Introduction
Colorectal cancer (CRC) is one of the most common
malignant cancers and is the second leading cause of cancer-related
mortality worldwide (1). The
five-year survival rate of patients in the early stage is high,
while that of late-stage patients is low due to metastasis. The
underlying molecular mechanism of metastasis is yet to be
elucidated, therefore, it is important to investigate and validate
the novel biomarkers that are involved in CRC development.
Recently, microRNAs (miRs) have been identified as novel molecules
that are crucial in cancer development (2–4).
miRs are a family of endogenous, small (containing
~22 nucleotides) non-coding RNA molecules, which are able to serve
as key regulators of gene expression at the post-transcriptional
level (5). They are able to bind to
the target gene mRNA in a complete or incomplete complementary
manner with the 3′ untranslated region (UTR) of the target gene
mRNA, leading to target mRNA degradation or translational
repression (5,6). Due to the incomplete base pair between
the miR and the 3′UTR of the target gene, one gene may be regulated
by multiple miRs, resulting in a complex regulation network of
miRs. Thus far, ~60% of the protein-coding genes are known to be
regulated by miRs and, depending on the roles of the target genes,
the miR functions as an oncogene or a tumor suppressor (7). Numerous studies have shown that miRs
participate in various biological processes, including cell growth,
timing development, apoptosis and differentiation (8–11).
Furthermore, studies indicated that the deregulation of miRs is
associated with cancer initiation and development, including
miR-122 in liver cancer (12,13),
miR-365 (14) and miR-25 (15) in colorectal cancer, and miR-125b in
breast cancer (16).
In the present study, the aim was to investigate the
putative roles of miR-708 and miR-31 in CRC. In addition,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and
colony formation assay, as well as other functional assays were
used to elucidate the miR-708 and miR-31 in CRC cells. Furthermore,
an attempt was made to identify the downstream genes of these two
miRs, which are likely to aid the identification of a novel
therapeutic strategy for patients with CRC.
Materials and methods
Tissue samples and cell culture
Five pairs of human CRC tissue and the adjacent
healthy tissue were supplied by The Second Xiangya Hospital of
Central South University (Changsha, China). The tissue samples were
confirmed by observation of the morphology and
immunohistochemistry, and consent was obtained from the patients
with CRC. The tissues were stored at −80°C.
The human CRC cell line, SW480, was cultured in
Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum (FBS) and 2 mM L-glutamine (Invitrogen Life
Technologies, Carlsbad, CA, USA). The SW620 cells were cultured in
L-15 medium (Shanghai Haoran Bio Technologies Co., Ltd., Shanghai,
China) supplemented with 10% FBS and all of the cells were
maintained at 37°C in a 5% CO2 atmosphere.
Transfection
The anti-sense oligonucleotides of miR-708, miR-31,
miR-708 and miR-31 mimics, and the controls [anti-negative control
(NC) or NC] were purchased from Genepharma Co., Ltd. (Shanghai,
China). The cells were transfected with the abovementioned
oligonucleotides by Lipofectamine™ 2000 (Invitrogen Life
Technologies) according to the manufacturer’s instructions.
RNA isolation and real-time polymerase
chain reaction (qPCR) analysis
The total RNA (including miR) was isolated using the
Trizol reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. The RNA concentration was determined
using a NanoDrop-1000 spectrophotometer (Thermo Scientific,
Rockford, IL, USA). For the reverse transcription (RT) reaction of
the miR, specific miR RT primers were used. U6 small nuclear B
non-coding RNA served as an internal control. qPCR was performed
using the SYBR Green PCR master mix (Applied Biosystems, Foster
City, CA, USA) according to the following conditions: 95°C for 5
min followed by 40 cycles of amplification at 95°C for 30 sec, 57°C
for 30 sec and 72°C for 30 sec.
Western blot analysis
The cells were plated into 6-well plates (Jixing
Biocompany, Shanghai, China) at a density of 30×104
cells/well and transfected on the second day when the cell
confluence reached ~80%. At 48 h after transfection, the cells were
lysed by radioimmunoprecipitation assay (RIPA) buffer (50 mM
Tris-HCl, pH 8.8, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and
0.1% SDS) for 30 min at 4°C. The protein concentration was measured
using the bicinchoninic acid method. The first antibody was rabbit
monoclonal anti-cyclin-dependent kinase inhibitor 2B (CDKN2B)
antibody (1:200 dilution; Abcam, Cambridge, MA, USA) and anti-GAPDH
antibody (1:1000 dilution; Abcam). The secondary antibody was goat
anti-rabbit immunoglobulin G conjugated with horseradish peroxidase
(1:1000 dilution). The bound antibodies were detected with the ECL
Plus Western Blotting Detection system (GE Healthcare, Princeton,
NJ, USA) and the chemiluminescent signals were detected with a
high-performance chemiluminescence film (GE Healthcare). GAPDH
served as an internal control to normalize the CDKN2B protein
levels.
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The transfected cells were plated into 96-well
plates (Jixing Biocompany) at a density of 5×103
cells/well. At 24, 48 and 72 h after transfection, the cells were
incubated with MTT reagent (Xinshiye Biocompany, Guangzhou, China)
for ~4 h at 37°C. Subsequently, the supernatant was replaced with
dimethyl sulphoxide to dissolve solid residues. A spectrophotometer
was used to measure the absorbance at 570 nm.
Apoptosis assay
Camptothecin (Sigma Aldrich, St. Louis, MO, USA) was
added to the cell medium following transfection with anti-miR-708
or anti-miR-31. Following incubation for 24 h, the cells were
collected and detected using an Annexin V fluorescein
isothiocyanate kit on a BD FACSCalibur™ system (Becton-Dickinson,
Franklin Lakes, NJ, USA) according to the manufacturer’s
instructions.
DNA constructs and luciferase reporter
assay
The 3′UTR of CDKN2B was amplified and inserted
downstream of the luciferase reporter gene. The mutant 3′UTR of
CDKN2B (various nucleotides within the binding sites were mutated)
was amplified using CDKN2B 3′UTR as the template. The cells were
co-transfected with miR mimics and CDKN2B 3′UTR or mutant 3′UTR,
together with the controls. At 48 h after transfection, the cells
were collected and lysed using the RIPA buffer. The luciferase
intensity was measured using the Dual Luciferase Reporter assay
system (Promega Corporation, Madison, WI, USA) according to the
manufacturer’s instructions.
Transwell invasion assay
The cell invasion ability was performed using the
Transwell chamber with Matrigel (Millipore, Billerica, MA, USA).
The transfected cells were plated into the upper chamber with 250
μl serum-free medium, while the lower chamber was filled with 750
μl cell medium with 10% FBS. When the cells had been invaded for
~20 h, the cells were wash, fixed and stained with 5% crystal
violet (Sigma Aldrich). The cells that had not invaded the membrane
were removed using cotton tips. Finally, the invasive cells were
imaged (EOS 500D, Canon, Tokyo, Japan) and counted under a
microscope (CSW-17AD, Cosway (China) Co., Ltd., Shenzen, China)
Statistical analysis
The difference between groups was analyzed by
Students’ t-test, and P<0.05 was considered to indicate a
statistically significant difference. All the data were represented
as means ± standard deviation.
Results
miR-708 and miR-31 are upregulated in CRC
tissues
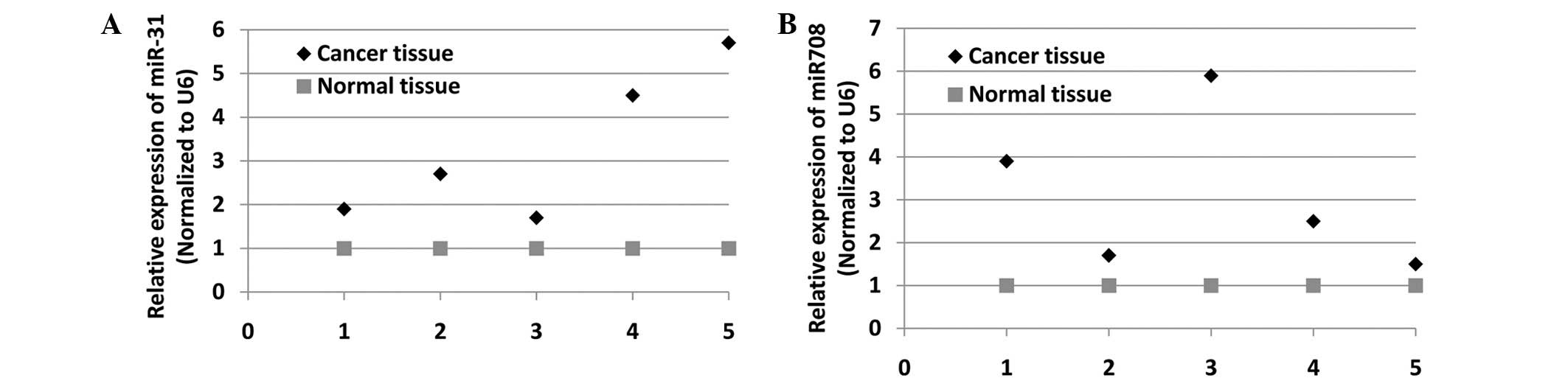
In order to determine the potential roles of miR-708
and miR-31 in CRC cells, the expression levels of the two miRs were
evaluated in five pairs of CRC tissue and the adjacent healthy
tissue using qPCR analysis. Fig. 1A and
B show that miR-31 and miR-708 were highly expressed in CRC
tissues, respectively. Therefore, these data indicate the possible
significance of miR-708 and miR-31 in CRC development.
Inhibition of miR-708 and miR-31
suppresses cell proliferation
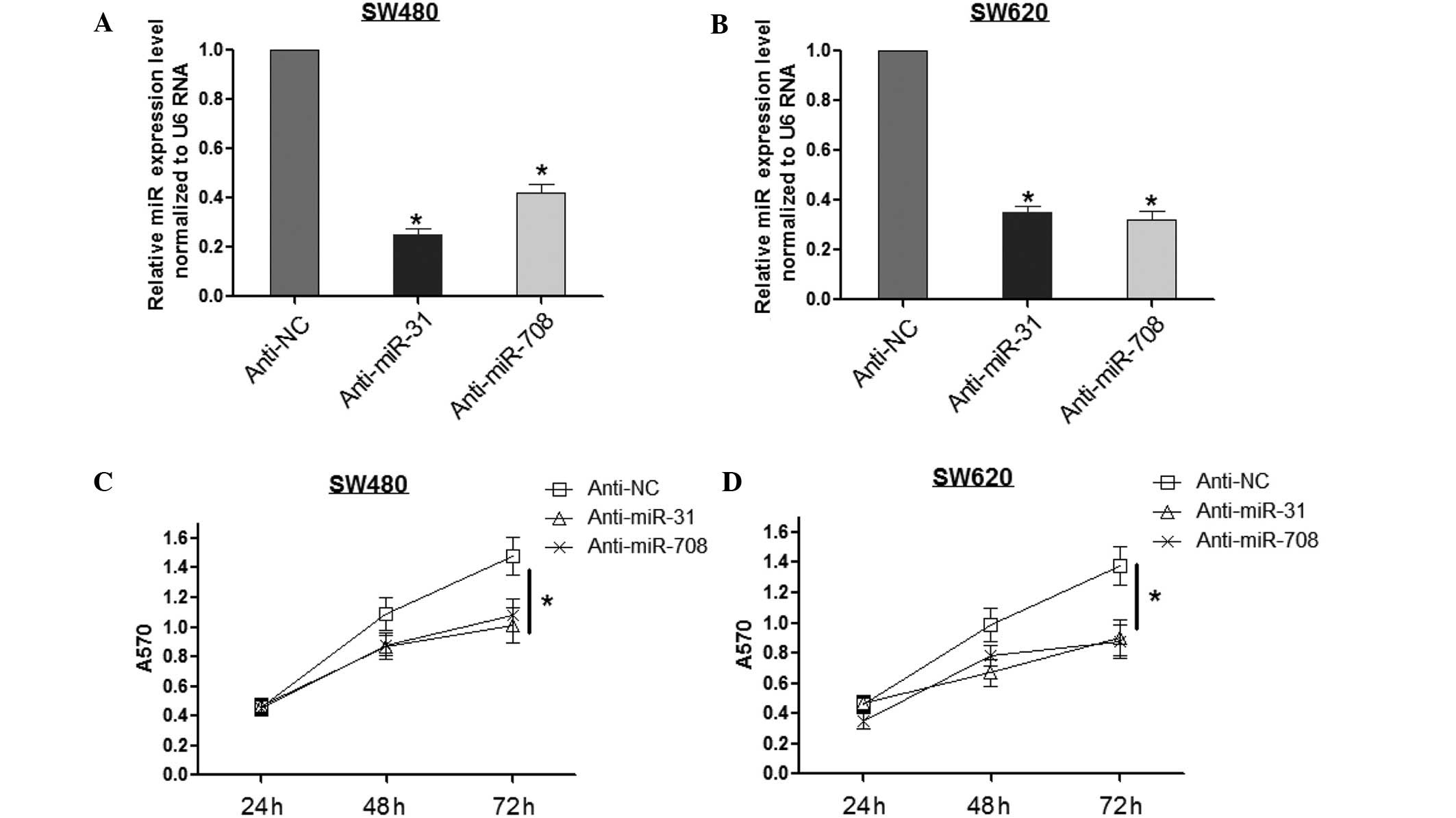
The MTT assay was performed to investigate the roles
of miR-708 and miR-31 in CRC cell proliferation. First, miR-708 and
miR-31 levels were inhibited by anti-miR transfection. Fig. 2A and B show that anti-miR-31 led to
the reduction of miR-31 by ~30% in the SW480 and SW620 cells, and
anti-miR-708 reduced the miR-708 expression by ~40%. Secondly, the
MTT assay was conducted in the SW480 and SW620 cells. As shown in
Fig. 2C, anti-miR-31 and
anti-miR-708 inhibited the SW480 cell viability at 72 h after
transfection, while no effect was observed at 24 and 48 h.
Consistently, there were similar effects of miR-708 and miR-31
identified in the SW620 cells (Fig.
2D), indicating that miR-708 and miR-31 may be significant in
CRC development.
Inhibition of miR-708 and miR-31 promotes
cell apoptosis
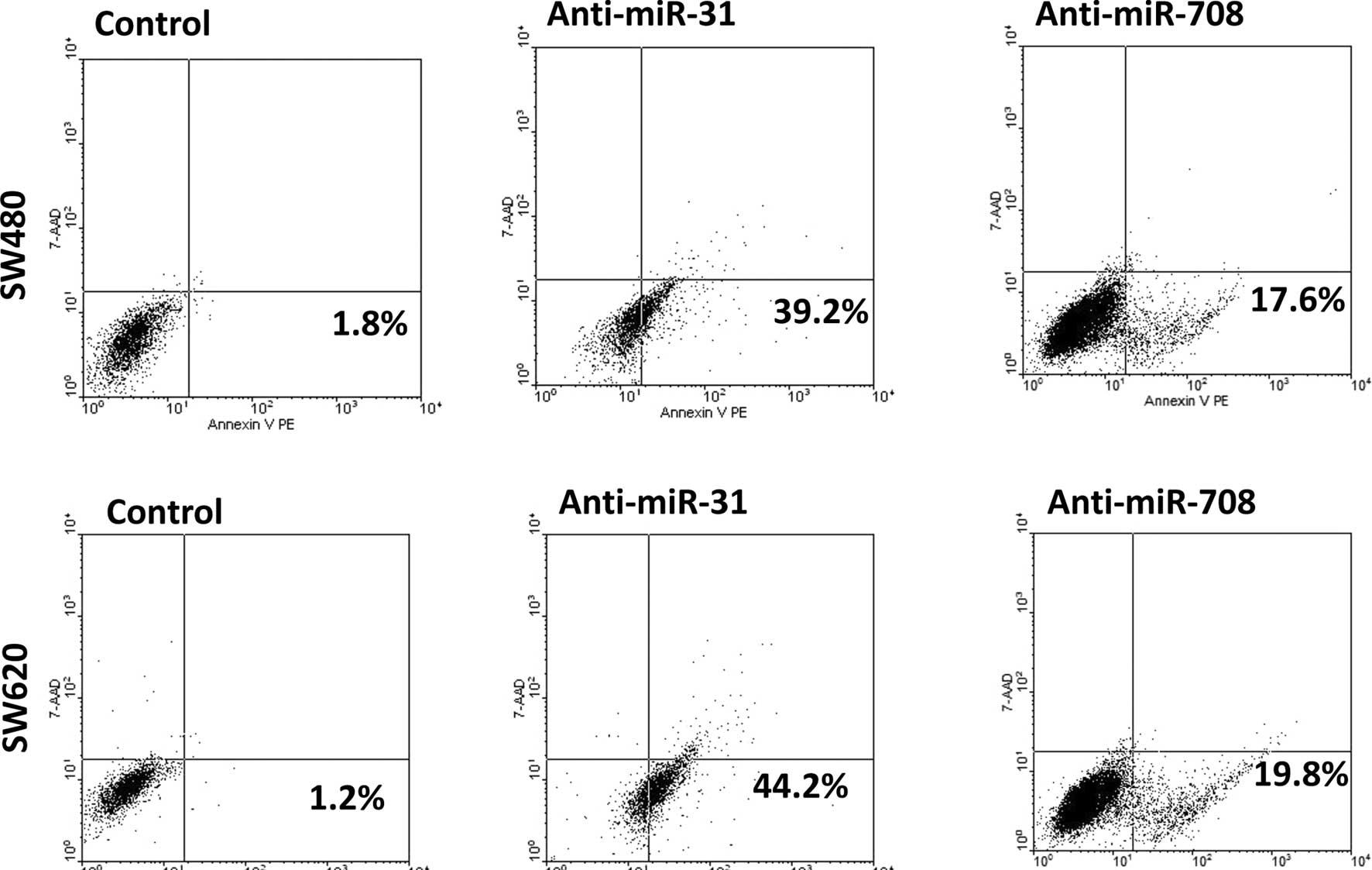
The effect of miR-708 and miR-31 on cell apoptosis
was further investigated using the Annexin V assay. Fig. 3 demonstrates that anti-miR-31
significantly increased the apoptosis of the SW480 and SW620 cells,
and anti-miR-708 led to similar results. Overall, these data
indicate that miR-708 and miR-31 may serve as significant
anti-apoptosis factors in CRC.
Inhibition of miR-708 and miR-31
suppresses cell invasion
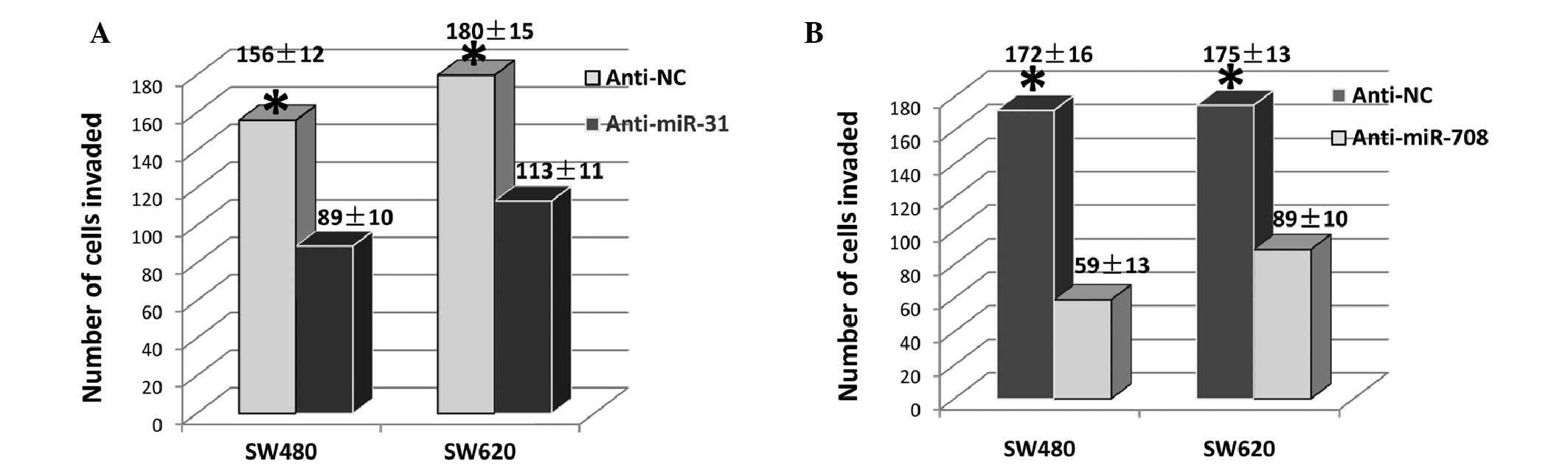
As cell invasion is an important factor for tumor
metastasis, the effect of miR-708 and miR-31 on cell invasion was
subsequently investigated. As shown in Fig. 4A, anti-miR-31 reduced the number of
invasive cells by ~40% in the SW480 and in the SW620 cells,
compared with the controls. Similarly, anti-miR-708 reduced the
invasive cells by ~80 and ~50%, in the SW480 and SW620 cells,
respectively (Fig. 4B). These
results indicate that miR-708 and miR-31 may be significant in CRC
metastasis.
miR-708 and miR-31 directly target CDKN2B
by binding to the 3′UTR
To investigate the regulatory mechanism of miR-708
and miR-31 in CRC, the target gene for these miRs was investigated
using bioinformatics. The target gene prediction was based on the
following principles: i) The candidate gene has binding sites with
miRs in the 3′UTR; ii) the candidate gene exerts similar roles to
that of the miRs; and iii) the candidate gene is expressed in CRC;
CDKN2B was selected as the target gene for further investigation.
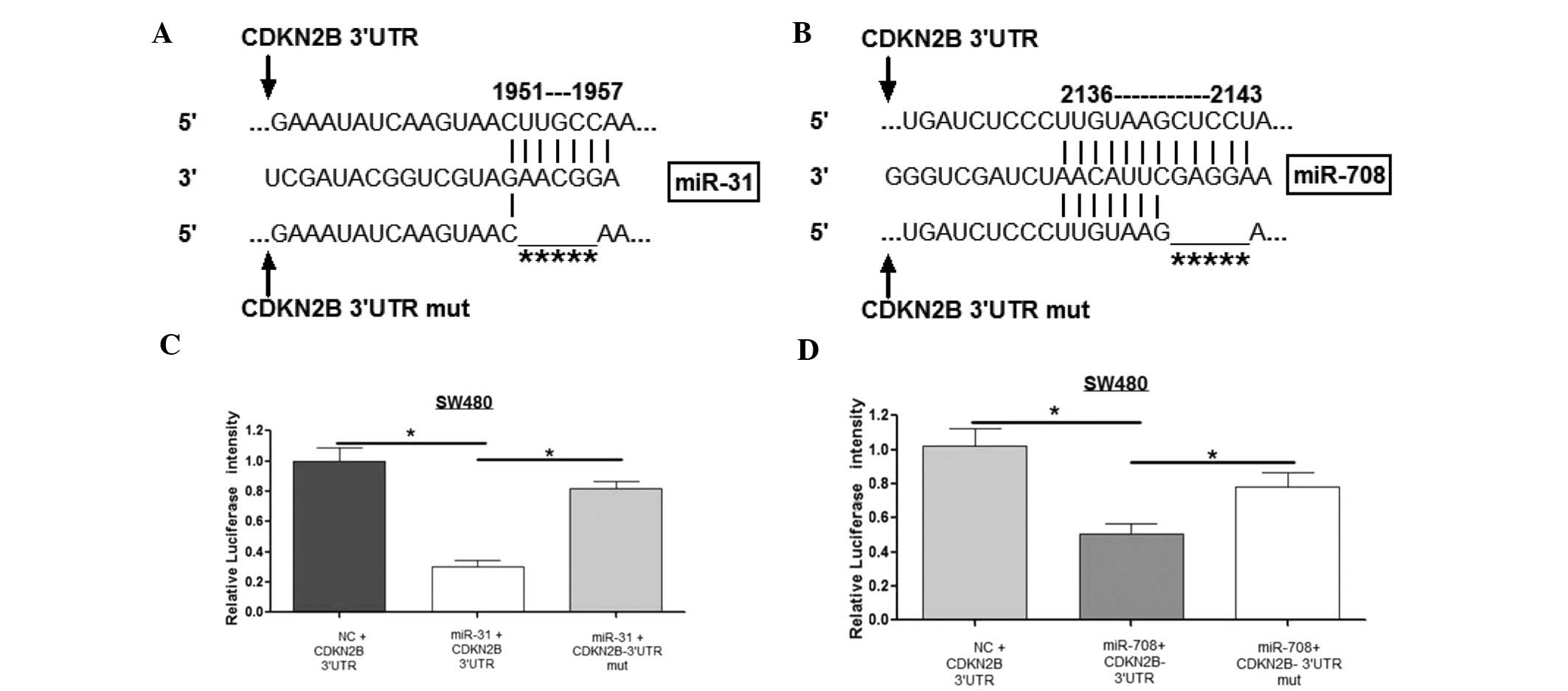
Fig. 5A and B show the alignment of
miR-31 and miR-708 with CDKN2B 3′UTR and mutant 3′UTR. To validate
whether the two miRs regulated CDKN2B 3′UTR directly through the
binding sites, the wild-type and mutant-type of CDKN2B were
constructed and cloned into the downstream of a luciferase reporter
gene. The results from the luciferase reporter assay demonstrated
that miR-31 and miR-708 reduced the luciferase intensity of
wild-type CDKN2B 3′UTR by ~70 and ~50%, respectively, compared with
the miR-NC cells. However, mutation in the binding sites of the
CDKN2B 3′UTR reduced the ability of these two miRs to inhibit
luciferase activity, indicating that they bind directly to the
3′UTR of CDKN2B (Fig. 5C and
D).
miR-708 and miR-31 negatively regulate
CDKN2B expression levels
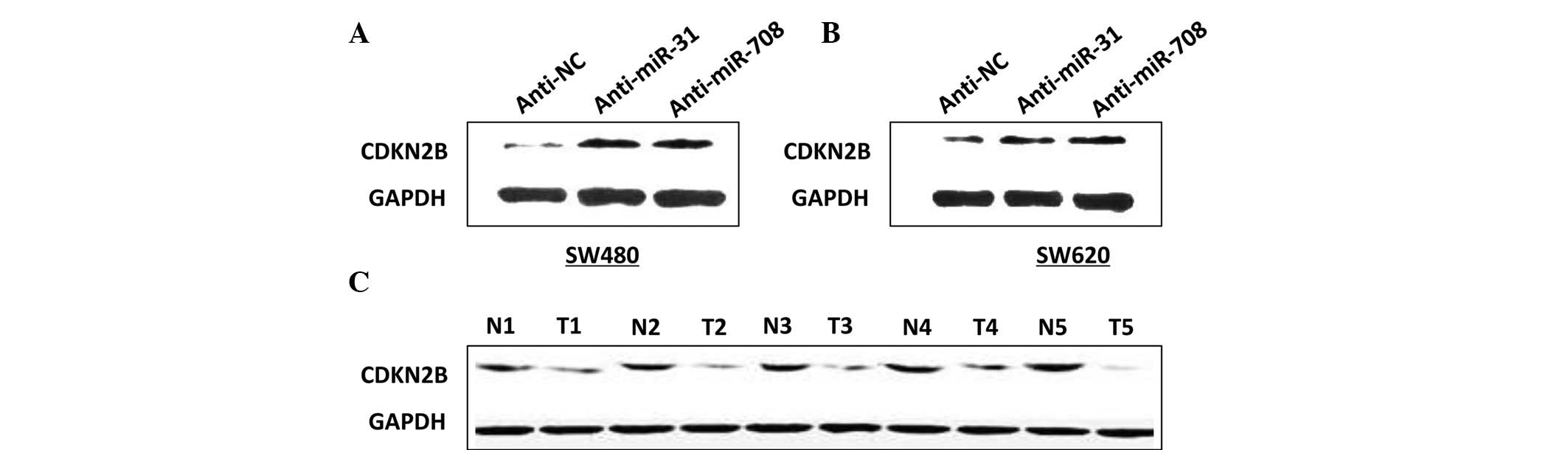
To determine the effect of the two miRs on CDKN2B
expression levels, western blot analysis was conducted. Fig. 6A shows that the expression of CDKN2B
protein levels was increased in the cells with anti-miR-31 or
anti-miR-708, compared with the anti-NC cells (Fig. 6A and B). In addition, CDKN2B protein
levels were investigated in five CRC tissues and the adjacent
healthy tissues. Fig. 6C indicates
that the CDKN2B levels were lower in the CRC tissue samples, which
contrasts with the miR-31 and miR-708 expression levels. Taken
together, these results indicate that CDKN2B is negatively
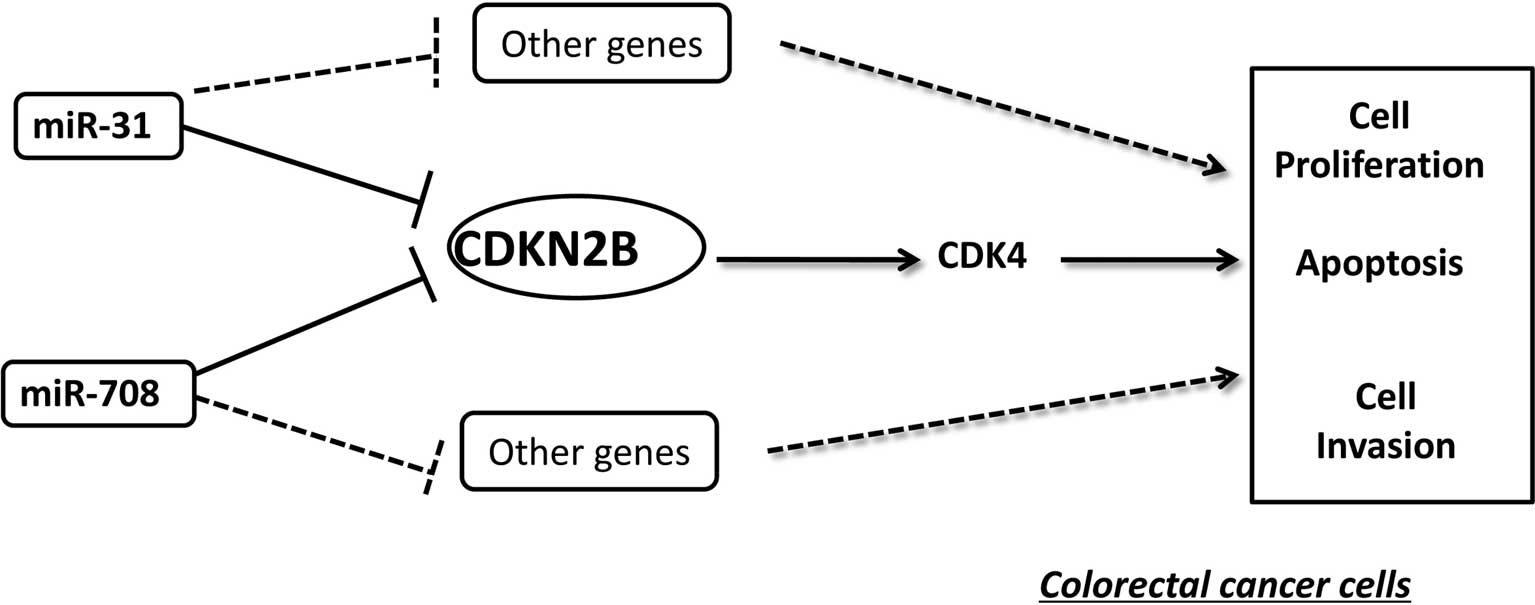
regulated by miR-31 and miR-708. This negative regulation is also
schematically demonstrated in Fig.
7.
Discussion
Increasing evidence shows that 50% of miRs are
located in the fragile or cancer-gene associated regions of
chromosomes (17), indicating that
aberrant miR expression is closely associated with cancer
development. Therefore, to investigate the roles of miRs in cancer
growth and metastasis, it is important to validate the deregulated
miRs. Using miR profiling, previous studies have demonstrated that
miR-708 and miR-31 are upregulated in CRC (18,19),
and the effect of miR-31 in CRC cells has been partially evaluated
(20). However, there are few
studies investigating the roles of miR-708 in CRC. Using qPCR
analysis, the present study identified that miR-708 and miR-31 were
overexpressed in CRC tissues, when compared with healthy colon
tissue samples, which was consistent with prior data (18).
Cell growth and invasion are important
characteristics for cancer cells. In the present study, cell growth
was initially investigated using the MTT assay and apoptosis
analysis. The MTT results indicated that inhibition of miR-31 and
miR-708 suppressed the cell viability. The Annexin V assay, which
was conducted for apoptosis analysis, found that inhibition of
miR-31 and miR-708 led to an induction of cell apoptosis. In
addition, a Transwell chamber assay was performed for the invasion
assay, indicating that anti-miR-31 and anti-miR-708 resulted in the
reduction of cell invasion. Accordingly, the effect of miR-31 and
miR-708 in CRC cells has been observed in other types of cancer.
For example, miR-31 is upregulated in esophageal cancer and
promotes cancer development via suppression of its target gene,
PPP2R2A (21). These data indicate
that miR-31 and miR-708 may be significant in tumor growth and
metastasis, however, this requires further investigation in
vivo.
The results of the functional investigation of the
two miRs in the present study indicate that miR-31 and miR-708
exert a similar effect on CRC cell growth and invasion. To validate
whether these two miRs cooperatively regulate the behaviors of CRC
cells, it was considered crucial to determine the common target
gene for the two miRs. Bioinformatics was used for target gene
prediction. CDKN2B was finally selected from the various candidate
genes. The luciferase reporter assay, a direct method for target
gene validation, subsequently identified that the two miRs reduced
the CDKN2B 3′UTR intensity, while neither miR-31 nor miR-708 had an
effect on CDKN2B 3′UTR that contained the mutant binding sites. The
western blot assay showed that miR-31 and miR-708 reduced CDKN2B
protein levels. In addition, there were low CDKN2B protein levels
in the CRC tissues that had high miR-31 and miR-708 expression
levels. These results identified that CDKN2B was a direct target
gene for miR-31 and miR-708, thus, indicating that one gene may be
regulated by multiple miRs. Certain studies also indicate that
multiple miRs regulate the same gene expression through binding to
various sites in the 3′UTR of a target gene. For example, miR-30d,
miR-181a and miR-199a-5p cooperatively target GRP78 in prostate,
colon and bladder tumors and cancer cell lines (22).
Furthermore, one gene may be modulated by multiple
miRs, however, one miR is also able to regulate numerous genes. The
present study showed that miR-31 and miR-708 target CDKN2B. A
previous study indicated that miR-31 is important in vascular
smooth muscle cell growth via the suppression of LATS2 (23). miR-708 promotes bladder cancer cell
growth and inhibits cell apoptosis by targeting caspase-2 (24). However, the present study indicated
that CDKN2B may partially mediate the functions of miR-31 and
miR-708 in CRC.
In the present study, CDKN2B expression was found to
be downregulated in CRC tissues, which is consistent with the
transcriptome profile of colorectal adenomas (25). Previous studies have identified that
there is a high frequency of methylation in the promoter of CDKN2B,
leading to the downregulation of CDKN2B in colon cancer (26,27).
Therefore, it was hypothesized that the low protein levels of
CDKN2B in the present study may also be due to hypermethylation,
however, this requires further investigation. Certain studies have
indicated that CDKN2B binds to cyclin-dependent kinase complexes
(CDKCs; CD/CDK4 or CD/CDK6) during the cell cycle transition, in
particular at G1/S, resulting in cell cycle arrest at the G1/S
transition and cell proliferation inhibition (28,29).
In addition, endoplasmic reticulum protein 29 suppresses breast
cancer cell invasion by upregulating numerous genes, including
CDKN2B, indicating that CDKN2B is involved in cell invasion
(30). The roles of CDKN2B oppose
those of miR-31 and miR-708, which further indicates that these two
miRs may regulate CRC cell growth and invasion through the
suppression of CDKN2B, however, this requires further
investigation.
In conclusion, it was demonstrated that miR-31 and
miR-708 were upregulated in CRC, and inhibition of the two miRs
induced the reduction of cell growth and invasion; the miRs
function as oncogenes. In addition, a direct target gene, CDKN2B,
was identified for miR-31 and miR-708 and these data indicate that
the two miRs may act via the suppression of CDKN2B (Fig. 7). Therefore, miR-31 and miR-708 may
be involved in a therapeutic strategy for patients as novel
biomarkers for the diagnosis and prognosis of CRC.
References
|
1
|
Zhang Y, He X, Liu Y, et al: microRNA-320a
inhibits tumor invasion by targeting neuropilin 1 and is associated
with liver metastasis in colorectal cancer. Oncol Rep. 27:685–694.
2012.
|
|
2
|
Baraniskin A, Birkenkamp-Demtroder K,
Maghnouj A, et al: MiR-30a-5p suppresses tumor growth in colon
carcinoma by targeting DTL. Carcinogenesis. 33:732–739. 2012.
|
|
3
|
Braconi C, Kogure T, Valeri N, et al:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
|
|
4
|
Chang Y, Yan W, He X, et al: miR-375
inhibits autophagy and reduces viability of hepatocellular
carcinoma cells under hypoxic conditions. Gastroenterology.
143:177–187. 2012.
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
|
|
6
|
Zeng Y, Yi R and Cullen BR: MicroRNAs and
small interfering RNAs can inhibit mRNA expression by similar
mechanisms. Proc Natl Acad Sci USA. 100:9779–9784. 2003.
|
|
7
|
Zhang LY, Liu M, Li X and Tang H:
miR-490-3p modulates cell growth and epithelial to mesenchymal
transition of hepatocellular carcinoma cells by targeting
endoplasmic reticulum-Golgi intermediate compartment protein 3
(ERGIC3). J Biol Chem. 288:4035–4047. 2012.
|
|
8
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
|
|
9
|
Xu P, Vernooy SY, Guo M and Hay BA: The
Drosophila microRNA Mir-14 suppresses cell death and is required
for normal fat metabolism. Curr Biol. 13:790–795. 2003.
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.
|
|
11
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004.
|
|
12
|
Bai S, Nasser MW, Wang B, et al:
MicroRNA-122 inhibits tumorigenic properties of hepatocellular
carcinoma cells and sensitizes these cells to sorafenib. J Biol
Chem. 284:32015–32027. 2009.
|
|
13
|
Tsai WC, Hsu PW, Lai TC, et al:
MicroRNA-122, a tumor suppressor microRNA that regulates
intrahepatic metastasis of hepatocellular carcinoma. Hepatology.
49:1571–1582. 2009.
|
|
14
|
Nie J, Liu L, Zheng W, et al:
microRNA-365, down-regulated in colon cancer, inhibits cell cycle
progression and promotes apoptosis of colon cancer cells by
probably targeting Cyclin D1 and Bcl-2. Carcinogenesis. 33:220–225.
2012.
|
|
15
|
Li Q, Zou C, Han Z, et al: MicroRNA-25
functions as a potential tumor suppressor in colon cancer by
targeting Smad7. Cancer Lett. 335:168–174. 2013.
|
|
16
|
Tang F, Zhang R, He Y, et al:
MicroRNA-125b Induces metastasis by targeting STARD13 in MCF-7 and
MDA-MB-231 breast cancer cells. PloS One. 7:e354352012.
|
|
17
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004.
|
|
18
|
Piepoli A, Tavano F, Copetti M, et al:
Mirna expression profiles identify drivers in colorectal and
pancreatic cancers. PLoS One. 7:e336632012.
|
|
19
|
Necela BM, Carr JM, Asmann YW and Thompson
EA: Differential expression of microRNAs in tumors from chronically
inflamed or genetic (APC(Min/+)) models of colon cancer. PLoS One.
6:e185012011.
|
|
20
|
Cekaite L, Rantala JK, Bruun J, et al:
MiR-9, -31, and -182 deregulation promote proliferation and tumor
cell survival in colon cancer. Neoplasia. 14:868–879. 2012.
|
|
21
|
Alder H, Taccioli C, Chen H, et al:
Dysregulation of miR-31 and miR-21 induced by zinc deficiency
promotes esophageal cancer. Carcinogenesis. 33:1736–1744. 2012.
|
|
22
|
Su SF, Chang YW, Andreu-Vieyra C, et al:
miR-30d, miR-181a and miR-199a-5p cooperatively suppress the
endoplasmic reticulum chaperone and signaling regulator GRP78 in
cancer. Oncogene. 32:4694–4701. 2012.
|
|
23
|
Liu X, Cheng Y, Chen X, et al: MicroRNA-31
regulated by the extracellular regulated kinase is involved in
vascular smooth muscle cell growth via large tumor suppressor
homolog 2. J Biol Chem. 286:42371–42380. 2011.
|
|
24
|
Song T, Zhang X, Zhang L, et al: miR-708
promotes the development of bladder carcinoma via direct repression
of Caspase-2. J Cancer Res Clin Oncol. 139:1189–1198. 2013.
|
|
25
|
Sabates-Bellver J, Van der Flier LG, de
Palo M, et al: Transcriptome profile of human colorectal adenomas.
Mol Cancer Res. 5:1263–1275. 2007.
|
|
26
|
Nieminen TT, Shoman S, Eissa S, Peltomäki
P and Abdel-Rahman WM: Distinct genetic and epigenetic signatures
of colorectal cancers according to ethnic origin. Cancer Epidemiol
Biomarkers Prev. 21:202–211. 2012.
|
|
27
|
Gonzalez-Zulueta M, Bender CM, Yang AS, et
al: Methylation of the 5′ CpG island of the p16/CDKN2 tumor
suppressor gene in normal and transformed human tissues correlates
with gene silencing. Cancer Res. 55:4531–4535. 1995.
|
|
28
|
Shi T, Mazumdar T, Devecchio J, et al:
cDNA microarray gene expression profiling of hedgehog signaling
pathway inhibition in human colon cancer cells. PLoS One.
5:e130542010.
|
|
29
|
Choi S, Kim TW and Singh SV: Ginsenoside
Rh2-mediated G1 phase cell cycle arrest in human breast cancer
cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of
cyclin-dependent kinases. Pharm Res. 26:2280–2288. 2009.
|
|
30
|
Bambang IF, Xu S, Zhou J, et al:
Overexpression of endoplasmic reticulum protein 29 regulates
mesenchymal-epithelial transition and suppresses xenograft tumor
growth of invasive breast cancer cells. Lab Invest. 89:1229–1242.
2009.
|