Introduction
The incidence of colorectal cancer (CRC) has been
increasing over the past few decades, resulting in the disease
being the fourth most common cancer in Asia (1). Although treatments with radical
surgery, radiotherapy and systemic chemotherapy are performed, the
clinical outcomes of CRC remain unsatisfactory. Adjuvant
chemotherapy is recommended universally for all patients with stage
III CRC, but the role of adjuvant chemotherapy in CRC patients at
stage II has not yet been well established (2). Only 3.6% of stage II patients benefit
from adjuvant chemotherapy; >80% of stage II patients are cured
only by surgery (3,4). At present, although numerous
prognostic markers, such as SMAD4 and CD44v6 (5,6), have
been investigated, only a few are clinically applied due to the
controversial value of prognostic factors. Furthermore, the high
expenses amassed in detecting potential prognostic markers prevent
them from being widely used clinically. Therefore, it is imperative
to identify the prognostic biomarkers that can be assessed by
low-expense methods widely used in clinics and screen for the CRC
subgroup that can benefit from adjuvant chemotherapy to avoid
unnecessary treatment with chemotherapy.
Aberrations in cell cycle regulatory proteins are
common in a number of tumors (7,8). The
cell cycle is a highly organized process regulated by cyclins,
cyclin-dependent kinases (CDKs) and CDK inhibitors (CKIs).
Overexpression of cyclins, CDKs or loss of CKIs, which can promote
the procession of cell cycle, may contribute to uncontrolled
proliferation (9). The cell cycle
comprises of four phases, the G1, S, G2 and M
phases. G1/S, as one of various important checkpoints,
plays a critical role in controlling cell cycle progression.
Aberrations of proteins regulating G1/S checkpoints may
be involved in tumorigenesis (10,11).
Numerous molecules are associated with regulating the cell cycle
transition from the G1 to the S phase. Of these
regulators, the tumor suppressor phosphatase and tensin homolog
(PTEN) and p27 (CDKI 1B, P27KIP-1) have a negative role, whereas
Cyclin D1 acts as a positive regulator (12,13).
The phosphatidylinositol 3-kinase (PI3K) signaling
pathway is a significant pathway regulating cell growth, survival
and proliferation (14). PTEN acts
as a key negative regulator of the PI3/Akt pathway (15) by dephosphorylating
phosphoinositol-3,4,5-triphosphate (PIP3), preventing Akt
activation and downregulating the pathway. Depletion of PTEN has
been reported to accelerate cell proliferation (12) and promote the G0 to
G1 cell cycle transition. Loss of PTEN expression has
been linked to a worse prognoses in patients with CRC (16). However Ghiţă et al (17) demonstrated that PTEN showed no
statistically prognostic value in CRC.
p27 belongs to the CKI family and plays a critical
role in inhibiting the transition from the G1 to S phase
by binding and inhibiting cyclin/CDKs (13). High expression levels of p27 are
present in the G0/G1 phase in the normal cell
cycle. Following mitogenic stimulation, rapid degradation of p27
occurs, allowing the promotion of cell proliferation via the action
of CDK2/cyclin. Thus, depletion of p27 may contribute to the
uncontrolled proliferation of malignant cells. A previous study
(17) showed that the decreased
expression of p27 was associated with a poor prognosis in CRC.
Al-Maghrabi et al (18)
showed that p27 positive expression was associated with a high
recurrence rate, in conflict with the findings by Zlobec et
al (19).
Cyclin D1 is an important cyclin that binds partners
cyclin-dependent kinase (CDK)4 and CDK6 and forms active complexes
that promote G1- to S-phase progression by
phosphorylating and inactivating the retinoblastoma (Rb) protein
(20). The ability of Cyclin D1 to
drive the cell cycle forward can be inhibited by CKIs, such as p21
and p27 (21). Increased levels of
Cyclin D1 may be associated with a shorter survival time (22). However, Belt et al (23) showed that Cyclin D1-positive
staining was associated with a good prognosis. The prognostic
significance of Cyclin D1 in CRC has yet to be resolved.
PTEN co-ordinates G1-phase arrest,
upregulating p27 through its lipid phosphatase activity and
downregulating Cyclin D1 through its phosphatase activity (24). These three proteins interact as a
network.
At present, none of these three proteins has been
clinically applied. The association of the combined detection of
PTEN/p27, PTEN/Cyclin D1 and p27/Cyclin D1 with survival in CRC has
not been documented. The prognostic value of PTEN, p27 and Cyclin
D1 should also be reevaluated. Whether joint examination of PTEN,
p27 and Cyclin D1 may predict the clinical outcome for CRC patients
requires investigation. The aim of the present study was to use
immunohistochemistry to determine the expression patterns of PTEN,
p27 and Cyclin D1 in normal and tumor epithelium in stage II/III
CRC patients. The correlation of the expression of the three
proteins with the clinicopathological characteristics and overall
survival time was analyzed respectively. The correlation between
concomitant expression of these markers and overall survival time
was assessed, and an analysis of whether combined detection can
provide additional prognostic clues in order to contribute to
identifying precise prognostic biomarkers was performed.
Materials and methods
Materials
The study group was comprised of 61 patients with
CRC in either stage II or III who underwent surgery between March
2005 and May 2006 at The Fourth People’s Hospital of Jinan (Jinan,
Shandong, China). A total of 32 patients were at stage II and 29 at
stage III, according to the 7th American Joint Committee on Cancer
staging system (25). Another 20
samples of corresponding adjacent non-cancerous tissues were used
as controls. All tissues were collected from the Pathology
Department (The Fourth People’s Hospital of Jinan), and all
patients were followed up until May 31, 2011. Tissue samples were
fixed in 10% formalin and embedded in paraffin. Of the 61 cases, 38
were male and 23 were female. The mean age at diagnosis was 64
years (range, 27–85 years). A total of 46 cases were of moderate
grade and 15 were of low grade, while 29 had pathological lymph
node metastasis and 32 did not. The number of pT3 and pT4 cases was
31 and 32, respectively. All cases were followed up for overall
survival analysis subsequent to being diagnosed until May 31, 2011.
The follow-up period was initiated on the date when the patient was
first diagnosed with CRC and lasted until mortality or the end of
follow-up. The median follow-up duration was 6.7 years. Patients
provided written informed consent and the study was approved by the
ethics committee of the Fourth People’s Hospital of Jinan.
Immunohistochemical staining
Immunohistochemical studies for PTEN, p27 and Cyclin
D1 were performed on formalin-fixed, paraffin-embedded surgical
sections, consisting of the 61 CRC and 20 normal tissues. The
tissue sections were deparaffinized and soaked in 0.01 M sodium
citrate buffer, and the cell antigens were retrieved. Subsequent to
blocking non-specific binding with 10% bovine serum, the sections
were incubated with anti-human mouse monoclonal antibodies against
PTEN (1:100), p27 (1:100) and cyclin D1 (1:100) (all Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C.
Anti-rabbit goat polyclonal antibodies (Vector Laboratories,
Burlingame, CA, USA) were used at dilutions of 1:50. The bound
antibodies were visualized using the avidin-biotin-peroxidase
method (Vector Laboratories). All sections were counterstained with
hematoxylin. Negative control sections were prepared using
Tris-buffered saline instead of primary antibody. Sections of
tonsil tissue obtained from the excision of the tonsils with known
PTEN expression were used as positive controls for PTEN.
Criteria for judging
The samples were assessed in a blind manner by two
investigators with no knowledge of the clinicopathological data.
Five microscopic fields were randomly viewed to calculate the mean
number of positive cells. The staining was evaluated only in the
areas with well-preserved tissue morphology and away from necrosis
or artefacts. PTEN expression was observed in the cytoplasm. The
intensity was scored according to a four-tier system, as follows:
0, no staining; 1, weak; 2, moderate; and 3, strong. Another 1, 2,
or 3 points was assigned if the percentage of positive cells was
<25, 25–50, or >50%, respectively. Specimens were defined as
positive if the score was ≥4 (12).
Staining for p27 and Cyclin D1 was found in the
nucleus. All cases were scored as either positive (≥10% of tumor
cells with strong nuclear staining), negative (<10% of tumor
cells with strong nuclear staining) or non-informative (26). A staining extent of >5% of the
tumor cells was considered positive for Cyclin D1, while ≤5% was
considered negative (22).
Statistical analysis
Fisher’s exact test was adopted to compare the
difference in expression levels of the three proteins between the
CRC and control groups. The χ2 test was used to examine
the association between PTEN, p27 and Cyclin D1 expression and the
clinicopathological parameters. Spearman’s linear regression test
was used to evaluate correlations among protein expression levels.
Survival curves were created using the Kaplan-Meier method, and any
differences in the survival curves were compared by the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
with SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL,
USA).
Results
Immunohistochemical expression of PTEN,
p27 and Cyclin D1
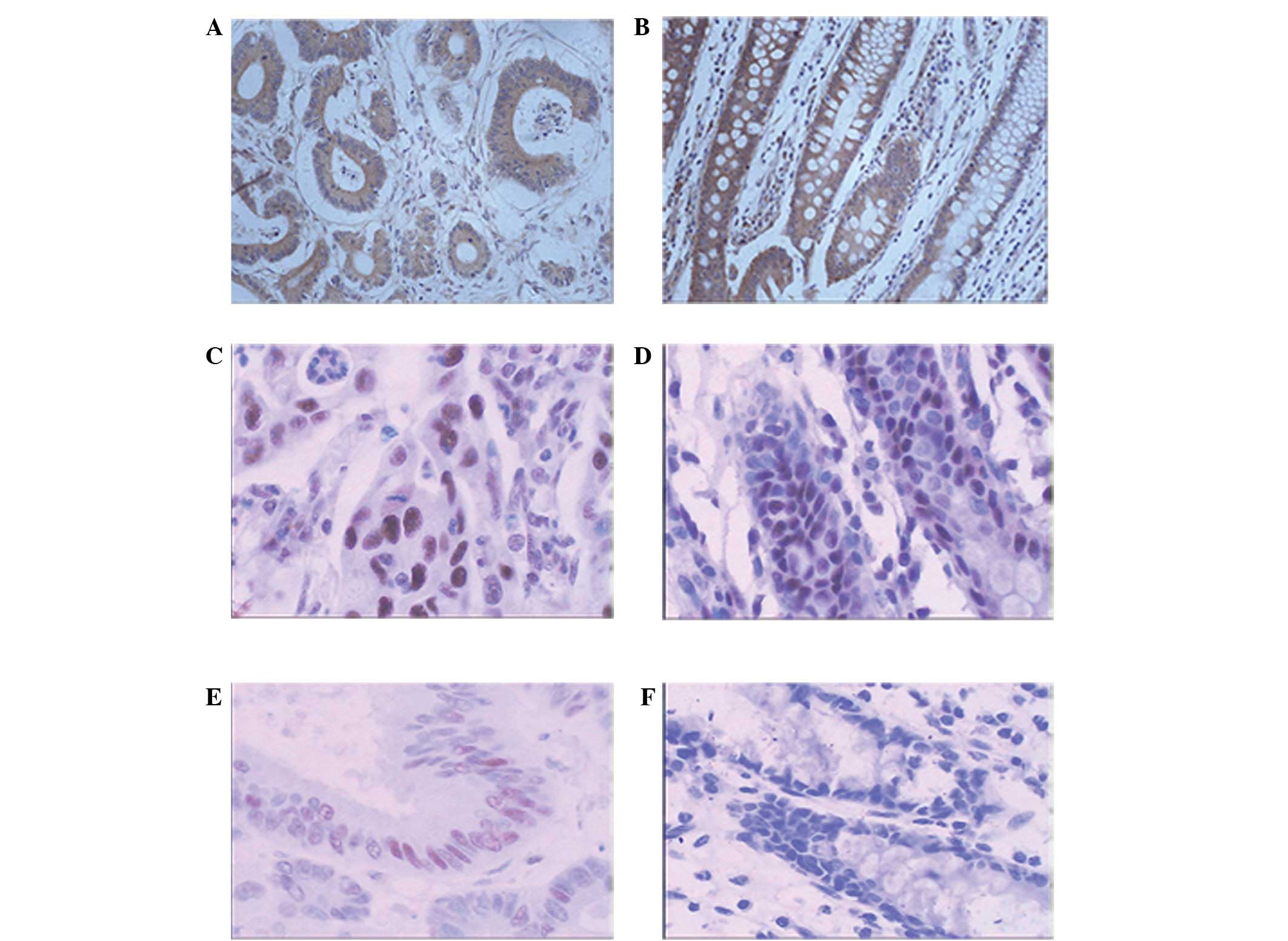
Positive staining for PTEN was detected in the
cytoplasm, but not in the nucleus. Of the 61 tumors analyzed, 26
(42.62%) demonstrated loss of PTEN expression, while 35 (57.38%)
retained PTEN expression (Fig. 1A).
All 20 (100%) in the control group tested positive (Fig. 1B). A total of 20 out of 61 (32.79%)
cases of CRC showed depleted p27 expression, and 41 out of 61
(67.21%) cases revealed p27 nuclear accumulation (Fig. 1C). However, all 20 (100%) showed
nuclear positivity in the control group (Fig. 1D). Cyclin D1 immunoreactivity was
confined to the nucleus. The positive expression of Cyclin D1 was
observed in 28 out of 61 (45.90%) cases of CRC (Fig. 1E), while no staining was observed in
the control group (Fig. 1F).
Expression levels of PTEN, p27 and Cyclin D1 between the CRC and
control groups were statistically different (P<0.05) (Table I).
 | Table IPTEN, p27 and Cyclin D1 expression in
the control and CRC groups. |
Table I
PTEN, p27 and Cyclin D1 expression in
the control and CRC groups.
| | PTEN | p27 | Cyclin D1 |
|---|
| |
|
|
|
|---|
| Group | Total | Positive, n (%) | P-value | Positive, n (%) | P-value | Positive, n (%) | P-value |
|---|
| Control | 20 | 20 (100.00) | 0.001 | 20 (100.00) | 0.008 | 0 (0.0) | 0.001 |
| CRC | 61 | 35 (57.38) | | 41 (67.21) | | 28 (45.90) | |
Correlation of PTEN, p27 and Cyclin D1
expression with the clinicopathological parameters
Table II shows that
the expression levels of PTEN and p27 were correlated with tumor
stage, histological grade and lymph node metastasis, and not with
gender, age or depth of tumor invasion. The depletion of PTEN and
p27 were more common in cases of stage III, low grade and lymph
node metastasis compared with those of stage II, moderate grade and
no lymph node metastasis (P<0.05, Table II). Similarly, the expression of
Cyclin D1 showed significant correlation with tumor stage, lymph
node metastasis and depth of tumor invasion, and no correlation
with gender, tumor grade and age. Cyclin D1-positive expression was
frequently detected in CRC of stage III, with lymph node metastasis
and deeper invasion (P<0.05, Table
II).
 | Table IICorrelation between expression of
PTEN, p27 and Cyclin D1 and clinicopathological characteristics in
61 patients with CRC. |
Table II
Correlation between expression of
PTEN, p27 and Cyclin D1 and clinicopathological characteristics in
61 patients with CRC.
| | PTEN | p27 | Cyclin D1 |
|---|
| |
|
|
|
|---|
| Variables | Total, n | Positive, n (%) | P-value | Positive, n (%) | P-value | Positive, n (%) | P-value |
|---|
| Age, years | | | 0.979 | | 0.796 | | 0.768 |
| ≥60 | 37 | 23 (62.2) | | 26 (70.3) | | 20 (54.1) | |
| <60 | 24 | 12 (50.0) | | 15 (62.2) | | 8 (33.3) | |
| Gender | | | 0.241 | | 0.052 | | 0.068 |
| Male | 38 | 24 (63.2) | | 29 (76.3) | | 14 (36.8) | |
| Female | 23 | 11 (47.8) | | 12 (52.2) | | 14 (60.9) | |
| Tumor grade | | | 0.001 | | 0.004 | | 0.207 |
| Moderate | 46 | 32 (69.6) | | 36 (78.3) | | 19 (41.3) | |
| Poor | 15 | 3 (20.0) | | 5 (33.3) | | 9 (60.0) | |
| Tumor invasion | | | 0.355 | | 0.316 | | 0.003 |
| T3 | 30 | 19 (63.3) | | 22 (73.3) | | 8 (26.7) | |
| T4 | 31 | 16 (51.6) | | 19 (61.3) | | 20 (64.5) | |
| Lymph
metastasis | | | 0.001 | | 0.001 | | 0.011 |
| N0 | 32 | 26 (81.3) | | 28 (87.5) | | 9 (28.1) | |
| N1 | 18 | 7 (38.9) | | 9 (50.0) | | 12 (66.7) | |
| N2 | 11 | 2 (18.2) | | 4 (36.4) | | 7 (63.6) | |
| Clinical stage | | | 0.002 | | 0.001 | | 0.002 |
| II | 32 | 26 (81.3) | | 28 (87.5) | | 9 (28.1) | |
| III | 29 | 9 (31.0) | | 13 (44.8) | | 19 (65.5) | |
Correlation among PTEN, p27 and Cyclin D1
expression
The depletion of PTEN expression was significantly
correlated with the loss of p27 (r=0.810; P<0.001) and with the
increased expression of Cyclin D1 (r=−0.470; P<0.001). However,
the decreased expression of p27 was inversely associated with the
Cyclin D1 level (r=−0.548; P<0.001).
Correlation of expression PTEN, p27 and
Cyclin D1 with prognosis
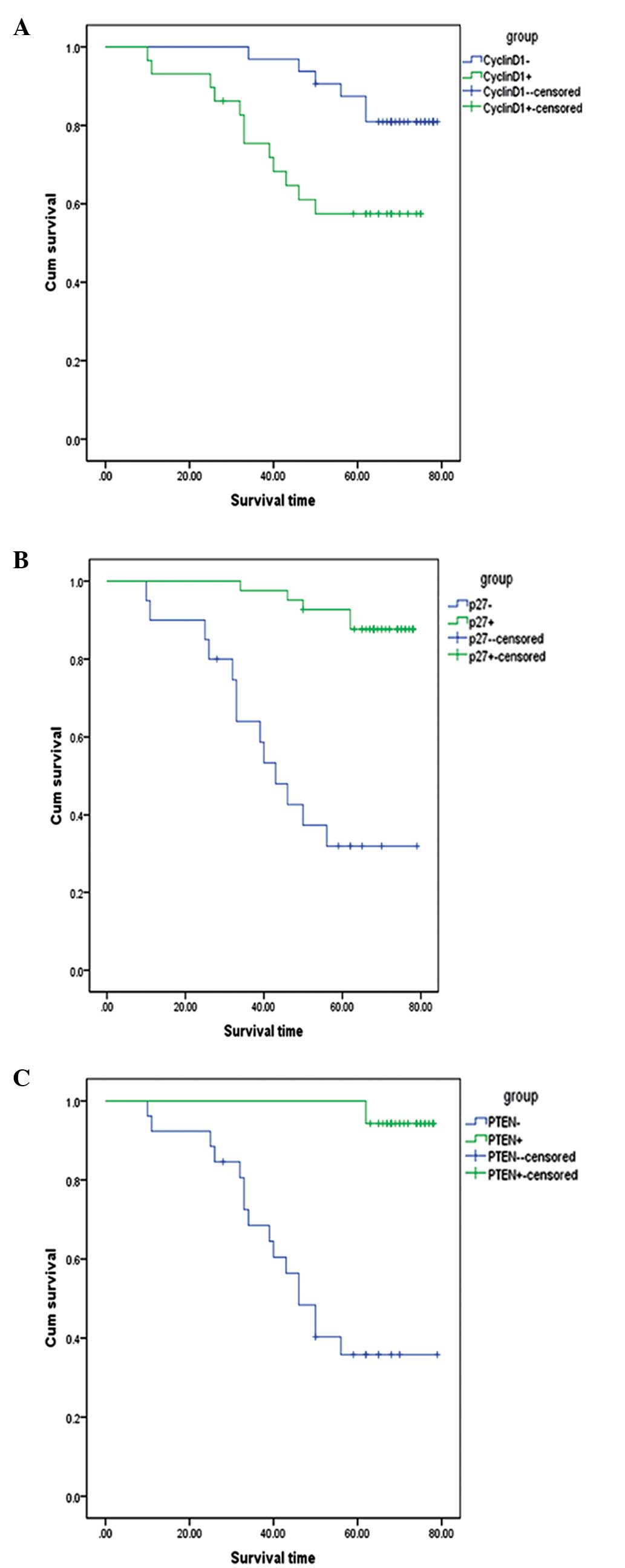
Of the 61 patients, 35 patients showed PTEN-positive
staining, with a mean survival time of 77.1 months, whereas PTEN
depletion was found in 26 patients, who exhibited a mean survival
time of 51.5 months (χ2=28.71; P<0.001) (Fig. 2A). A total of 41 patients expressed
high p27 levels, and 20 patients demonstrated p27 depletion, with a
mean survival rate of 74.7 and 48.7 months, respectively
(χ2=26.88 ; P<0.001) (Fig. 2B). In total, 29 patients showed
Cyclin D1-positive expression and 32 exhibited no staining, with a
mean survival time of 56.9 and 73.8 months, respectively
(χ2=5.36; P=0.021) (Fig.
2C).
To determine whether joint examination of these
proteins could provide additional information for prognosis, the
survival Kaplan-Meier test for the combination of PTEN/p27,
PTEN/Cyclin D1 and p27/Cyclin D1 was analyzed. The results
indicated that PTEN and p27 are markers for good prognosis, whereas
Cyclin D1 predicts adverse prognosis. In Fig. 3A, the survival curves among the
patients with PTEN(+), PTEN(−), p27(+), p27(−), PTEN(+)/p27(+) and
PTEN(−)/p27(−) expression was assessed. The patients with PTEN(+),
PTEN(−), p27(+), p27(−), PTEN(+)/p27(+) and PTEN(−)/p27(−)
expression exhibited a mean survival time of 77.1, 51.5, 74.7,
48.7, 77.1, and 48.7 months, respectively. The patients with
PTEN(+)/p27(+) expression exhibited a 2.4-months longer survival
time than the patients with p27(+) expression alone. Notably, the
patients with PTEN(−)/p27(−) expression exhibited a 2.8-months
shorter survival time compared with those with PTEN(−) expression
alone. As expected, the patients with PTEN(+)/p27(+) expression
exhibited the best overall survival time, whereas the patients with
PTEN(−)/p27(−) expression exhibited the worst survival time.
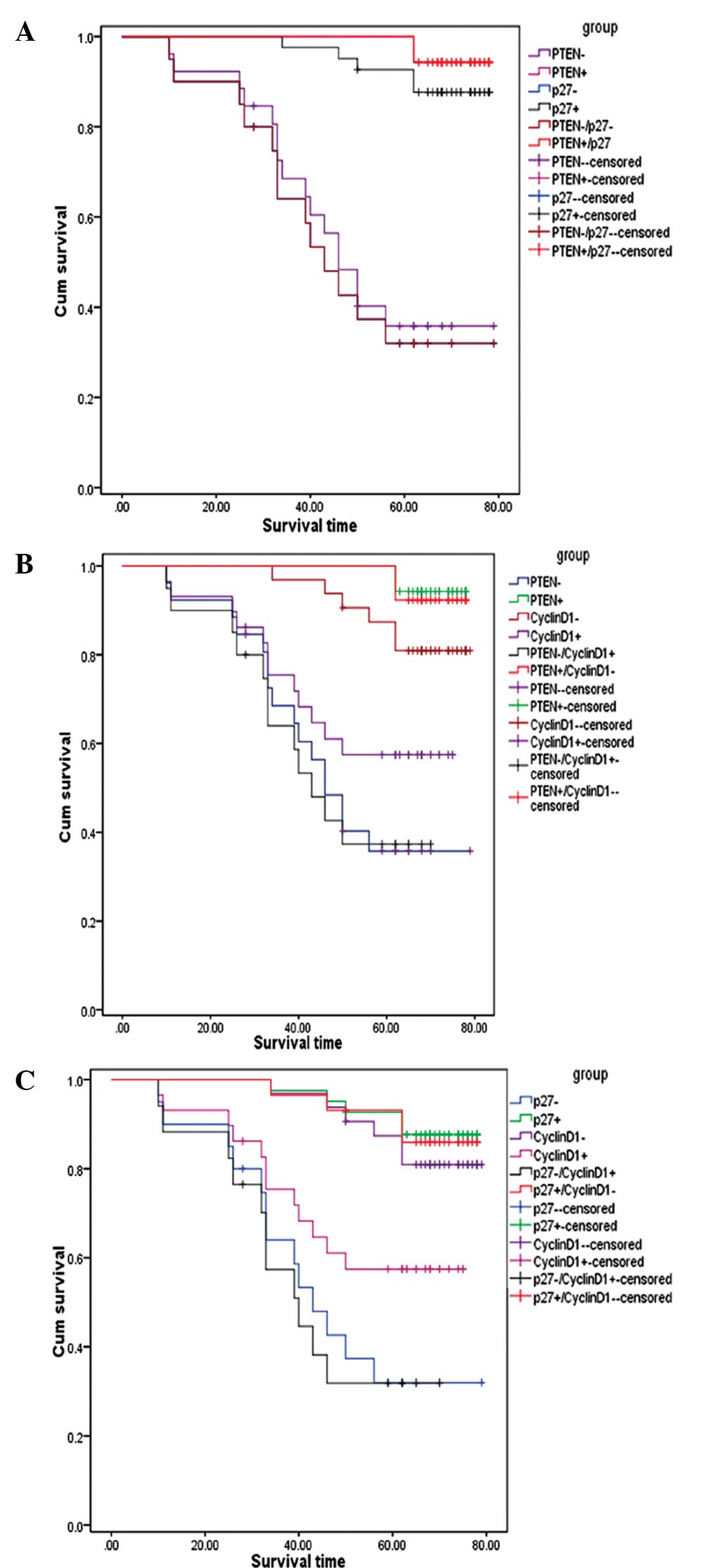 | Figure 3Overall survival curves for CRC
patients based on combined detection of PTEN/p27, PTEN/Cyclin D1
and p27/Cyclin D1 expression. (A) Patients with PTEN(+), PTEN(−),
p27(+), p27(−), PTEN(+)/p27(+), and PTEN(−)/p27(−) exhibited a mean
survival time of 77.1, 51.5, 74.7, 48.7, 77.1 and 48.7 months,
respectively. Patients with PTEN(−)/p27(−) expression exhibited the
worst survival times. (B) Patients with PTEN(+), PTEN(−), Cyclin
D1(−), Cyclin D1(+), PTEN(+)/Cyclin D1(−) and PTEN(−)/Cyclin D1(+)
expression had a mean survival time of 77.1, 51.5, 73.8, 56.9, 76.8
and 46.6 months, respectively. Patients with PTEN(+) expression
exhibited the best survival times, while joint examination of
PTEN(+)/Cyclin D1(−) expression did not provide extra prognostic
information. However, combinations of PTEN(−)/Cyclin D1(+)
expression provided a more adverse prognosis compared with
detection of PTEN(−) and Cyclin D1(+) expression alone. (C)
Patients with p27(+), p27(−), Cyclin D1(−), Cyclin D1(+),
p27(+)/Cyclin D1(−) and p27(−)/Cyclin D1(+) expression had a mean
survival time of 74.7, 48.7, 73.8, 56.9, 74.2 and 43.5 months,
respectively. Combination of p27(+)/Cyclin D1(−) expression did not
provide additional predictive information for clinical outcome
compared with detection of p27(+) and Cyclin D1(−) expression
alone. Notably, patients with p27(−)/Cyclin D1(+) expression had
worse survival times. PTEN, phosphatase and tensin homolog; CRC,
colorectal cancer. |
Fig. 3B shows that
the patients with PTEN(+), PTEN(−), Cyclin D1(−), Cyclin D1(+),
PTEN(+)/Cyclin D1(−) and PTEN(−)/Cyclin D1(+) expression had a mean
survival time of 77.1, 51.5, 73.8, 56.9, 76.8 and 46.6 months,
respectively. Evidently, those patients with PTEN(+), Cyclin D1(−)
and PTEN(+)/Cyclin D1(−) expression had improved clinical outcomes.
The patients with PTEN(+) expression showed the best survival
times, while the joint examination of PTEN(+)/Cyclin D1(−)
expression did not provide extra prognostic information. However,
combinations of PTEN(−)/Cyclin D1(+) expression provided a more
adverse prognosis compared with detection of PTEN(−) and Cyclin
D1(+) expression alone. Patients with PTEN(−)/Cyclin D1(+)
expression exhibited a 4.9- and 10.3-months shorter survival time
compared with those with PTEN(−) and Cyclin D1(+) expression alone,
respectively.
Fig. 3C shows that
the patients with p27(+), p27(−), Cyclin D1(−), Cyclin D1(+),
p27(+)/Cyclin D1(−) and p27(−)/Cyclin D1(+) expression had a mean
survival time of 74.7, 48.7, 73.8, 56.9, 74.2 and 43.5 months,
respectively. It was observed that the patients with p27(+)/Cyclin
D1(−) expression survived only 0.4 months longer than those with
Cyclin D1(−) expression and 0.5 months less than those with p27(+)
expression alone. Thus, the combination of p27(+)/Cyclin D1(−)
expression did not provide additional predictive information to the
clinical outcome compared with detection of p27(+) and Cyclin D1(−)
expression alone. However, the study indicated that the expression
of p27(−), PTEN(+) and p27(−)/Cyclin D1(+) were associated with a
poor survival time. Notably, patients with p27(−)/Cyclin D1(+) had
worse survival. Those patients with p27(−)/Cyclin D1(+) expression
had mean survival times of 5.2 and 13.4 months in contrast to those
with p27(−) and Cyclin D1(+) alone, respectively.
Discussion
The gene product of PTEN, lipid phosphatase, can
dephosphorylate PIP3 (15). The
lipid phosphatase can inhibit PI3K activity, which is normally
essential for activation of protein kinase B, a serine/threonine
kinase involved in cell growth and survival (15). PTEN can also induce
G1-phase cell cycle arrest by negatively regulating the
PI3K/Akt signaling pathway (27).
Loss of PTEN results in increased Akt activity and uncontrolled
cell proliferation. Depletion of PTEN has been associated with a
large number of human cancers, including bladder cancer, CRC and
breast cancer (12,19,28).
In the present study, PTEN was frequently deleted in CRC of poor
grade, with lymph metastasis and high clinical stage, indicating
the involvement of PTEN deletion in colorectal carcinogenesis. At
present, the prognostic value of PTEN has not been documented in
CRC. Jin et al (16)
reported significantly higher three- and five-year survival rates
in PTEN protein-positive patients than in PTEN protein-negative
patients. However, in a multivariate analysis, Hsu et al
(29) demonstrated that PTEN
expression was not of independent prognostic value in CRC. In the
present study, it was observed that patients with PTEN-positive
expression exhibited improved prognoses compared with those with
PTEN-negative staining, which was in line with the study by Jin
et al (16).
p27 is a member of the cip1/kip1 family of CDKIs
that regulate the progression of cells from late G1-phase into the
S-phase (30). Loss of p27 may
contribute to the uncontrolled proliferation of malignant cells. It
is known that p27 expression is lower in a series of human cancers,
including CRC and breast and lung cancer (20,31,32).
In the present study, 32.79% of tumors showed lower or depleted p27
expression, in agreement with the report of Al-Maghrabi et
al (18). The present study
found that there were no significant correlations between the loss
of p27 expression and gender, age and depth of invasion. Depletion
of p27 revealed highly significant correlation with tumor grade,
lymph metastasis and clinical stage. This observation is in
agreement with the study by Shapira et al (33), who reported low p27 levels
associated with poorly-differentiated tumors, but not with age,
gender and clinical stage in CRC. However, Al-Maghrabi et al
(18) showed that p27 expression
was only associated with depth of invasion and not with tumor
grade, lymph metastasis and clinical stage in CRC. Although p27 may
be significantly associated with tumor invasiveness
characteristics, including grade, depth of invasion and clinical
stage, the prognostic value of p27 remains controversial.
Al-Maghrabi et al (18)
reported that colorectal tumors with increased p27 expression
showed a higher recurrence rate and shorter disease-free patient
survival time than those with lower level or completely depleted
p27 expression. However, in the present study, it was observed that
patients with p27 expression had longer survival times than those
with no p27 expression, which was consistent with the study by
Bertagnolli et al (26),
which described that loss of p27 as associated with reduced
survival in colon cancer patients. Differences in the technical
aspects of recording p27 expression or in the interpretation of its
expression may result in these apparently discrepant findings.
Cyclin D1 is an important regulator of G1
to S-phase progression. Together with its binding partners, CDK4
and CDK6, Cyclin D1 forms active complexes that promote cell cycle
progression by phosphorylating and inactivating the Rb protein
(20). Defective regulation of this
important checkpoint may result in uncontrolled cellular
proliferation. The overexpression of Cyclin D1 has been found in a
variety of tumors types, including breast, oral and oropharyngeal
squamous cell carcinoma and CRC (13,34,35).
In the present study, increased expression of Cyclin D1 was
observed, in line with the findings of Ioachim (35). However, the expression of Cyclin D1
and its correlation with the clinicopathological parameters remain
unclear. Ioachim (35) found that
the overexpression of Cyclin D1 was significantly associated with
tumor stage and lymph node involvement in CRC. Meanwhile, Mao et
al (22) found no association
between Cyclin D1 expression and gender, age, depth of invasion,
tumor differentiation, clinical stage and lymph node metastasis in
colonic adenocarcinoma. In the present study, it was observed that
increased Cyclin D1 expression was correlated with advanced
clinical stage, lymph node metastasis and deeper tumor invasion
depth, but not with age, gender or histological grade. These
results indicate that Cyclin D1 may be associated with the more
aggressive phenotype of CRC. The association between Cyclin D1
expression and overall survival is controversial. A previous study
found that Cyclin D1-positive expression was associated with
shorter survival times in patients with CRC, but that it was not an
independent predictor of survival (22). However, according to the study by
Hwang et al (36), the
overexpression of Cyclin D1was associated with improved outcomes in
breast cancer. In the present study, it was found that patients
with Cyclin D1-positive expression had a poorer prognosis, in
contrast to the findings of Hwang et al (36) and in line with those of Mao et
al (22).
PTEN, p27 and Cyclin D1 belong to the cell cycle
regulatory proteins. PTEN and p27 play a significant role in
regulating the transition from the G1 phase to the S
phase. PTEN negatively mediates p27 activation and inhibits the
binding of Cyclin D1 with CDK. Depletion of PTEN contributes to
deactivating p27 through the suppression of AFX/FKHR (37). Similarly, loss of PTEN can activate
pAKT, which may activate Cyclin D1 through mTOR (37). A previous study observed that p27
and Cyclin D1 may be the crucial downstream targets of the
PTEN-mediated pathway. Thus, concomitant expression of PTEN and p27
exhibit a synergistic role in the progression from G1 to
S phase. PTEN expression has been positively correlated with p27
(31). The present study also
observed a positive correlation between PTEN and p27 expression, in
line with the study by Tsutsui et al (31). Certain studies have evaluated the
prognostic value of the combination of PTEN and p27 protein
expression in breast cancer and prostate cancer. The combined loss
of PTEN and p27 expression was associated with an aggressive
phenotype and a poor prognosis in breast and prostate carcinomas
(31,38). However, the joint detection of PTEN
and p27 in CRC has not been documented. In the present study,
patients with joint loss of PTEN and p27 had worse survival times
compared with those with PTEN and p27 loss alone. Patients with the
combined deletion of PTEN and p27 had a 2.8-months shorter survival
time than those with PTEN deletion alone. These results indicate
that a combined loss of PTEN and p27 function strongly promotes the
progression of CRC. These findings are consistent with the results
of the studies on breast and prostate carcinoma (31,38).
There was, however, no definite association between
PTEN and Cyclin D1. In breast cancer studies, statistically
significant associations have been found between PTEN and cyclin D1
expression patterns (34). However,
Fiano et al (39) observed
no direct correlation between Cyclin D1 overexpression and the loss
of PTEN in CRC. In the present study, an inverse association
between Cyclin D1 and loss of PTEN was observed. The prognostic
value of the combined examination of PTEN and Cyclin D1 in CRC has
not been documented. In the present study, patients with the
combined detection of Cyclin D1(+)/PTEN, PTEN(−) and CylinD1(+)
exhibited survival times of 46.6, 51.5 and 56.9 months,
respectively. Joint detection provided a worse prognostic value in
CRC, indicating that loss of PTEN exhibited a synergistic role with
Cyclin D1-positive expression in tumor progression.
p27 interacts with Cyclin D1 in the progression of
the cell cycle. As a potential inhibitor of the cyclin-CDK complex,
p27 can negatively regulate the ability of the complex to
phosphorylate other proteins like pRb, preventing Cyclin D1-CDK4
from binding. Thus, the decreased expression of p27 has been
correlated with Cyclin D1 overexpression. However, according to the
findings of Engin et al (34) in breast cancer, p27 expression was
not associated with Cyclin D1. With regard to CRC, Ioachim
(35) reported that overexpression
of Cyclin D1 was not associated with p27. Ye et al (40) found that the expression of Cyclin D1
observably increased, while p27 expression markedly decreased in
vitro in gastric cancer cells. In the present study, p27 was
inversely correlated with Cyclin D1. At present, there are no
reports stating that the concomitant detection of p27 and Cyclin D1
is associated with the prognosis of CRC. In the present study, it
was observed that the coexistence of p27-negative and Cyclin
D1-positive staining showed worse patient survival times compared
with p27-negative and Cyclin D1-positive expression alone (43.5 vs.
48.7 vs. 56.9 months). Patients in the combined group had a
13.4-months shorter survival time than those with Cyclin
D1-positive expression, indicating that p27 may coordinate with
Cyclin D1 in the progression of colorectal carcinogenesis.
In conclusion, the findings of the present study
confirmed the prognostic value of PTEN, p27 and Cyclin D1 in CRC.
Furthermore, PTEN was found to positively correlate with p27 and
negatively correlate with Cyclin D1, indicating the combined
regulation of these cell cycle checkpoints during the process of
neoplastic progression. Moreover, joint examination can provide
more adverse prognostic clues to those patients with a poor
prognosis, not improved prognostic indexes to those with a good
prognosis. These joint examinations may assist in the selection of
patients with a worse prognosis; these patients can then be
directed to the more intensive treatment of adjuvant chemotherapy.
Thus, these selections may enable more accurate subgrouping of
patients in terms of potential benefit from adjuvant therapy to
avoid unnecessary excessive chemotherapy. The present study
indicated that single prognostic markers often provide inaccurate
and incomplete prognostic indexes. Therefore, combined detection
provides significant insight into an accurate prognosis. To confirm
the accurate prognostic value of PTEN, p27 and Cyclin D1, a larger
group of patients should be enrolled in future blinded, prospective
studies.
References
|
1
|
Wang N, Zhu WX, Xing XM, et al: Time
trends of cancer incidence in urban Beijing, 1998–2007. Chin J
Cancer Res. 23:15–20. 2011.
|
|
2
|
Goodwin RA and Asmis TR: Overview of
systemic therapy for colorectal cancer. Clin Colon Rectal Surg.
22:251–256. 2009.
|
|
3
|
Quasar Collaborative Group. Gray R,
Barnwell J, et al: Adjuvant chemotherapy versus observation in
patients with colorectal cancer: a randomised study. Lancet.
370:2020–2029. 2007.
|
|
4
|
André T, Boni C, Navarro M, et al:
Improved overall survival with oxaliplatin, fluorouracil, and
leucovorin as adjuvant treatment in stage II or III colon cancer in
the MOSAIC trial. J Clin Oncol. 27:3109–3116. 2009.
|
|
5
|
Voorneveld PW, Jacobs RJ, De Miranda NF,
et al: Evaluation of the prognostic value of pSMAD
immunohistochemistry in colorectal cancer. Eur J Cancer Prev.
22:420–424. 2013.
|
|
6
|
Saito S, Okabe H, Watanabe M, et al:
CD44v6 expression is related to mesenchymal phenotype and poor
prognosis in patients with colorectal cancer. Oncol Rep.
29:1570–1578. 2013.
|
|
7
|
Bahnassy AA, Zekri AR, Alam El-Din HM, et
al: The role of cyclins and cyclins inhibitors in the multistep
process of HPV-associated cervical carcinoma. J Egypt Natl Canc
Inst. 18:292–302. 2006.
|
|
8
|
Taghavi N, Biramijamal F, Sotoudeh M, et
al: p16INK4a hypermethylation and p53, p16 and MDM2 protein
expression in esophageal squamous cell carcinoma. BMC Cancer.
10:1382010.
|
|
9
|
Bartkova J, Rajpert-De Meyts E, Skakkebaek
NE, et al: Deregulation of the G1/S-phase control in human
testicular germ cell tumours. APMIS. 111:252–266. 2003.
|
|
10
|
Macaluso M, Montanari M, Cinti C and
Giordano A: Modulation of cell cycle components by epigenetic and
genetic events. Semin Oncol. 32:452–457. 2005.
|
|
11
|
Gordon GM, Zhang T, Zhao J and Du W:
Deregulated G1-S control and energy stress contribute to the
synthetic-lethal interactions between inactivation of RB and
TSC1/TSC2. J Cell Sci. 126:2004–2013. 2013.
|
|
12
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011.
|
|
13
|
Perisanidis C, Perisanidis B, Wrba F, et
al: Evaluation of immunohistochemical expression of p53, p21, p27,
cyclin D1, and Ki67 in oral and oropharyngeal squamous cell
carcinoma. J Oral Pathol Med. 41:40–46. 2012.
|
|
14
|
Strimpakos AS, Karapanagiotou EM, Saif MW
and Syrigos KN: The role of mTOR in the management of solid tumors:
an overview. Cancer Treat Rev. 35:148–159. 2009.
|
|
15
|
Asano T, Yao Y, Zhu J, et al: The PI
3-kinase/Akt signaling pathway is activated due to aberrant Pten
expression and targets transcription factors NF-kappaB and c-Myc in
pancreatic cancer cells. Oncogene. 23:8571–8580. 2004.
|
|
16
|
Jin C, Wang A, Chen J, et al: Relationship
between expression and prognostic ability of PTEN, STAT3 and VEGF-C
in colorectal cancer. Exp Ther Med. 4:633–639. 2012.
|
|
17
|
Ghiţă C, Vîlcea ID, Dumitrescu M, et al:
The prognostic value of the immunohistochemical aspects of tumor
suppressor genes p53, bcl-2, PTEN and nuclear proliferative antigen
Ki-67 in resected colorectal carcinoma. Rom J Morphol Embryol.
53:549–556. 2012.
|
|
18
|
Al-Maghrabi J, Al-Ahwal M, Buhmeida A, et
al: Expression of cell cycle regulators p21 and p27 as predictors
of disease outcome in colorectal carcinoma. J Gastrointest Cancer.
43:279–287. 2012.
|
|
19
|
Zlobec I, Minoo P, Baumhoer D, et al:
Multimarker phenotype predicts adverse survival in patients with
lymph node-negative colorectal cancer. Cancer. 112:495–502.
2008.
|
|
20
|
Wang MT, Chen G, An SJ, et al: Prognostic
significance of cyclinD1 amplification and the co-alteration of
cyclinD1/pRb/ppRb in patients with esophageal squamous cell
carcinoma. Dis Esophagus. 25:664–670. 2012.
|
|
21
|
Sugimoto M, Martin N, Wilks DP, et al:
Activation of cyclin D1-kinase in murine fibroblasts lacking both
p21(Cip1) and p27(Kip1). Oncogene. 21:8067–8074. 2002.
|
|
22
|
Mao Y, Li Z, Lou C and Zhang Y: Expression
of phosphorylated Stat5 predicts expression of cyclin D1 and
correlates with poor prognosis of colonic adenocarcinoma. Int J
Colorectal Dis. 26:29–35. 2011.
|
|
23
|
Belt EJ, Brosens RP, Delis-van Diemen PM,
et al: Cell cycle proteins predict recurrence in stage II and III
colon cancer. Ann Surg Oncol. 19(Suppl 3): S682–S692. 2012.
|
|
24
|
Weng LP, Brown JL and Eng C: PTEN
coordinates G(1) arrest by down-regulating cyclin D1 via its
protein phosphatase activity and up-regulating p27 via its lipid
phosphatase activity in a breast cancer model. Hum Mol Genet.
10:599–604. 2001.
|
|
25
|
Lan YT, Yang SH, Chang SC, et al: Analysis
of the seventh edition of American Joint Committee on colon cancer
staging. Int J Colorectal Dis. 27:657–653. 2011.
|
|
26
|
Bertagnolli MM, Warren RS, Niedzwiecki D,
et al: p27Kip1 in stage III colon cancer: implications for outcome
following adjuvant chemotherapy in cancer and leukemia group B
protocol 89803. Clin Cancer Res. 15:2116–2122. 2009.
|
|
27
|
Li D, Zhang Y, Xie Y, et al: Enhanced
tumor suppression by adenoviral PTEN gene therapy combined with
cisplatin chemotherapy in small-cell lung cancer. Cancer Gene Ther.
20:251–259. 2013.
|
|
28
|
Zhu L, Loo WT and Louis WC: PTEN and VEGF:
possible predictors for sentinel lymph node micro-metastasis in
breast cancer. Biomed Pharmacother. 61:558–561. 2007.
|
|
29
|
Hsu CP, Kao TY, Chang WL, et al: Clinical
significance of tumor suppressor PTEN in colorectal carcinoma. Eur
J Surg Oncol. 37:140–147. 2011.
|
|
30
|
Jung SM, Park SS, Kim WJ and Moon SK:
Ras/ERK1 pathway regulation of p27KIP1-mediated G1-phase cell-cycle
arrest in cordycepin-induced inhibition of the proliferation of
vascular smooth muscle cells. Eur J Pharmacol. 681:15–22. 2012.
|
|
31
|
Tsutsui S, Inoue H, Yasuda K, et al:
Inactivation of PTEN is associated with a low p27Kip1 protein
expression in breast carcinoma. Cancer. 104:2048–2053. 2005.
|
|
32
|
Zolota VG, Tzelepi VN, Leotsinidis M, et
al: Histologic-type specific role of cell cycle regulators in
non-small cell lung carcinoma. J Surg Res. 164:256–265. 2010.
|
|
33
|
Shapira M, Ben-Izhak O, Linn S, et al: The
prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in
colorectal carcinoma. Cancer. 103:1336–1346. 2005.
|
|
34
|
Engin H, Baltali E, Güler N, et al:
Expression of PTEN, cyclin D1, P27/KIP1 in invasive ductal
carcinomas of the breast and correlation with clinicopathological
parameters. Bull Cancer. 93:E21–E26. 2006.
|
|
35
|
Ioachim E: Expression patterns of cyclins
D1, E and cyclin-dependent kinase inhibitors p21waf1/cip1, p27kip1
in colorectal carcinoma: correlation with other cell cycle
regulators (pRb, p53 and Ki-67 and PCNA) and clinicopathological
features. Int J Clin Pract. 62:1736–1743. 2008.
|
|
36
|
Hwang TS, Han HS, Hong YC, et al:
Prognostic value of combined analysis of cyclin D1 and estrogen
receptor status in breast cancer patients. Pathol Int. 53:74–80.
2003.
|
|
37
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999.
|
|
38
|
Halvorsen OJ, Haukaas SA and Akslen LA:
Combined loss of PTEN and p27 expression is associated with tumor
cell proliferation by Ki-67 and increased risk of recurrent disease
in localized prostate cancer. Clin Cancer Res. 9:1474–1479.
2003.
|
|
39
|
Fiano V, Ghimenti C, Imarisio S, et al:
pAkt, cyclin D1 and p27/Kip. 1 in glioblastomas with and without
EGFR amplification and PTEN mutation. Anticancer Res. 24:2643–2647.
2004.
|
|
40
|
Ye YW, Wu JH, Wang CM, et al: Sox17
regulates proliferation and cell cycle during gastric cancer
progression. Cancer Lett. 307:124–131. 2011.
|