Fig. 6A and B) and was positive for p53. A
retrospective pathological analysis of the initially resected
specimens demonstrated a component of a poorly differentiated
tubular adenocarcinoma in the UC-OGC. The final diagnosis of the
second cancer of the pancreatic remnant was an intra-pancreatic
metastasis of the component of ductal adenocarcinoma (DAC)
originating from the UC-OGC, rather than a multi-focal second
pancreatic carcinoma. To date, 18 months subsequent to the second
surgery, the patient has survived without recurrence.
A meta-analysis of patients with UC-OGC who
underwent surgical resection was conducted in the current study.
The inclusion criteria for the meta-analysis were as follows: i)
Reports of UC-OGC published in English; ii) cases of patients
surviving more than two years following surgical resection
(long-term survivors); and iii) cases of patients who succumbed
less than one year following surgical resection (short-term
survivors). A statistical comparison between the long- and
short-term survivors was performed.
Thirteen cases were identified as the short-term
survivors and 15 cases, including the present case, were identified
as long-term survivors (Table I)
(4–24). At the time of surgery, the patients
were identified to be significantly older in the short-term
survivor group compared with those in the long-term survivor group
(64.7±14.3 vs. 50.6±14.0 years, P=0.034; Mann-Whitney-U test).
There were fewer females in the short-term survivor group than in
the long-term survivor group (33 vs. 67%, P=0.085; χ2
test). The localization of the tumor did not differ between the two
groups. The maximum diameter of the tumor was found to be smaller
in the short-term survivor group compared with those of the
long-term survivors (8.7±5.2 vs. 12.3±7.0 cm, P=0.213). The number
of patients with a solid mass was greater in the short-term
survivor group than in the long-term survivor group (60 vs. 25%,
P=0.231). The value of CA19-9 was not mentioned for all of the
cases; however, the level of CA19-9 was increased in two of the
three patients in the short-term survivor group, and one of two
patients in the long-term survivor group for which the values were
mentioned. The incidence of lymph node metastasis was identified to
be significantly higher in the short-term survivor group compared
with that of the long-term survivor group (50 vs. 7%, P=0.039). A
second surgery was performed on only one patient in the short-term
survivor group and on three patients in the long-term survivor
group; one patient from the long-term survivor group succumbed
shortly after the surgery. One patient in the short-term survivor
group did not undergo any surgical resection. Dworak et al
(6) reported a patient who
underwent five surgeries, and who survived for 40 months following
surgery without recurrence. To the best of our knowledge, the
present patient is the first five-year survivor after undergoing a
second curative resection. The incidence of a concomitant component
of mucinous cystic neoplasm (MCN) did not significantly differ
between the two groups (two cases in the short-term and three cases
in the long-term survivors). The incidence of a component of the
concomitant DAC in the UC-OGC was significantly higher in the
short-term survivor group compared with that in the long-term
survivor group (50 vs. 7%, P=0.039) and the present case was the
only long-term survivor who presented with a concomitant component
of DAC. The incidence of a concomitant component of pleomorphic
giant cell carcinoma (PGC) in the UC-OGC was higher in the
short-term survivor group than that in the long-term survivor group
(63 vs. 21%, P=0.143).
 | Table ILiterature review regarding patients
exhibiting undifferentiated carcinoma with osteoclast-like giant
cell tumors, who survived for two year or more and those who
succumbed within one year following resection. |
Table I
Literature review regarding patients
exhibiting undifferentiated carcinoma with osteoclast-like giant
cell tumors, who survived for two year or more and those who
succumbed within one year following resection.
| A, Short-term
survivors |
|---|
|
|---|
| Year | First author
(ref) | Age,
years/Gender | Pancreatic
location | Max. diameter,
cm | Surgery | Lymph node
metastasis | Survival, months | Second surgery | Pathological
features |
|---|
| 1990 | Lewandrowski
(11) | 60/M | Tail | 13.0 | DP+S | Negative | 4 | No | PGC |
| 1994 | Martin (12) | 57/M | Tail | 7.0 | DP+S | Negative | 4 | Yes | PGC and DAC |
| 1995 | Gatteschi (13) | 72/M | Head | 6.0 | PD | Negative | 4 | No | PGC |
| 1997 | Watanabe (10) | 76/M | Head | 5.0 | PD | Negative | 3 | No | PGC and DAC |
| 1998 | Molberg (4) | 62/F | Head | 6.0 | PD | Nm | 11 | No | Nm |
| 1998 | Molberg (4) | 43/F | Tail | 7.0 | DP+S | Nm | 8 | No | Nm |
| 1998 | Molberg (4) | 88/F | Tail | 14.0 | DP+S | Nm | 2 | No | Nm |
| 1998 | Molberg (4) | 63/M | Head | 5.0 | PD | Nm | 11 | No | Nm |
| 1998 | Molberg (4) | 85/F | Head | 3.5 | PD | Nm | 6 | No | Nm |
| 2005 | Nai1 (5) | 69/M | Head | 4.7 | PD | Positive | 12 | No | MCN and DAC |
| 2010 | Singhal (14) | 42/M | Tail | 14.0 | DP+S | Positive | 4 | No | PGC and DAC |
| 2011 | Hur (15) | 77/M | Tail | 10.0 | DP+S | Negative | 3 | No | Nm |
| 2011 | Wada (9) | 59/M | Tail | 20.0 | DP+S+TG | Positive | 4 | No | MCN |
|
| B, Long-term
survivors |
|
| Year | First author
(ref) | Age,
years/Gender | Pancreatic
location | Max. diameter,
cm | Surgery | Lymph node
metastasis | Survival, months | Second surgery | Pathological
features |
|
| 1966 | Shamblin (16) | 49/M | Head | 8.0 | TP | Negative | 180 | No | Nm |
| 1987 | Baniel (17) | 65/F | Tail | 23.0 | DP, distal
gastrectomy | Negative | 72 | No | Nm |
| 1993 | Scott (18) | 63/M | Head | 24.0 | Local resection | Negative | 24 | Yes | Nm |
| 1993 | Dworak (6) | 44/F | Tail | 13.0 | DP | Negative | 40 | Yes | Nm |
| 1998 | Molberg (4) | 58/F | Head | 13.0 | PD | Nm | 168 | No | Nm |
| 2001 | Suda (8) | 35/F | Tail | 11.0 | DP+S Positive
168 | No | MCC | | |
| 2002 | Shiozawa (7) | 45/F | Tail | 4.0 | DP+S | Negative | 30 | No | Nm |
| 2004 | Osaka (19) | 57/M | Tail | 20.0 | DP+S+TG | Negative | 36 | No | Nm |
| 2005 | Sedivy (20) | 44/F | Tail | 12.0 | DP+S | Negative | 48 | No | MCC |
| 2006 | Lukas (21) | 27/M | Head | 22.0 | PD | Negative | 30 | No | PGC |
| 2006 | Lukas (21) | 59/F | Head | 8.0 | PD | Negative | 40 | No | PGC |
| 2006 | Sautot-Vial
(22) | 74/M | Head | 10.0 | PD | Negative | 26 | No | Nm |
| 2009 | Burkadze (23) | 34/F | Tail | 11.0 | DP+S | Negative | 48 | No | MCN |
| 2011 | Maksymov (24) | 68/F | Head | 2.0 | PD | Negative | 36 | No | PGC |
| 2012 | Present case | 37/F | Head | 4.0 | PpPD | Negative | 66 | Yes | DAC |
Discussion
The present study reported the case of a patient who
exhibited UC-OGC of the pancreas and underwent two surgical
resections, which resulted in a favorable long-term outcome. A
meta-analysis using previous reports showed that the
characteristics of the short-term survivors following surgical
resection were an older age, males, and those exhibiting smaller
tumors, positive lymph node metastasis and a concomitant component
of DAC. The concomitant component of an MCN was not considered to
be a prognostic factor. The current patient, to the best of our
knowledge, is the first five-year survivor after undergoing a
second curative resection.
Giant cell tumors of the pancreas are rare
neoplasms, which present as two variations. One variation is UC
with a pleomorphic/sarcomatoid growth pattern and multinucleated
tumor giant cells (1,2). UC-OGC, the second variant, was
initially reported by Rosai (25)
in 1968 as a variant tumor of UC, which exhibited conspicuous giant
cells that resembled osteoclasts. UC-OGC of the pancreas is
characterized by a well-delineated tumor, which frequently contains
bleeding areas and central necrotic foci. Therefore, CT and
magnetic resonance imaging demonstrated lobular cystic findings or
bleeding and necrosis within the solid tumor (26). In the present patient, cystic and
solid components exhibiting enhancement were observed. In addition,
the resected specimen contained bleeding areas and central necrotic
foci. Histopathologically, the tumor in the current case consisted
of polymorphic cells with a small number of nuclei and
multinucleated giant cells that resembled osteoclasts.
The prognosis of UC-OGC is particularly variable,
ranging from four months to 10 years in the published literature
(4). Molberg et al (4) reported that five out of six patients,
who were followed up post-surgery, succumbed due to the primary
disease within one year. Shiozawa et al (7) summarized the prognosis using the
literature that was reported until 1997, and found that only three
out of 32 patients survived for two years or more without
recurrence. Contrary to these reports, Strobel et al
(6) reported the improved survival
of patients with UC-OGC, indicating that 80% of the patients who
underwent curative surgery survived for at least two years. As
shown in the literature review of the present report, there were 15
patients who survived for two years or longer and 13 patients who
succumbed within one year following surgery. Based on the
literature review, the prognosis of patients with UC-OGC does not
appear to be as poor as that of patients with
pleomorphic/sarcomatoid giant cells, in whom there were no one-year
survivors following surgical resection in the report by Strobel
et al (5).
UC-OGC has been identified to present with
concomitant components of DAC or MCN (7,27,28)
and an improved prognosis was described for the combination of
UC-OGC with DAC (29). In addition,
UC-OGC associated with MCN appears to have a markedly more
favorable prognosis (28,8). However, the present literature review
indicated that the concomitant component of DAC in UC-OGC was a
significant negative prognostic factor. In addition, the present
results do not demonstrate that the combination of UC-OGC and MCN
predicts an improved prognosis following surgery, as Wada et
al (9) and Nai et al
(30) reported. According to a case
report by Molberg et al (4),
although only one of the 10 reported patients survived more than
two years, the incidence of a mixture of concomitant DAC with
UC-OGC was 30%. In addition, the results reported by Molberg et
al (4) indicated the
significance of concomitant DAC as a prognostic factor.
Furthermore, the current literature review demonstrated that the
coincidence of PGC, which is considered to be a sarcomatous
metaplasia of DAC (10), indicates
a poorer prognosis compared with UC-OGC alone, as was recently
shown by Strobel et al (5).
There are two possibilities concerning the recurrent
tumor of the remnant pancreas in the current patient: i) The tumor
was a metachronous metastasis in the remnant pancreas; or ii) the
tumor was a multifocal secondary carcinoma. As a poorly
differentiated tubular adenocarcinoma was retrospectively
identified in a section of the initially resected specimens, it was
speculated that the tumor of the remnant pancreas was an
intra-pancreatic metastasis.
In conclusion, the meta-analysis demonstrated that
the characteristics of the patients in the short-term survivor
group following surgical resection were those of an older age,
males, and those exhibiting smaller tumors, positive lymph node
metastasis and a concomitant component of DCA. The current patient,
to the best of our knowledge, was the first five-year survivor
following a curative second resection, which has been reported thus
far in the English literature.
References
|
1
|
Chen J and Baithun SI: Morphological study
of 391 cases of exocrine pancreatic tumours with special reference
to the classification of exocrine pancreatic carcinoma. J Pathol.
146:17–29. 1985.
|
|
2
|
Morohoshi T, Held G and Klöppel G:
Exocrine pancreatic tumours and their histological classification.
A study based on 167 autopsy and 97 surgical cases. Histopathology.
7:645–661. 1983.
|
|
3
|
Jo S: Huge undifferentiated carcinoma of
the pancreas with osteoclast-like giant cells. World J
Gastroenterol. 20:2725–2730. 2014.
|
|
4
|
Molberg KH, Heffess C, Delgado R and
Albores-Saavedra J: Undifferentiated carcinoma with osteoclast-like
giant cells of the pancreas and periampullary region. Cancer.
82:1279–1287. 1998.
|
|
5
|
Strobel O, Hartwig W, Bergmann F, et al:
Anaplastic pancreatic cancer: Presentation, surgical management,
and outcome. Surgery. 149:200–208. 2011.
|
|
6
|
Dworak O, Wittekind C, Koerfgen HP and
Gall FP: Osteoclastic giant cell tumor of the pancreas. An
immunohistological study and review of the literature. Pathol Res
Pract. 189:228–234. 1993.
|
|
7
|
Shiozawa M, Imada T, Ishiwa N, et al:
Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol.
7:376–380. 2002.
|
|
8
|
Suda K, Takase M, Oyama T, et al: An
osteoclast-like giant cell tumor pattern in a mucinous
cystadenocarcinoma of the pancreas with lymph node metastasis in a
patient surviving over 10 years. Virchows Arch. 438:519–520.
2001.
|
|
9
|
Wada T, Itano O, Oshima G, et al: A male
case of an undifferentiated carcinoma with osteoclast-like giant
cells originating in an indeterminate mucin-producing cystic
neoplasm of the pancreas. A case report and review of the
literature. World J Surg Oncol. 9:1002011.
|
|
10
|
Watanabe M, Miura H, Inoue H, et al: Mixed
osteoclastic/pleomorphic-type giant cell tumor of the pancreas with
ductal adenocarcinoma: histochemical and immunohistochemical study
with review of the literature. Pancreas. 15:201–208. 1997.
|
|
11
|
Lewandrowski KB, Weston L, Dickersin GR,
et al: Giant cell tumor of the pancreas of mixed osteoclastic and
pleomorphic cell type: evidence for a histogenetic relationship and
mesenchymal differentiation. Hum Pathol. 21:1184–1187. 1990.
|
|
12
|
Martin A, Texier P, Bahnini JM and Diebold
J: An unusual epithelial pleomorphic giant cell tumour of the
pancreas with osteoclast-type cells. J Clin Pathol. 47:372–374.
1994.
|
|
13
|
Gatteschi B, Saccomanno S, Bartoli FG, et
al: Mixed pleomorphic-osteoclast-like tumor of the pancreas. Light
microscopical, immunohistochemical, and molecular biological
studies. Int J Pancreatol. 18:169–175. 1995.
|
|
14
|
Singhal A, Shrago SS, Li SF, et al: Giant
cell tumor of the pancreas: a pathological diagnosis with poor
prognosis. Hepatobiliary Pancreat Dis Int. 9:433–437. 2010.
|
|
15
|
Hur YH, Kim HH, Seoung JS, et al:
Undifferentiated carcinoma of the pancreas with osteoclast-like
giant cells. J Korean Surg Soc. 81:146–150. 2011.
|
|
16
|
Shamblin WR, Priestley JT, Sprague RG and
Harrison EG Jr: Total pancreatectomy for pleomorphic carcinoma. A
five-year cure. Arch Surg. 92:315–317. 1966.
|
|
17
|
Baniel J, Konichezky M and Wolloch Y:
Osteoclast-type giant cell tumor of the pancreas. Case report. Acta
Chir Scand. 153:67–69. 1987.
|
|
18
|
Scott R, Jersky J and Hariparsad G: Case
report: malignant giant cell tumour of the pancreas presenting as a
large pancreatic cyst. Br J Radiol. 66:1055–1057. 1993.
|
|
19
|
Osaka H, Yashiro M, Nishino H, et al: A
case of osteoclast-type giant cell tumor of the pancreas with
high-frequency microsatellite instability. Pancreas. 29:239–241.
2004.
|
|
20
|
Sedivy R, Kalipciyan M, Mazal PR, et al:
Osteoclast-like giant cell tumor in mucinous cystadenocarcinoma of
the pancreas: an immunohistochemical and molecular analysis. Cancer
Detect Prev. 29:8–14. 2005.
|
|
21
|
Lukás Z, Dvorák K, Kroupová I and Habanec
B: Immunohistochemical and genetic analysis of osteoclastic giant
cell tumor of the pancreas. Pancreas. 32:325–329. 2006.
|
|
22
|
Sautot-Vial N, Rahili A, Karimdjee-Soihili
B, et al: Hepatobiliary and pancreatic: Osteoclast-like giant cell
tumor of the pancreas. J Gastroenterol Hepatol. 21:10722006.
|
|
23
|
Burkadze G and Turashvili G: A case of
osteoclast-like giant cell tumor of the pancreas associated with
borderline mucinous cystic neoplasm. Pathol Oncol Res. 15:129–131.
2009.
|
|
24
|
Maksymov V, Khalifa MA, Bussey A, et al:
Undifferentiated (anaplastic) carcinoma of the pancreas with
osteoclast-like giant cells showing various degree of pancreas duct
involvement. A case report and literature review. JOP. 12:170–176.
2011.
|
|
25
|
Rosai J: Carcinoma of pancreas simulating
giant cell tumor of bone. Electron-microscopic evidence of its
acinar cell origin. Cancer. 22:333–344. 1968.
|
|
26
|
Zou XP, Yu ZL, Li ZS and Zhou GZ:
Clinicopathological features of giant cell carcinoma of the
pancreas. Hepatobiliary Pancreat Dis Int. 3:300–302. 2004.
|
|
27
|
Jalloh SS: Giant cell tumour
(osteoclastoma) of the pancreas - an epithelial tumour probably of
pancreatic acinar origin. J Clin Pathol. 36:1171–1175. 1983.
|
|
28
|
Sedivy R, Peters K and Klöppel G:
Osteopontin expression in ductal adenocarcinomas and
undifferentiated carcinomas of the pancreas. Virchows Arch.
446:41–45. 2005.
|
|
29
|
Klöppel G, Hruban RH, Longnecker D, et al:
Ductal adenocarcinoma of the pancreas. Pathology and Genetics of
Tumours of the Digestive System. Hamilton S and Aaltonen L:
Illustrated reprint. IARC Press; Lyon: pp. 221–230. 2000
|
|
30
|
Nai GA, Amico E, Gimenez VR and Guilmar M:
Osteoclast-like giant cell tumor of the pancreas associated with
mucus-secreting adenocarcinoma. Case report and discussion of the
histogenesis. Pancreatology. 5:279–284. 2005.
|