Introduction
Over the last two decades, breast cancer has
remained the most common type of cancer in females in China
(1). The incidence of breast cancer
has significantly increased, but that of breast cancer-associated
mortality is decreasing over time due to the development of new
diagnostic approaches, the release of new drugs and the
understanding of the molecular pathology of this disease (2). This has encouraged research on novel
and more efficient treatments that overcome the limitations of
conventional chemotherapy. Although adjuvant therapy results in a
survival advantage, the toxicity associated with these therapies is
significant. Thus, the balance between the risks, costs and the
potential benefits for breast cancer patients is of importance
(3). Patients with lymph
node-negative breast cancer exhibit good biological behaviors, but
how these patients will be benefit from cytotoxicity treatments is
unknown. Therefore, it is important to evaluate the association
between prognosis and clinical pathological factors, and to
establish an appropriate treatment regimen.
The potential factors affecting the prognosis of
lymph node-negative breast cancer include tumor diameters (<1
cm) and the status of hormone receptors (4). The prognosis for lymph node-negative
females with small tumors is very good, with a 5-year disease free
survival (DFS) rate of 100%, which decreases with an increase in
tumor size (5). Hormone receptors,
such as estrogen receptor (ER) and progesterone receptor (PR), are
considered not only prognostic factors, but also as biomarkers to
evaluate the efficiency of adjuvant therapy (6). The survival rate of ER-positive
patients is higher as compared with that of ER-negative patients
(7). Other factors associated with
lymph node-negative breast cancer include the age at which the
disease is diagnosed, tissue type of tumor and classification
(8). The identification of
additional prognostic factors will assist physicians in determining
the appropriate therapeutic approach to follow.
Overexpression of cancer-related genes is
characteristic of cancer cells and allows overproduction of
proteins responsible for the acquisition and maintenance of
malignant phenotypes (9). Oncogenes
function in the progression of breast cancer (10,11).
Previous studies have shown that HER2, an epidermal growth factor
(EGF), is detected in ~25% breast cancer patients, and is
associated with a poor prognosis (12,13).
However, the association between HER2 and lymph node-negative
breast cancer is controversial. Other potential genes, such as
TOP2A and CCND1, have been suggested (9,14), but
their association with lymph node-negative breast cancer are in
disagreement. Enhanced understanding of the prognostic implication
of oncogenes in patients with lymph node-negative breast cancer
will provide more accurate prognostic information, and may
influence the treatment options followed.
The present study aimed to investigate the
association between clinicopathological factors and prognosis, as
well as the association between tumor-related gene expression and
prognosis for 341 patients with lymph node-negative breast
cancer.
Patients and methods
Study population
The subjects of the present study included a cohort
of 341 patients with lymph node-negative breast cancer from a total
of 1347 breast cancer patients admitted to the Cancer Hospital of
the Chinese Academy of Medical Sciences (Beijing, China) from 1995
to 1999. All 341 patients were treated with surgery in the early
stages of cancer, and were followed up until 2005.
Clinicopathological factors, including the age at which the
diagnosis was made, menopausal status, tumor diameter, lymph node
dissection, histopathological type, and ER and PR status, were
collected. The 43 patients who exhibited recurrence were considered
as the poor prognosis group, and 40/268 surviving patients were
considered as the good prognosis group for gene and protein
expression analysis. A total of 3/43 cases with poor prognosis were
excluded as they only received a modified radical mastectomy. A
total of 43 cases of breast fibroadenoma tissue were used as
controls. The study was approved by the ethics committee of the
Chinese Academy of Medical Sciences Cancer Hospital (no.
NCC2013-038; Beijing, China).
Immunohistochemistry (IHC)
Normal and tumor tissues were embedded in paraffin
(35×27 mm), and the subsequent paraffin slices were observed under
a microscope [BX46; Olympus (China) Co., Ltd., Beijing, China] to
ensure that the samples had ≥50% of the tumor tissue. A tissue
array was constructed using a tissue microarrayer (ATA-27; Beecher
Instruments, Inc., Sun Prairie, WI, USA). The slides were stained
by immunohistochemical methods, as previously described (15). The monoclonal mouse anti-human HER2
antibody (DakoCytomation, Glostrup, Denmark) was diluted 1:150 in
phosphate-buffered saline (PBS). The monoclonal rabbit anti-human
CCND1 and monoclonal mouse anti-human TOP2A primary antibodies were
purchased from Zhongshan Biotech Co., Ltd., (ZA-0101; Zhongshan,
China) and Fuzhou Maixin Biotech Co., Ltd (MAB-0588; Fuzhou,
China), respectively. Control sections were incubated with PBS
instead of the primary antibody as a negative control in each set
of slides stained. The biotinylated polyclonal goat
anti-mouse/rabbit IgG (ZB-2305, ZB-2301; Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) was used as secondary
antibody with a 1:2,000 dilution. Following
streptavidin-biotinylated horseradish peroxidase complex
incubation, the slides were stained with 3,3′-diaminobenzidine. The
slides were then counterstained with hematoxylin, and mounted with
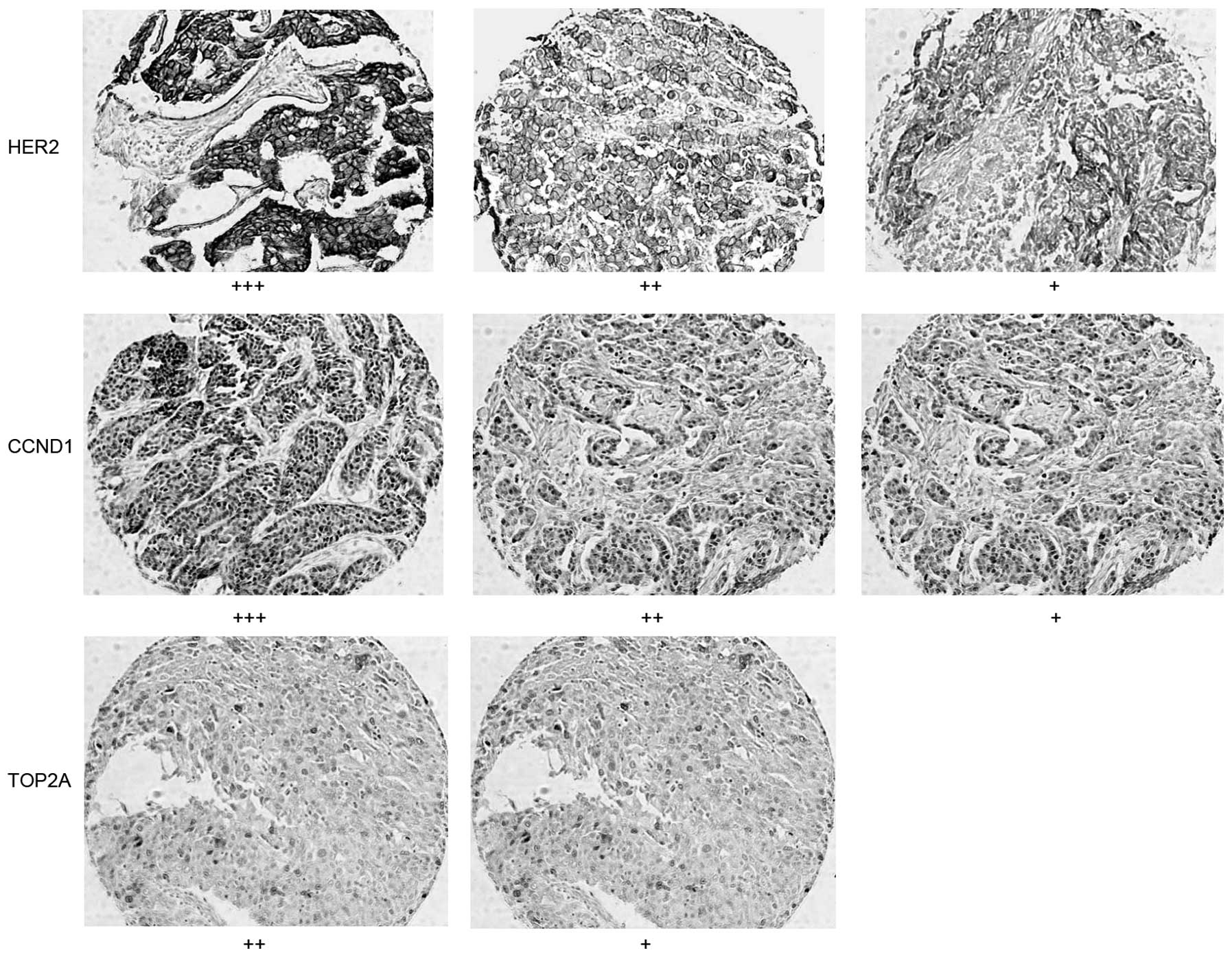
neutral balsam. HER2 was stained brown in the cell membrane.
For data analysis, the Hercep Test Score method was
used as follows (15): Membrane
staining in <10% of tumor cells was defined as 0; weak and
incomplete membrane staining in >10% of cells was defined as
1+; moderate and complete membrane staining in >10%
of tumor cells was defined as 2+; strong and complete
membrane staining in >10% of tumor cells was defined as
3+. Samples with a score of 0 or 1+ were
considered negative, and samples with a score of 2+ or
3+ were considered positive. Cells were considered
positive for CCND1 and TOP2A staining when brown particles were
observed on the nuclei. The percentage of positive cells in a slice
was calculated. Positive staining was defined at three levels:
10–20% was considered as 1+, 20–50% was considered as
2+, and >50% was considered as 3+.
HER2, CCND1, and TOP2A DNA
expression
DNA was extracted from paraffin-embedded samples by
dewaxing, hydration and digestion. The digestion buffer comprising
comprising 50 mmol/l Tris HCl, 1 mmol/l EDTA, 0.5% Tween 20 and 1
mg/ml proteinase K was purchased from Millipore (#39450-01-6;
Billerica, MA, USA). Tissues were centrifuged at 13,000 × g for 3
min after being immersed in dimethylbenzene overnight, and the
supernatant was discarded (repeated for three cycles). The samples
were then hydrated in sequential concentrations of ethanol (100, 95
and 70%). Digestion was performed with four to five volumes of
digestion buffer (50 mmol/l Tris-HCl, 1 mmol/l EDTA, 0.5% Tween 20
and 1 mg/ml proteinase K) and incubated in a water bath for 48 h at
56°C, followed by 95°C for 8–10 min. The supernatant was collected
following centrifugation at 13,000 × g for 3 min and stored at
−20°C for polymerase chain reaction (PCR) analysis. Quantitative
PCR (qPCR) was performed using an ABI Prism 7300 system (Life
Technologies, Grand Island, NY, USA). The master mix included 12.5
μl SYBR® Premix Ex Taq™ (Takara Bio, Inc., Shiga,
Japan), 0.5 μl forward primer, 0.5 μl reverse primer, 1 μl DNA and
10.5 μl ddH2O. The PCR primer sequences were as follows:
Her2-F,5′-GAACTGGTG TATGCAGATTGC-3′; Her2-R,
5′-AGCAAGAGTCCCCAT CCTA-3′. Ccnd1-F,
5′-GGGCAGTTTTCTAATGGAATGG-3′; Ccnd1-R,
5′-CACCACAGTGGCCCACACT-3′. Top2a-F:
5′-GCCAGAATCTGTTCGGTTCAAC-3′; Top2a-R: 5′-AGG
AAACTGAGTGCCGGCTT-3′. GAPDH-F, 5′-CCCCA CACACATGCACTTAC-3′;
GAPDH-R, 5′-CCTAGTCC CAGGGCTTTGAT-3′.
The samples were run in triplicate. The PCR
conditions were as follows: Predenaturation for 10 sec at 95°C, 45
cycles of 95°C for 5 sec, 56°C for 31 sec for HER2 and 60°C for 31
sec for CCND1 and TOP2A, with an added dissociation stage. The
relative gene expression was calculated relative to GAPDH according
to the following equations:
ΔCt = Ct (Target gene) − Ct (GAPDH)
ΔΔCt = ΔCt (samples) − ΔCt (adjust samples)
Gene expression = 2−ΔΔCt
2−ΔΔCt ≥3 was considered to indicate gene
overexpression, and 2−ΔΔCt <3 was not considered to
indicate overexpression.
Statistical analysis
All statistical analyses were performed using SPSS,
version 10.0 (SPSS, Inc., Chicago, IL, USA). The survival rate was
analyzed using the Kaplan-Meier method, and the correlation between
the clinicopathological factors and prognosis were performed by
log-rank test. A χ2 test was used to determine whether
there were significant differences in the mRNA and protein
expression of HER2, CCND1 and TOP2A between good and poor prognosis
patients. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological factors and patient
survival rate, and their association with prognosis
The 341 patients with lymph node-negative breast
cancer were diagnosed between the ages of 18 and 82 years. Among
them, 57.5% were premenopausal and 18.5% had a family history of
tumors. The tumor diameters ranged from 0.5 to 8 cm, the median
value was 2 cm and the average diameter was 2.6 cm. Among these 341
patients, 50% had small tumor (≤2 cm in diameter), and the tumors
were located in the upper outer quadrant of the breast. According
to the classification of the tumor in its pathology, 62.2% were
simplex carcinomas, 25.2% were breast invasive ductal carcinomas
and ~81.5% of the patients had more than 10 lymph node dissections
(Table I).
 | Table IClinicopathological factors of 341
lymph node-negative breast cancer patients. |
Table I
Clinicopathological factors of 341
lymph node-negative breast cancer patients.
| Patients, n (%) | Recurrence patients,
n (%) | No disease survival,
n (%) |
|---|
| Age of Diagnosis,
years |
| ≤35 | 32 (9.4) | 11 (15.1) | 21 (7.8) |
| 36–59 | 244 (71.6) | 53 (72.6) | 191 (71.3) |
| ≥ 60 | 65 (19.1) | 9 (12.3) | 56 (20.9) |
| Menopausal
status |
| Premenopausal | 196 (57.5) | 47 (64.4) | 149 (55.6) |
| Postmenopausal | 145 (42.5) | 26 (35.6) | 119 (44.4) |
| Tumor diameter,
cm |
| ≤2 | 176 (51.6) | 29 (39.7) | 147 (55) |
| 2–5 | 142 (41.6) | 39 (53.4) | 103 (38.3) |
| ≥5 | 23 (6.7) | 5 (6.8) | 18 (6.7) |
| Tumor site |
| Upper out | 160 (46.9) | 37 (50.7) | 123 (45.9) |
| Upper in | 29 (8.5) | 6 (8.2) | 23 (8.6) |
| Bottom out | 18 (5.3) | 4 (5.4) | 14 (5.3) |
| Bottom in | 82 (24) | 15 (20.5) | 67 (25.1) |
| Around the
areola | 52 (15.3) | 11 (15.1) | 41 (15.3) |
| Lymph node
dissection |
| <10 | 63 (18.5) | 13 (17.8) | 50 (18.7) |
| ≥10 | 278 (81.5) | 60 (82.2) | 218 (81.3) |
| Histopathological
type |
| Carcinoma
simplex | 212 (62.2) | 53 (72.6) | 159 (59.3) |
| Invasive ductal | 86 (25.2) | 13 (17.8) | 73 (27.2) |
| Other types | 43 (12.6) | 7 (9.6) | 36 (13.5) |
| ER |
| Positive | 199 (58.4) | 33 (45.2) | 166 (61.9) |
| Negative | 96 (28.2) | 29 (39.7) | 67 (25.0) |
| Unknown | 46 (13.5) | 11 (15.1) | 35 (13.1) |
| PR |
| Positive | 214 (62.8) | 40 (50.8) | 174 (64.9) |
| Negative | 80 (23.5) | 22 (30.1) | 58 (21.6) |
| Unknown | 47 (13.8) | 11 (15.1) | 36 (13.4) |
Approximately 50% of patients received adjuvant
chemotherapy, including cyclophosphamide, cisplatin, vincristine,
methotrexate, fluorouracil, epirubicin, adriamycin and pirarubicin;
while the remaining ~50% of patients received cyclophosphamide
methotrexate fluorouracil. The proportion of patients that received
radiotherapy and hormone therapies were 52.5 and 54.9%,
respectively.
The 5-year DFS and overall survival (OS) rate in
patients >35 years was 85.1 and 95.1%, respectively, which was
significantly higher as compared with patients <35 years (DFS,
P=0.01; OS, P=0.07). The diameter of the tumor significantly
affected the 5-year DFS rate, and patients with small tumors (≤2 cm
in diameter) had significantly higher DFS rates as compared with
patients with large tumors (P=0.02). However, the diameter of the
tumor had no significant effect on the OS rate (P=0.1). Patients
who were ER-positive had a significantly higher 5-year DFS
(P=0.006) and OS rate (P=0.0009) as compared with ER-negative
patients. By contrast, there was no significant difference in the
5-year DFS (P=0.1) or OS rate (P=0.09) between PR-positive and
-negative patients (Table II).
 | Table IIAssociation between
clinicopathological factors and survival for 341 lymph
node-negative breast cancer patients. |
Table II
Association between
clinicopathological factors and survival for 341 lymph
node-negative breast cancer patients.
| DFS | OS |
|---|
|
|
|
|---|
| 5-year, % | P-value | 5-year, % | P-value |
|---|
| Age at diagnosis,
years |
| >35 | 85.1 | 0.0100a | 95.1 | 0.0700 |
| ≤35 | 75.0 | | 90.6 | |
| Menopausal
status |
|
Postmenopausal | 81.6 | 0.2000 | 93.1 | 0.8000 |
| Premenopausal | 84.1 | | 94.9 | |
| Tumor diameter,
cm |
| ≤2 | 86.9 | 0.0200a | 94.3 | 0.1000 |
| >2 | 78.1 | | 93.9 | |
| ER |
| Positive | 87.4 | 0.0060a | 95.9 | 0.0009a |
| Negative | 73.9 | | 89.5 | |
| PR |
| Positive | 85.5 | 0.1000 | 95.3 | 0.0900 |
| Negative | 77.5 | | 92.5 | |
Overall, the patients who received hormone therapy
in an adjuvant setting exhibited a significant improvement in both
the mean DFS (P=0.003) and mean OS (P=0.002), as compared with
those who did not receive hormone therapy. Further analysis
indicated that patients who were premenopausal, had a large tumor
(>2 cm) or were ER-positive were most likely to benefit from
hormone therapy, as compared with patients who were postmenopausal,
had a small tumor (≤2 cm) or were ER-negative (Table III).
 | Table IIIAssociation between hormone therapy
and survival for 341 lymph node-negative breast cancer
patients. |
Table III
Association between hormone therapy
and survival for 341 lymph node-negative breast cancer
patients.
| Mean DFS,
years | Mean OS, years |
|---|
|
|
|
|---|
| Hormone
therapy | Yes | No | P-value | Yes | No | P-value |
|---|
| All patients | 8.3 | 9.3 | 0.003a | 10.2 | 9.4 | 0.002a |
| Menopausal
status |
|
Postmenopausal | 9.4 | 8.7 | 0.100 | 10.1 | 9.4 | 0.200 |
| Premenopausal | 9.2 | 8.1 | 0.008a | 10.1 | 8.5 | 0.006a |
| Tumor diameter,
cm |
| ≤2 | 9.4 | 8.7 | 0.050 | 10.1 | 9.5 | 0.020a |
| >2 | 9.0 | 7.9 | 0.040a | 10.1 | 9.0 | 0.030a |
| ER |
| Positive | 9.6 | 8.6 | 0.010a | 10.1 | 9.5 | 0.004a |
| Negative | 8.9 | 7.8 | 0.070 | 10.1 | 9.0 | 0.200 |
HER2, CCND1 and TOP2A protein
expression
HER2 was stained brown in the cell membrane, whereas
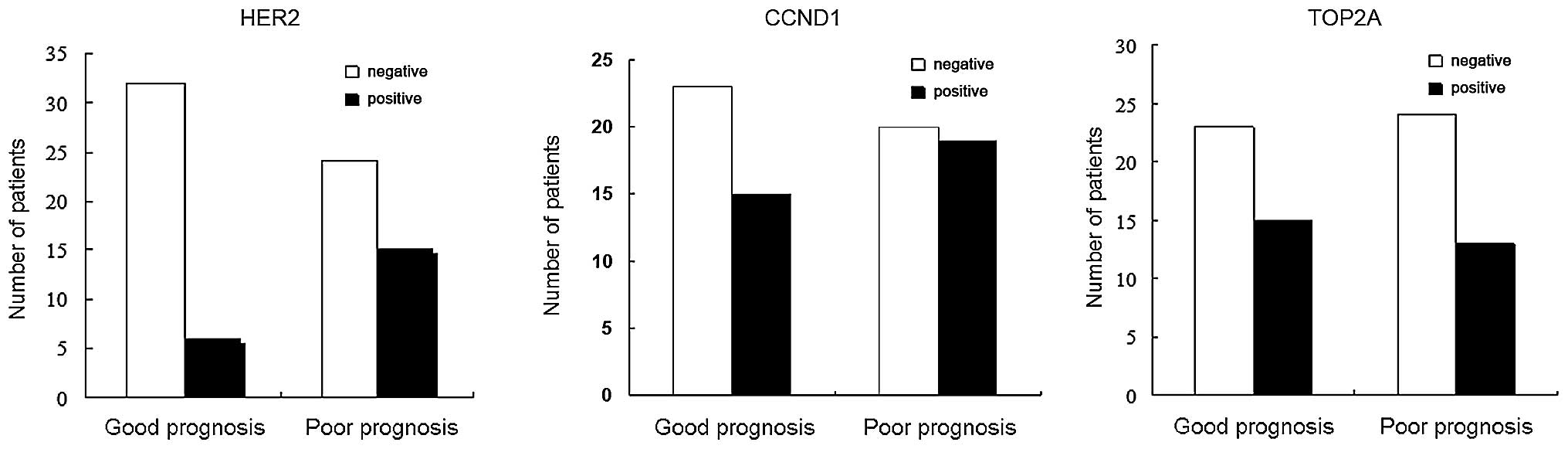
CCND1 and TOP2A were detected in the cell nuclei (Fig 1). Staining was performed on 77 cases
of breast tumors, including 38 cases with a good prognosis and 39
cases with a poor prognosis. In addition, 43 cases of normal breast
tissue were stained successfully in the prepared tissue array. IHC
results showed that 27.2% (21/77) of patients with breast cancer
expressed HER2, while no HER2 expression was detected in normal
breast tissues. HER2 expression was detected in 15.8% (6/38) of the
patients with a good prognosis, which was significantly lower than
that in patients with a poor prognosis (38.5%, 15/39) (P=0.04). A
total of 34/77 patients (44.2%) exhibited positive CCND1 protein
expression. CCND1 expression in normal tissue was detected in only
one case. There was no significant difference in the CCND1 protein
expression between patients with a good (39.5%, 15/38) or poor
(48.7%, 19/39) prognosis (P=0.5). TOP2A protein expression was
detected in 36.4% (28/77) of tumor tissues, which was significantly
higher as compared with that observed in normal tissues. However,
there was no significant difference in TOP2A protein expression
between patients with a good (39.5%, 15/38) or poor (33.3%, 13/39)
prognosis (P=0.6) (Fig. 2).
HER2, CCND1 and TOP2A gene
expression
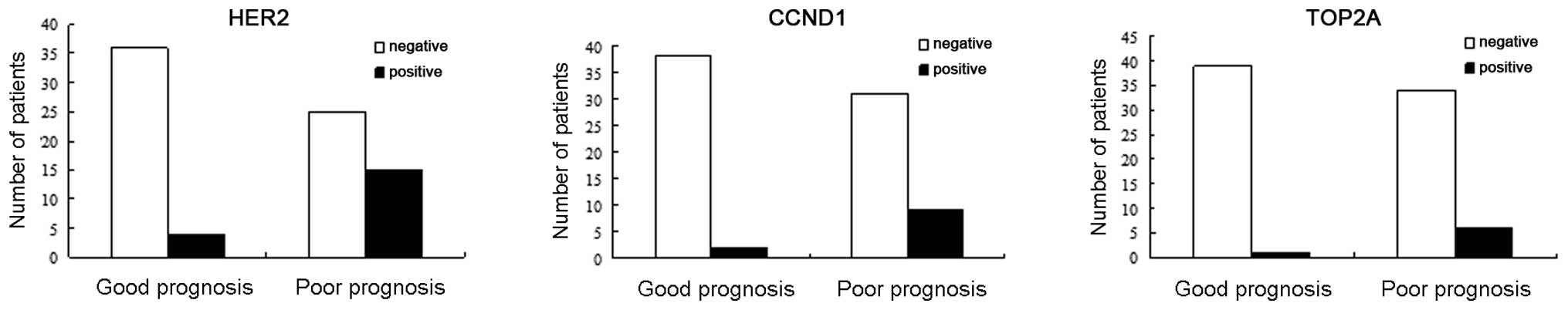
HER2 was expressed in 23.75% of the 80 patients with
lymph node-negative breast cancer, in 37.5% (15/40) of patients in
the poor prognosis group and in 10% (4/40) of patients in the good
prognosis group. HER2 gene expression was detected at a higher
frequency in the poor prognosis group as compared with the good
prognosis group. The expression of CCND1 was significantly
different between the good (22.5%, 9/40) and poor (5%, 2/40)
prognosis groups (P=0.048). TOP2A expression was detected in 7/80
patients (8.75%). There was no significant difference in TOP2A
expression between the good (2.5%, 1/40) and poor (15%, 6/40)
prognosis groups (P=0.108) (Fig.
3). HER2, CCND1 and TOP2A gene expression were not associated
with diagnosis age, menopausal status, tumor diameter or ER
status.
Discussion
The present study investigated the association
between clinicopathological factors and survival rate in 341
patients with lymph node-negative breast cancer. To the best of our
knowledge, the present study is the first to report that the
expression of HER2, but not CCND1 or TOP2A, may be a critical
predictor of a poor prognosis in the Chinese patients with lymph
node-negative breast cancer.
In western countries, the majority of patients are
diagnosed with lymph node-negative breast cancer after the age of
35, and only 4% of the patients are diagnosed with lymph
node-negative breast cancer before the age of 35 (16). In Asian countries, lymph
node-negative breast cancer is diagnosed at younger ages. The
percentage of patients who are diagnosed with lymph node-negative
breast cancer before the age of 35 has been reported to be 11.5% in
the South Korean population (17),
and 8.9% in the Chinese population (18). In the present study, it was
identified that 9.4% of the patients with lymph node-negative
breast cancer were diagnosed under 35 years old, which was
consistent with the results of a previous study (18). In concordance with a study by Chung
et al (19), the DFS rate
was lower in patients <35 years as compared with patients >35
years, which implied that the younger age when lymph node-negative
breast cancer was diagnosed, the worse prognosis. In a separate
study, however, no clear association was identified between the age
of diagnosis and prognosis (20).
This disagreement may result from sample size, standardization and
the range of age variations. It has been well documented that tumor
size is a good predicator for prognosis, and it was considered that
patients with a tumor diameter >2 cm were considered as at high
risk of tumor recurrence (4). The
5-year OS of lymph node-negative breast cancer patients with small
tumors (1 cm in diameter) has been reported as ~100%, and 75% of
patients had no tumor recurrence or metastases 30 years following
the initial diagnosis (5). In the
present study, it was found that tumor size was associated with
DFS, and patients with large tumors had a lower DFS and poor
prognosis. No association was identified between tumor size and OS,
which may be caused by the small population size and the relatively
short follow-up period of the present study. Furthermore, it was
identified that ER-positive patients exhibited a relatively longer
DFS and OS as compared with ER-negative patients. This suggested
that ER status was associated with prognosis, which was in
agreement with previous results (21). ER status is a considered a good
predictor for hormone therapy, and the death rate of ER-positive
patients has been reduced by 5.6% after 5 years of hormone therapy
(6). In the present study, there
was no significant association between lymph node dissection and
prognosis. A previous study, however, showed that patients with
<10 lymph node dissections had a low DFS (22). The discrepancy of lymph node
dissection and prognosis requires further investigation.
In 1998, the Early Breast Cancer Trialist’s
Collaborative Group performed a meta-analysis of 30,000 cancer
patients who received chemotherapy, and found that the mortality
rate of patients <50 years old was reduced by 7%, and the
mortality rate of patients between the ages of 51 and 69 years old
was reduced by 2% (23). A similar
report from the National Surgical Adjuvant Breast and Bowel Project
showed that adjuvant chemotherapy improved the DFS and OS in lymph
node-negative breast cancer and ER-negative patients (24). Chemotherapy, however, was not
beneficial to patients with lymph node-negative breast cancer in
the present study, which may be explained by the short period of
chemotherapy and the limitation of drug application. By contrast,
adjuvant hormone therapy improved the survival rate in the patients
of the present study, which was reflected by the extension of the
DFS and OS rate. Further analysis indicated that adjuvant hormone
therapy had a combined effect on ER-positive and premenopausal
patients, which was consistent with the results of previous studies
(6,24,25).
Hormone therapy was not observed to improve the survival status in
postmenopausal patients; 81.4% of the postmenopausal patients in
the present study had small tumors (tumor diameter, ≤2 cm), and all
of these patients had a good prognosis. Differences in survival
status were not apparent during the relatively short period.
The HER2 gene is located on human chromosome
17q12.1-q12.2. It encodes a 185-kDa transmembrane protein that
belongs to the family of epidermal growth factor receptors.
Approximately 30% of breast cancer primary lymph node-positive
patients have been reported to exhibit HER2 overexpression
(26), and ~60% of in situ
carcinoma patients also have HER2 gene overexpression. Therefore,
HER2 may be an early predictor of breast cancer (27). Ross et al (2003) demonstrated
that HER2 overexpression was associated with poor prognosis in
lymph node-negative breast cancer patients (28). In the present study, HER2 gene
expression was analyzed in Chinese patients with lymph
node-negative breast cancer. High HER2 gene and protein levels were
shown to be associated with poor prognosis. In contrast to HER2,
the association between TOP2A expression and lymph node-negative
breast cancer patients was poor. As a critical protein in the
regulation of DNA replication, TOP2A may be associated with the
prognosis of lymph node-negative breast cancer (29). Previous studies, however, have
suggested that there is no correlation between TOP2A expression and
lymph node-negative breast cancer (30,31).
CCND1 is another potential molecular biomarker as a
predictor of cancer prognosis. The CCND1 gene encodes cyclinD1,
which is an initiation factor controlling G1 to S phase cell cycle
transition. The CCND1 gene is located on human chromosome 11q13 and
has been associated with numerous types of cancer, and has been
shown to be expressed in 5–23% of breast cancer patients (32). CCND1 gene expression is also
associated with estrogen and progesterone (33,34),
but there has been disagreement concerning the association between
CCND1 and prognosis (35,36), although CCND1 is expressed in
ER-positive patients. In the present study, there was no
association between CCND1 protein expression and prognosis in lymph
node-negative breast cancer, but CCND1 gene expression was
associated with poor prognosis. There have been other reports of
inconsistent results between gene and protein expression in several
other types of cancer (37,38). Possible reasons for a discrepancy
include complex gene recombination, posttranscriptional control and
protein translation.
In conclusion, the age at diagnosis, tumor diameter,
ER status and hormone therapy increased the DFS and OS rate in
Chinese patients with lymph node-negative breast cancer. Molecular
biomarker HER2, but not CCND1 and TOP2A, may be a critical factor
as a predictor of breast cancer prognosis.
References
|
1
|
Wang YC, Wei LJ, Liu JT, Li SX and Wang
QS: Comparison of Cancer Incidence between China and the USA.
Cancer Biol Med. 9:128–132. 2012.
|
|
2
|
Tinoco G, Warsch S, Glück S, et al:
Treating breast cancer in the 21st centrury: emerging biological
therapies. J Cancer. 4:117–132. 2013.
|
|
3
|
Alphandéry EL: Perspectives of breast
cancer thermotherapies. J Cancer. 5:472–479. 2014.
|
|
4
|
Goldhirsch A, Glick JH, Gelber RD, Coates
AS and Senn HJ: Meeting highlights: International Consensus Panel
on the Treatment of Primary Breast Cancer. Seventh International
Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin
Oncol. 19:3817–3827. 2001.
|
|
5
|
Rosen PP, Groshen S, Saigo PE, Kinne DW
and Hellman S: Pathological prognostic factors in stage I (T1N0M0)
and stage II (T1N1M0) breast carcinoma: a study of 644 patients
with median follow-up of 18 years. J Clin Oncol. 7:1239–1251.
1989.
|
|
6
|
Tamoxifen for early breast cancer: an
overview of the randomised trials. Early Breast Cancer Trialists’
Collaborative Group. Lancet. 351:1451–1467. 1998.
|
|
7
|
Fisher B, Redmond C, Fisher ER and Caplan
R: Relative worth of estrogen or progesterone receptor and
pathologic characteristics of differentiation as indicators of
prognosis in node negative breast cancer patients: findings from
National Surgical Adjuvant Breast and Bowel Project Protocol B-06.
J Clin Oncol. 6:1076–1087. 1988.
|
|
8
|
Saimura M, Fukutomi T, Tsuda H, et al:
Prognosis of a series of 763 consecutive node-negative invasive
breast cancer patients without adjuvant therapy: analysis of
clinicopathological prognostic factor. J Surg Oncol. 71:101–105.
1999.
|
|
9
|
Hui R, Campbell DH, Lee CS, et al: EMS1
amplification can occur independently of CCND1 or INT-2
amplification at 11q13 and may identify different phenotypes in
primary breast cancer. Oncogene. 15:1617–1623. 1997.
|
|
10
|
Kallioniemi OP, Kallioniemi A, Kurisu W,
et al: ERBB2 amplification in breast cancer analyzed by
fluorescence in situ hybridization. Proc Natl Acad Sci USA.
89:5321–5325. 1992.
|
|
11
|
Rummukainen JK, Salminen T, Lundin J, et
al: Amplification of c-myc by fluorescence in situ hybridization in
a population-based breast cancer tissue array. Mod Pathol.
14:1030–1035. 2001.
|
|
12
|
Jerjees DA, Alabdullah M, Green AR, et al:
Prognostic and biological significance of proliferation and HER2
expression in the luminal class of breast cancer. Breast Cancer Res
Treat. 145:317–330. 2014.
|
|
13
|
Figueroa-Magalhães MC, Jelovac D, Connolly
RM and Wolff AC: Treatment of HER2-positive breast cancer. Breast.
23:128–136. 2014.
|
|
14
|
Bautista S and Theillet C: CCND1 and FGFR1
coamplification results in the colocalization of 11q13 and 8p12
sequences in breast tumor nuclei. Genes Chromosomes Cancer.
22:268–277. 1998.
|
|
15
|
Sidoni A, Ferri I, Cavaliere A, et al:
Detection of HER-2/neu (c-erbB-2) overexpression and amplification
in breast carcinomas with ambiguous immunohistochemical results. A
further contribution to defining the role of fluorescent in situ
hybridization. Anticancer Res. 26:2333–2337. 2006.
|
|
16
|
Winchester DP: Breast cancer in young
women. Surg Clin North Am. 76:279–287. 1996.
|
|
17
|
Han W, Kim SW, Park IA, et al: Young age:
an independent risk factor for disease-free survival in women with
operable breast cancer. BMC Cancer. 4:822004.
|
|
18
|
Chen WG, Li JW, Zhu L, Li YF and Zhu JX:
Analysis of prognosis of breast cancer in women under 35 years of
age (report of 157 cases). Zhong Liu. 2:135–137. 2001.(In
Chinese).
|
|
19
|
Chung M, Chang HR, Bland KI and Wanebo HJ:
Younger women with breast carcinoma have a poorer prognosis than
older women. Cancer. 77:97–103. 1996.
|
|
20
|
Fowble BL, Schultz DJ, Overmoyer B, et al:
The influence of young age on outcome in early stage breast cancer.
Int J Radiat Oncol Biol Phys. 30:23–33. 1994.
|
|
21
|
Mirza AN, Mirza NQ, Vlastos G and
Singletary SE: Prognostic factors in node-negative breast cancer: a
review of studies with sample size more than 200 and follow-up more
than 5 years. Ann Surg. 235:10–26. 2002.
|
|
22
|
Salama JK, Heimann R, Lin F, et al: Does
the number of lymph nodes examined in patients with lymph
node-negative breast carcinoma have prognostic significance?
Cancer. 103:664–671. 2005.
|
|
23
|
No authors listed. Polychemotherapy for
early breast cancer: an overview of the randomised trials. Early
Breast Cancer Trialists’ Collaborative Group. Lancet. 352:930–942.
1998.
|
|
24
|
Fisher B, Jeong JH, Anderson S and Wolmark
N: Treatment of axillary lymph node-negative, estrogen
receptor-negative breast cancer: updated findings from National
Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl
Cancer Inst. 96:1823–1831. 2004.
|
|
25
|
Fisher B, Jeong JH, Bryant J, et al:
Treatment of lymph-node-negative, oestrogen-receptor-positive
breast cancer: long-term findings from National Surgical Adjuvant
Breast and Bowel Project randomised clinical trials. Lancet.
364:858–868. 2004.
|
|
26
|
Slamon DJ, Clark GM, Wong SG, et al: Human
breast cancer: correlation of relapse and survival with
amplification of the HER-2/neu oncogene. Science. 235:177–182.
1987.
|
|
27
|
van de Vijver MJ, Peterse JL, Mooi WJ, et
al: Neu-protein overexpression in breast cancer. Association with
comedo-type ductal carcinoma in situ and limited prognostic value
in stage II breast cancer. N Engl J Med. 319:1239–1245. 1988.
|
|
28
|
Ross JS, Fletcher JA, Linette GP, et al:
The Her-2/neu gene and protein in breast cancer 2003: biomarker and
target of therapy. Oncologist. 8:307–325. 2003.
|
|
29
|
Hajduk M: Topoisomerase II alpha - a
fundamental prognostic factor in breast carcinoma. Pol J Pathol.
60:67–75. 2009.
|
|
30
|
Rudolph P, MacGrogan G, Bonichon F, et al:
Prognostic significance of Ki-67 and topoisomerase IIalpha
expression in infiltrating ductal carcinoma of the breast. A
multivariate analysis of 863 cases. Breast Cancer Res Treat.
55:61–71. 1999.
|
|
31
|
Depowski PL, Rosenthal SI, Brien TP,
Stylos S, Johnson RL and Ross JS: Topoisomerase IIalpha expression
in breast cancer: correlation with outcome variables. Mod Pathol.
13:542–547. 2000.
|
|
32
|
Schwab M: Amplification of oncogenes in
human cancer cells. Bioessays. 20:473–479. 1998.
|
|
33
|
Casimiro MC, Wang C, Li Z, et al: Cyclin
D1 determines estrogen signaling in the mammary gland in vivo. Mol
Endocrinol. 27:1415–1428. 2013.
|
|
34
|
Hernández-Hernández OT and Camacho-Arroyo
I: Regulation of gene expression by progesterone in cancer cells:
effects on cyclin D1, EGFR and VEGF. Mini Rev Med Chem. 13:635–642.
2013.
|
|
35
|
Cheng CW, Liu YF, Yu JC, et al: Prognostic
significance of cyclin D1, β-catenin, and MTA1 in patients with
invasive ductal carcinoma of the breast. Ann Surg Oncol.
19:4129–4139. 2012.
|
|
36
|
Mylona E, Tzelepis K, Theohari I, et al:
Cyclin D1 in invasive breast carcinoma: favourable prognostic
significance in unselected patients and within subgroups with an
aggressive phenotype. Histopathology. 62:472–480. 2013.
|
|
37
|
Vogel C, de Abreu RS, Ko D, et al:
Sequence signatures and mRNA concentration can explain two-thirds
of protein abundance variation in a human cell line. Mol Syst Biol.
6:4002010.
|
|
38
|
Schwanhäusser B, Busse D, Li N, et al:
Global quantification of mammalian gene expression control. Nature.
473:337–342. 2011.
|