Introduction
Hepatocellular carcinoma (HCC) exhibits one of the
highest incidences of morbidity and mortality worldwide (1), and presents the predominant
histological subtype of primary liver cancer (2). As postsurgical recurrence of HCC is
frequent and often fatal, surgery and liver transplant offer
limited treatment options for HCC (3,4).
Consequently, it is important to identify an effective drug therapy
for the treatment of HCC. The anticancer ability of certain
traditional Chinese medicines has been accepted in cancer therapy
(4).
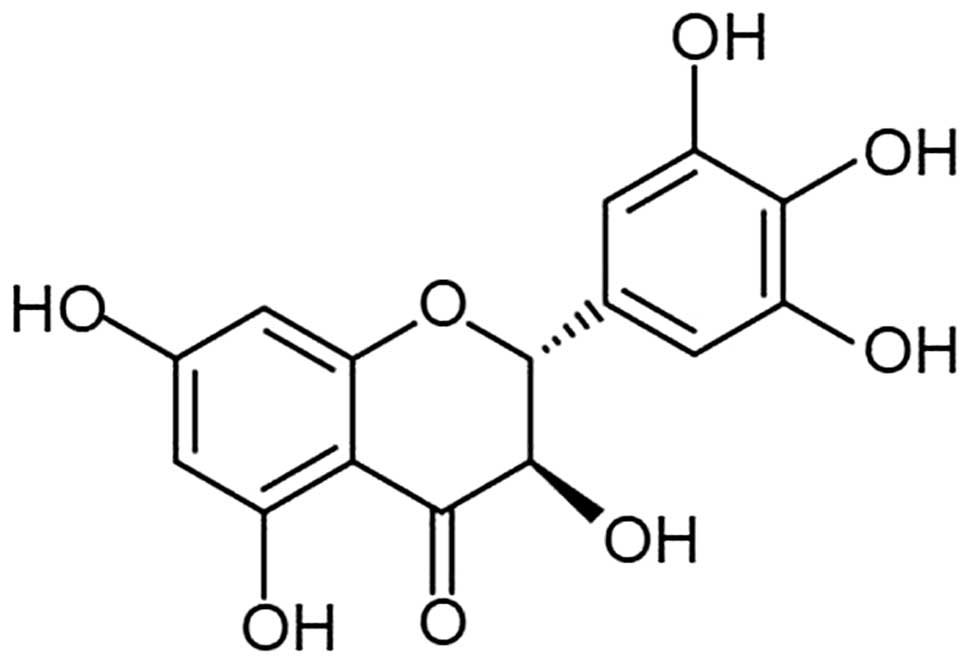
Dihydromyricetin (DHM) is a type of flavonoid
extracted from Ampelopsis grossedentata (Fig. 1), which exhibits pharmacodynamic
effects, including scavenging of free radicals, anti-oxidative,
antithrombotic and anti-inflammatory effects (5–7). In
addition, DHM has been found to exhibit anti-alcoholic and
anti-lipid peroxidation effects (8,9).
Previous studies have suggested that DHM exhibits a protective
ability on the liver. In this study, the suppression of
proliferation and induction of apoptosis by DHM was investigated in
hepatocellular carcinoma cell lines.
Materials and methods
Main reagents
DHM (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethylsulfoxide (DMSO) at a concentration of 50 mM,
and diluted to a working concentration using culture medium, prior
to use. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) and propidium iodide (PI) were also purchased from
Sigma-Aldrich. RNase A was purchased from Thermo Fisher Scientific
(Rockford, Waltham, MA, USA). The In Situ Cell Death Detection kit,
POD was purchased from Roche (Basel, Switzerland), and the
fluorescein isothiocyanate (FITC)-Annexin V staining kit was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). Monoclonal
rabbit anti-human primary antibodies against Bax, Bak, p53,
caspase-3, Caspase-9 and β-actin were obtained from Cell Signaling
Technology, Inc. (Boston, MA, USA). The polyclonal goat anti-rabbit
secondary antibody was purchased from EarthOx Life Science
(Millbrae, CA, USA).
Cell lines and cell culture
Human hepatocellular carcinoma cell lines (HepG2,
QGY7701, QGY7703, QSG7701, Huh-7, MHcc97L, MHcc97H and SK-HEP-1),
one mouse hepatocellular carcinoma Hepal-6 cell line and
immortalized normal human liver HL7702 and L-02 cell lines were
used in this study. HepG2, QGY7701, Hepal-6 and QGY7703 cell lines
were obtained from Shanghai Maternal and Child Health Hospital
(Shanghai, China). L-02, MHcc97L, MHcc97H, QSG7701, Huh-7 and
SK-HEP-1 cell lines were purchased from the Shanghai Cell Bank of
Chinese Academy of Science (Shanghai, China). The HL7702 cell line
was obtained from the Chinese Academy of Science (Kunming, China).
HepG2, QSG7701, L-02, HL7702 and Hepal-6 cells were maintained in
RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco-BRL). QGY7701, QGY7703,
Huh-7, SK-HEP-1, MHcc97L and MHcc97H cells were maintained in
Dulbecco’s modified Eagle’s medium (Gibco-BRL) supplemented with
10% FBS. All cell lines were cultured at 37°C in a humidified
incubator with an atmosphere of 5% CO2.
Cell proliferation assay
The effects of DHM treatment on the cell
proliferation of hepatic cancer cell lines was detected by MTT
assay. Cells were seeded in 96-well plates at a density of
1×104 cells/well with 100 μl culture medium. Following
24 h, culture medium was removed and the same volume of medium,
containing various concentrations of DHM, was added, respectively.
Cells cultured in medium containing DMSO were used as a vehicle
control. Following various periods of time, 5 mg/ml MTT solution
was added to each group (20 μl/well), and then incubated at 37°C
for 4 h. Next, the liquid in each well was removed and replaced
with 150 μl DMSO. The absorbance was then detected using a
microplate reader (PerkinElmer, Waltham, MA, USA) at a wavelength
of 570 nm and the percentages of viable cells were compared with
the control. The experiments were performed independently and at
least in triplicate.
Cell cycle assay
Cells were plated onto 60-mm dishes at a density of
3×106cells/dish. Following overnight growth, the cells
were exposed to various concentrations of DHM, and then harvested
when significant proliferation inhibition was observed. Cells were
fixed in 70% ethanol water at 4°C overnight, followed by incubation
with 100 μg/ml PI and 100 μg/ml RNase A in PBS at 37°C for 1 h. DNA
content was then determined by flow cytometry for the cell cycle
distribution assay. The experiments were performed independently
and at least in triplicate.
Annexin V staining
Annexin V-FITC/PI staining was used to detect
apoptosis induced by DHM according to the manufacturer’s
instructions. Cells were cultured in six-well plates at a density
of 1×105 cells/well. Following overnight growth, cells
were treated with various DHM concentrations and harvested for the
apoptosis assay. Untreated cells were used as a negative control.
The experiments were performed independently and in triplicate.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The late-stage apoptosis of hepatic cell lines was
detected using the In Situ Cell Death Detection kit, POD (Roche).
Cells were seeded in 96-well plates and, following DHM treatment,
cells were fixed with 4% paraformaldehyde according to the
manufacturer’s instructions. The cells were then counterstained
with 4′,6-diamidino-2-phenylindole for 5 min at room temperature in
the dark and observed under a fluorescence microscope (Olympus
IX70; Olympus Corporation, Tokyo, Japan) to detect the apoptotic
cells (TUNEL-positive cells).
Western blot analysis
The expression of the apoptosis-associated proteins
Bak, Bax, p53, caspase-3 and -9 were detected in hepatic cancer
cell lines. Cells were suspended in lysis buffer on ice for 30 min
and the cell lysates were cleared by centrifugation at 13,000 × g
at 4°C for 10 min. The supernatants were then collected and
detected by bicinchoninic assay. Next, the cellular lysates
containing equal amounts of total protein were separated by
SDS-PAGE and transferred to polyvinylidene difluoride membranes.
Membranes were then blocked using 5% non-fat milk in Tris-buffered
saline and Tween 20 (TBST) at room temperature for 1 h, and the
membranes were incubated at 4°C overnight with the primary
antibodies. The membranes were washed three times with TBST for 5
min each and incubated for 1 h with horseradish
peroxidase-conjugated secondary antibodies. The bands were then
analyzed by an enhanced chemiluminescence blotting detection system
(FluorChem E; Proteinsimple, Santa Clara, CA, USA).
Statistical analysis
The data were obtained from at least three
independent experiments and all results are presented as the mean ±
standard deviation. The differences between the groups were
assessed using Student’s t-test. Comparisons were relative to
untreated controls. P<0.05, P<0.01 and P<0.001 were
considered to indicate a statistically significant difference.
Results
DHM inhibits the proliferation of HCC
cell lines
MTT assay was used to investigate the potential
inhibition of cell growth in HCC cells. As shown in Fig. 2, in HCC cells treated with various
concentrations of DHM for 24, 48 and 72 h, cell viability was
significantly inhibited in a dose- and time-dependent manner.
Following 24 h of treatment with 100 μM DHM, the proliferation of
HepG2, QSG7701, QGY7701 and Hepal-6 cells was significantly
inhibited (P<0.01). Furthermore, following 48 h of treatment
with 50 μM DHM, the proliferation of HepG2, Huh7, QSG7701, QGY7701
and Hepal-6 cells was markedly suppressed (P<0.001). The results
showed that the inhibitory rate of MHcc97H, QGY7703 and SK-HEP-1
cells was significantly higher than the control group (0 μM)
following 48 h of treatment with 50 μM DHM (P<0.05), while
MHcc97L cells were significantly inhibited following treatment with
25 μM DHM for 72 h (P<0.01). Since the results revealed that the
inhibitory rates were <50% following treatment with 150 μM DHM
for 72 h, these cell lines were considered to be less sensitive to
DHM administration. Notably, DHM did not affect the cell growth of
immortalized normal human liver HL7702 and L-02 cell lines.
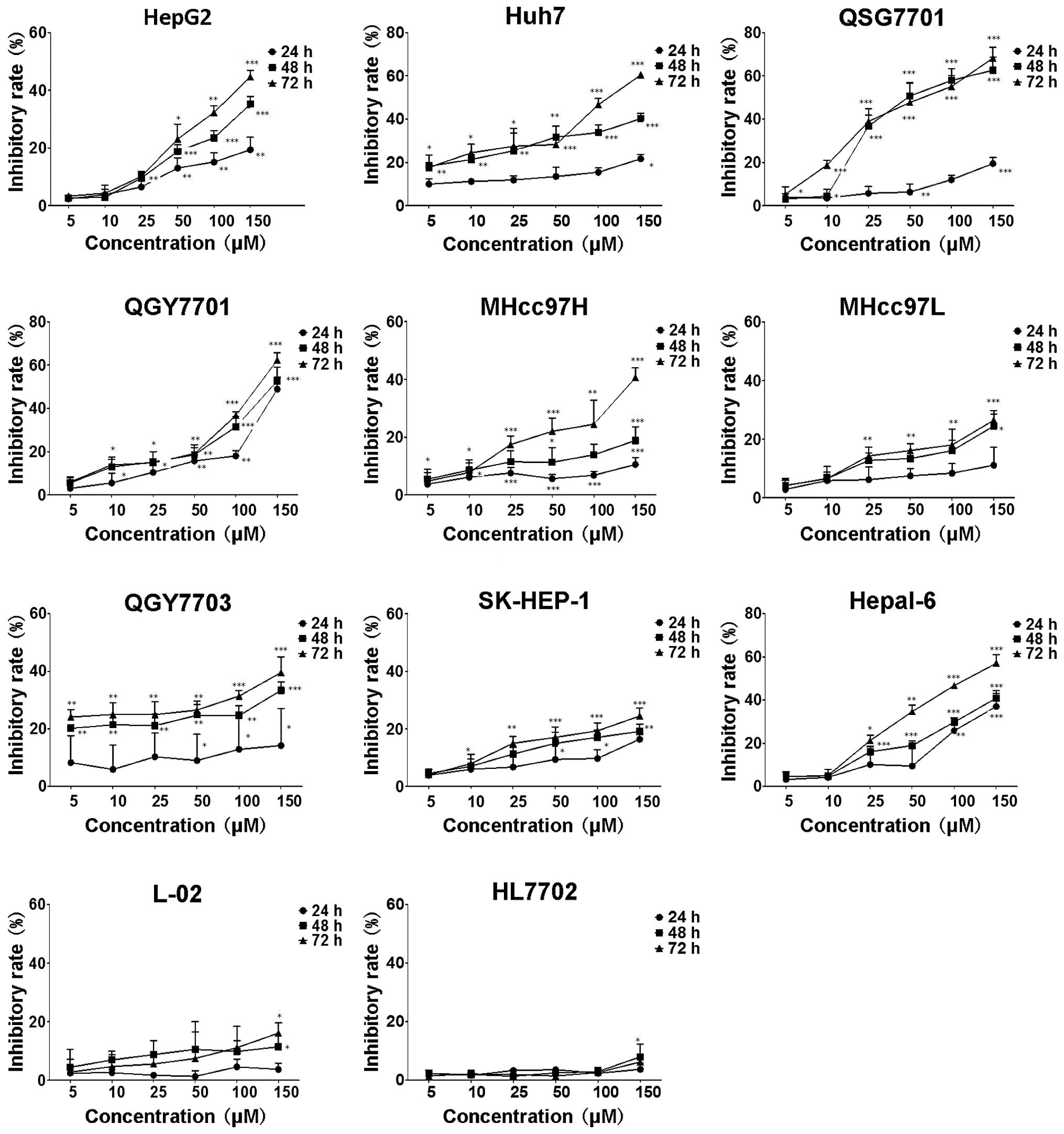 | Figure 2Cell growth inhibition rate of eleven
different types of liver cell lines following DHM treatment. Cells
were exposed to various DHM concentrations (5, 10, 25, 50, 100 and
150 μM) for 24, 48 and 72 h, and the inhibition rate of cells
without DHM treatment was defined as 0. Each sample was duplicated,
and the figures present three independent assays (n=4). Values are
presented as the mean ± standard deviation for at least three
independent experiments performed in triplicate.
*P<0.05, **P<0.01 and
***P<0.001, compared with the untreated (0 μm)
control. DHM, Dihydromyricetin. |
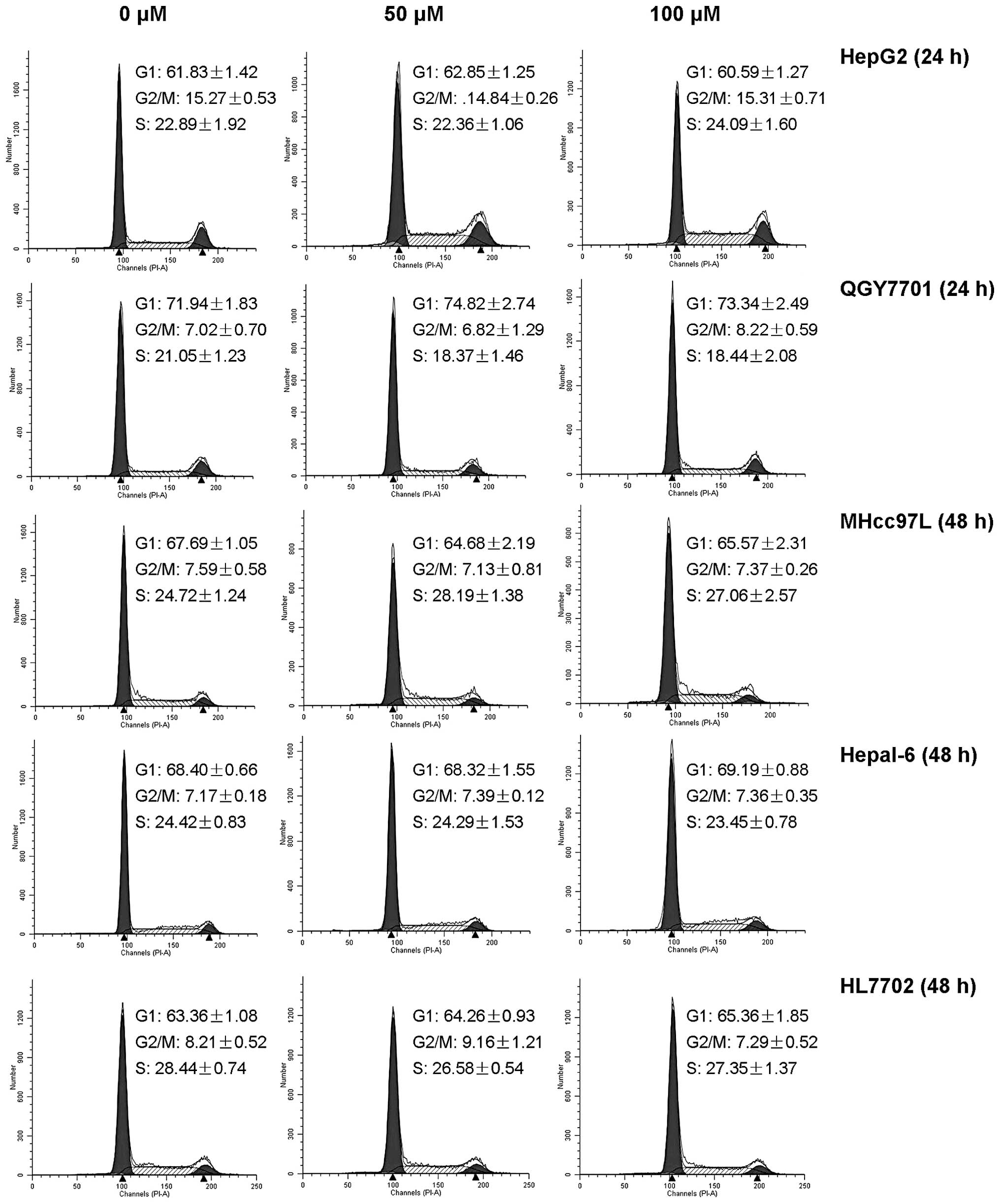
DHM does not induce cell cycle arrest in
HCC cell lines
The cell cycle arrest of HepG2, QGY7701, MHcc97L,
Hepal-6 and HL7702 was detected by flow cytometry. No significant
differences were identified between DHM-treated groups and the
control (Fig. 3).
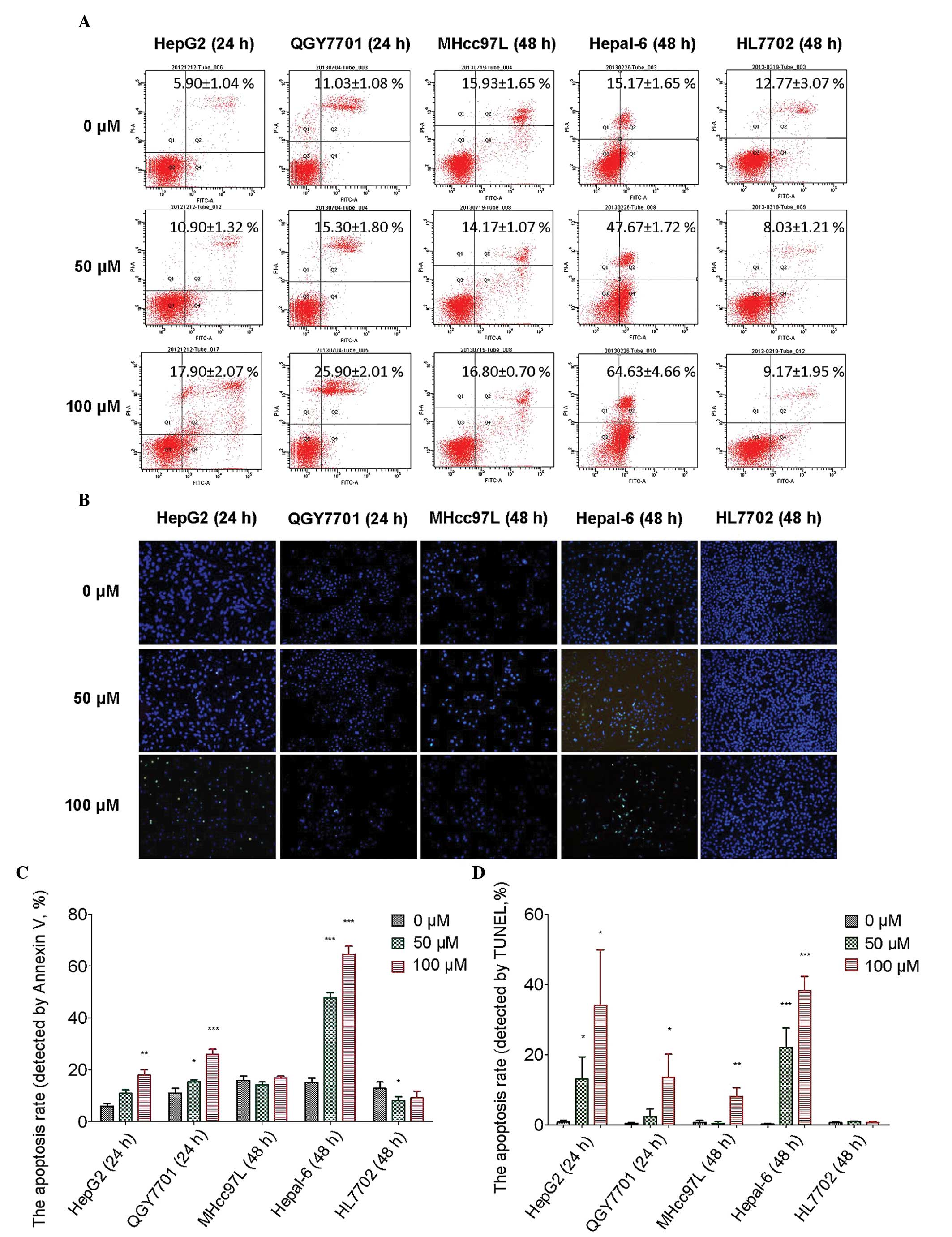
DHM promotes apoptosis in HCC cells
Flow cytometry was used to detect the apoptosis of
HCC cells following DHM treatment. In the present study, HepG2 and
QGY7701 cells were sensitive to DHM treatment. MHcc97L cells were
insensitive to DHM treatment when compared with HepG2 and QGY7701
cells. Hepal-6 is a mouse hepatoma carcinoma cell line, in which a
higher apoptotic rate may be induced than in human HCC cell lines.
HL7702 is an immortalized normal human hepatocyte cell line that
was used as the control. Apoptosis was detected in all cell lines
by Annexin V staining following treatment with DHM (0, 50 and 100
μM) for 24 and 48 h. The results revealed that following 24 h of
treatment with DHM, apoptosis was induced in HepG2 and QGY7701 cell
lines. DHM treatment for 48 h induced very high levels of apoptosis
in Hepal-6 cells. However, apoptosis was not observed in MHcc97L
and HL7702 cells following 48 h of DHM treatment. Notably, the
apoptosis rate significantly decreased in the HL7702 cell line
following treatment with 50 μM DHM, which indicated that lower
concentrations of DHM exhibit protective effects on normal liver
cells (Fig. 4A and B).
A TUNEL assay was used to detect the late-stage
apoptosis of the cell lines following DHM treatment. Late-stage
apoptotic cells were detected in the HepG2 and QGY7701 cell lines
following treatment with DHM for 24 h, and in the remaining cell
lines following treatment with DHM for 48 h. These results were
consistent with the flow cytometry results (Fig. 4C and D).
DHM induces cell apoptosis via the
activation of caspase-3 and subsequent upregulation of p53, Bax and
Bak
In this study, western blot analysis was used to
detect the expression of apoptosis-associated proteins in the
QGY7701, HepG2, MHcc97L, Hepal-6 and HL7702 cell lines. The results
demonstrated that DHM upregulates the levels of Bax, Bak, p53 and
cleaved caspase-3 (p19) proteins in QGY7701, HepG2, MHcc97L and
Hepal-6 cells. However, no significant differences in the
expression of caspase-9 were identified. Furthermore, DHM did not
affect the expression of p53, Bax, Bak or caspase-3 in HL7702 cells
(Fig. 5).
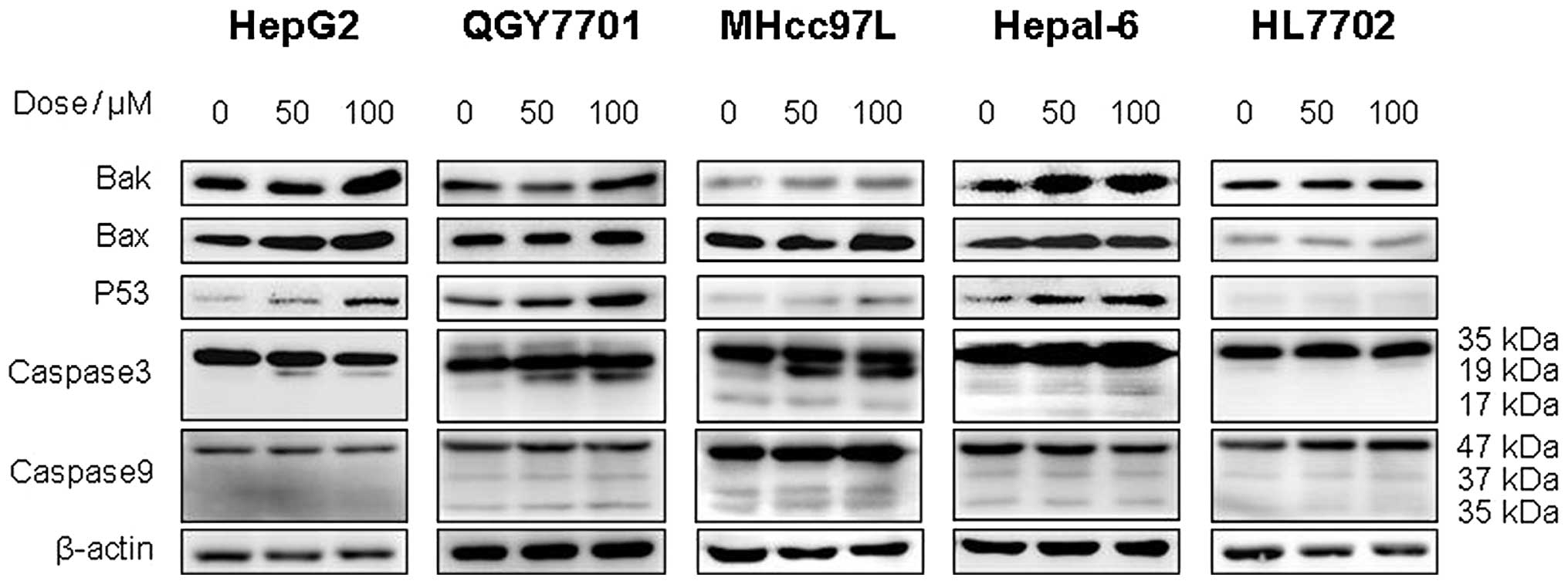 | Figure 5DHM induces cell apoptosis of HCC
possibly via the p53/Bax and caspase-3 signaling pathways. (A)
QGY7701, (B) HepG2, (C) MHcc97L, (D) Hepl-6 and (E) HL7702 cells
were treated with DHM, and the levels of p53, Bax, Bak, caspase-3
and -9 proteins were detected by western blot analysis. DHM
upregulated p53, then p53 recruited the activated form of
caspase-3, which induced cell apoptosis of HCC in a
concentration-dependent manner. No significant differences in the
apoptosis-associated proteins (p53 and cleaved caspase-3) were
identified following DHM treatment of the normal hepatic HL7702
cell line. DHM, dihydromyricetin; HCC, hepatocellular
carcinoma. |
Discussion
In the present study, DHM was found to significantly
inhibit the proliferation of nine different types of HCC cell line
when compared with two immortalized normal human hepatic cell
lines, HL7702 and L-02. The cell cycle assay demonstrated that DHM
did not induce cell cycle arrest in HCC cell lines or normal human
hepatic cell lines. In addition, various concentrations of DHM did
not induce apoptosis in HCC cells. Notably, DHM did not induce
apoptosis in the normal human liver cell lines, which suggested
that DHM may present a novel candidate for the treatment of HCC
based on its selective effects on liver cancer cells rather than
normal liver cells.
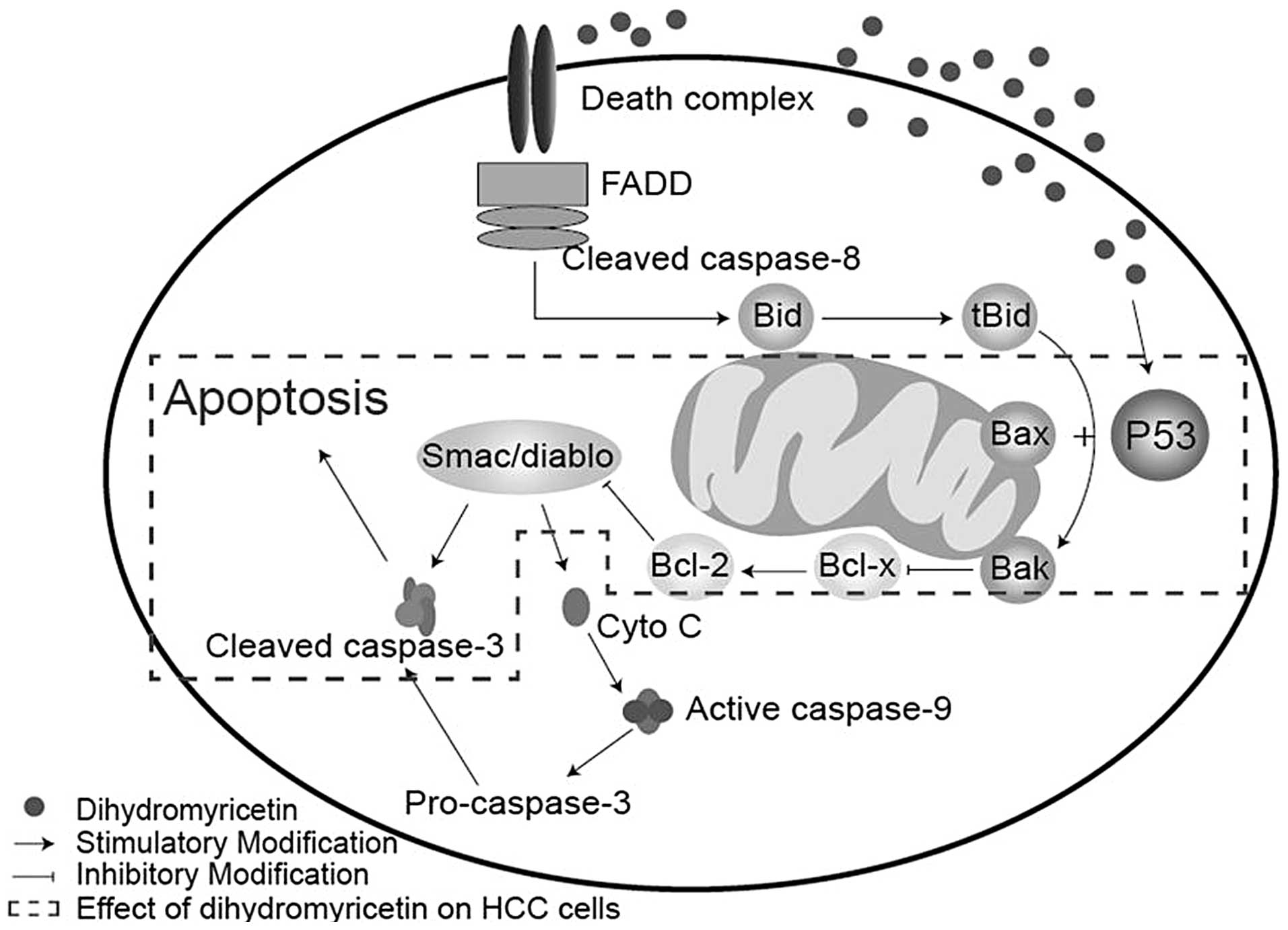
Cell apoptosis is a complex biological process
linked with intricate pathways, whereby the activation of cysteine
proteases (caspases) acts as a key intracellular regulator of cell
apoptosis (10,11). Specifically, caspase-3 is a key
mediator in the caspase family (12). Caspase-3 may be activated by a
variety of activators, which are classified into two predominant
pathways: The death receptor-mediated pathway, involving caspase-8
and-10, and the mitochondrion-mediated pathway, involving caspase-9
(13,14). In the present study, no significant
differences in caspase-9 expression were identified following DHM
treatment, which indicated that DHM selectively induces apoptosis
in HCC cells directly via the death receptor-mediated pathway.
p53 is an important protein in the death receptor
pathway, which is as an upstream regulator of pro-apoptotic Bax and
Bak in mitochondria (15–17). Previous studies have demonstrated
that p53 activates the transcription of Bax and Bak, whereby the
activated Bax and Bak may coordinate with the release of cytochrome
c and Smac/diablo from the mitochondria, leading to the
induction of caspase-9 and -3 activation and/or cleavage, directly
(17,18). In the present study, p53, Bax and
Bak were significantly upregulated following DHM treatment in
different HCC cell lines; however, caspase-9 was not activated. The
mechanism of DHM-induced cell apoptosis may occur as follows, the
upregulation of p53 positively increases the expression of Bax/Bak,
whilst simultaneously inhibiting Bcl-2 protein. This then results
in the activation of caspase-3, leading to cell apoptosis (Fig. 6). In addition, it was found that DHM
did not affect the growth of immortalized normal human liver cells
lines.
In conclusion, the results of the present study
revealed that DHM effectively inhibits proliferation and induces
apoptosis in HCC cells. In addition, DHM exhibited no significant
hepatotoxicity to normal liver cells, which supports the
possibility of DHM serving as a therapeutic candidate for HCC.
Acknowledgements
This study was supported by the National Natural
Science Fund (grant no. 81041099) and the Guangdong Province
Natural Science Fund (grant no. S2011010003750).
References
|
1
|
Chan SL and Yeo W: Targeted therapy of
hepatocellular carcinoma: present and future. J Gastroenterol
Hepatol. 27:862–872. 2012.
|
|
2
|
Perz JF, Armstrong GL, Farrington LA, et
al: The contributions of hepatitis B virus and hepatitis C virus
infections to cirrhosis and primary liver cancer worldwide. J
Hepatol. 45:529–538. 2006.
|
|
3
|
Takayama T, Sekine T, Makuuchi M, et al:
Adoptive immunotherapy to lower postsurgical recurrence rates of
hepatocellular carcinoma: a randomised trial. Lancet. 356:802–807.
2000.
|
|
4
|
Hu Y, Wang S, Wu X, et al: Chinese herbal
medicine-derived compounds for cancer therapy: A focus on
hepatocellular carcinoma. J Ethnopharmacol. 149:601–612. 2013.
|
|
5
|
Chen YQ, Ni DJ, Cheng Q, et al: Study on
the hypolipidemic effect of flavones and dihydromyricetin From
Tengcha. J Tea Sci. 3:221–225. 2422007.
|
|
6
|
Zhong ZX, Zhou GF, Chen XF and Qin JP:
Experimental study on the protective effect of dihydromyricetin
from Guangxi Ampelopsis grossepentata on liver. Chin J
Tradit Med Sci Technol. 9:155–156. 2002.
|
|
7
|
Xu JJ, Yao MJ and Wu MC: Study on
biological efficacy of dihydromyricetin. Food Sci. 29:622–625.
2008.
|
|
8
|
Shen Y, Lindemeyer AK, Gonzalez C, et al:
Dihydromyricetin as a novel anti-alcohol intoxication medication. J
Neurosci. 32:390–401. 2012.
|
|
9
|
He GX, Yang WL, Pei G, et al: Studies on
the effect of dihydromyricetin on antilipid-peroxidation. Zhongguo
Zhong Yao Za Zhi. 28:1188–1190. 2003.(In Chinese).
|
|
10
|
Mu R, Lu N, Wang J, et al: An oxidative
analogue of gambogic acid-induced apoptosis of human hepatocellular
carcinoma cell line HepG2 is involved in its anticancer activity
in vitro. Eur J Cancer Prev. 19:61–67. 2010.
|
|
11
|
Alenzi FQ, Alenazi BQ, AL-Anazy FH, et al:
The role of caspase activation and mitochondrial depolarisation in
cultured human apoptotic eosinophils. Saudi J Biol Sci. 17:29–36.
2010.
|
|
12
|
Wolf BB, Schuler M, Echeverri F and Green
DR: Caspase-3 is the primary activator of apoptotic DNA
fragmentation via DNA fragmentation factor-45/inhibitor of
caspase-activated DNase inactivation. J Biol Chem. 274:30651–30656.
1999.
|
|
13
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005.
|
|
14
|
Ling Y, Lu N, Gao Y, et al: Endostar
induces apoptotic effects in HUVECs through activation of caspase-3
and decrease of Bcl-2. Anticancer Res. 29:411–417. 2009.
|
|
15
|
Cheng EHY, Wei MC, Weiler S, et al: BCL-2,
BCL-X(L) sequester BH3 domain-only molecules preventing BAX-and
BAK-mediated mitochondrial apoptosis. Mol Cell. 8:705–711.
2001.
|
|
16
|
Hussein MR: Analysis of p53, BCL-2 and
epidermal growth factor receptor protein expression in the partial
and complete hydatidiform moles. Exp Mol Pathol. 87:63–69.
2009.
|
|
17
|
Degenhardt K, Chen G, Lindsten T and White
E: BAX and BAK mediate p53-independent suppression of
tumorigenesis. Cancer Cell. 2:193–203. 2002.
|
|
18
|
Henry H, Thomas A, Shen Y and White E:
Regulation of the mitochondrial checkpoint in p53-mediated
apoptosis confers resistance to cell death. Oncogene. 21:748–760.
2002.
|