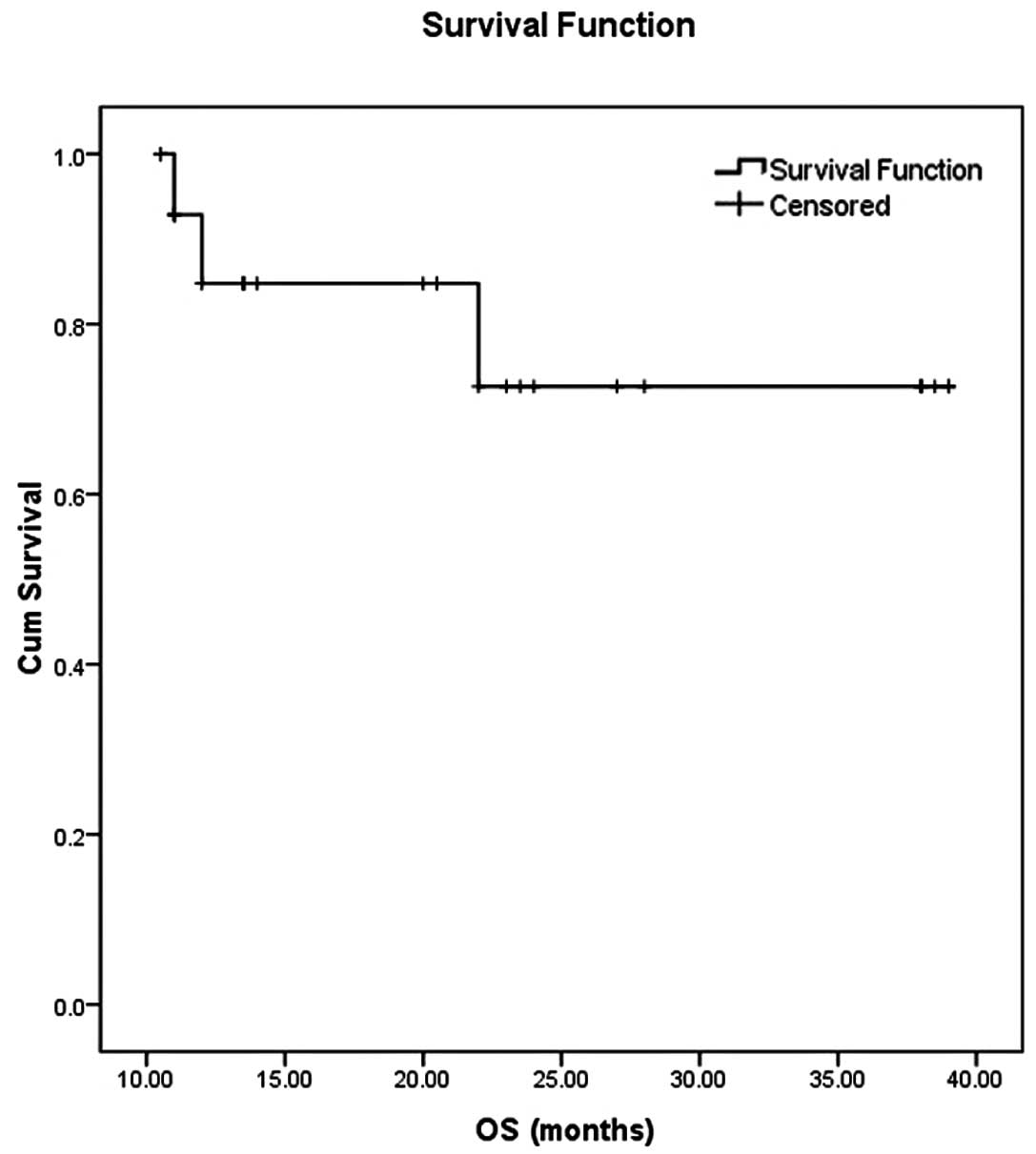
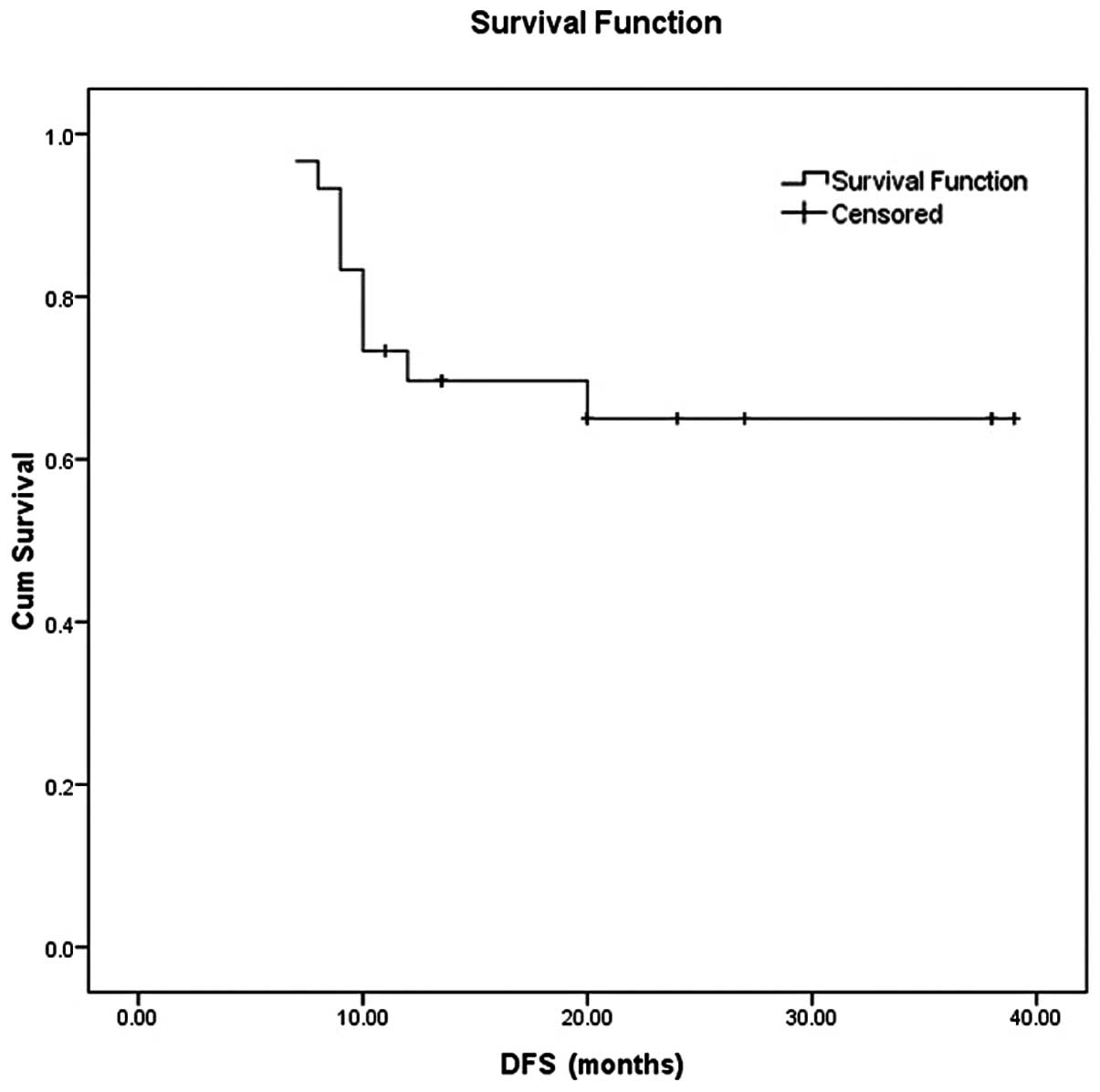
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
|
|
2
|
Park SH, Kim DY, Heo JS, et al:
Postoperative chemoradiotherapy for gastric cancer. Ann Oncol.
14:1373–1377. 2003.
|
|
3
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group. Paoletti X, Oba
K, Burzykowski T, et al: Benefit of adjuvant chemotherapy for
resectable gastric cancer: a meta-analysis. JAMA. 303:1729–1737.
2010.
|
|
4
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001.
|
|
5
|
Smalley SR, Benedetti JK, Haller DG, et
al: Updated analysis of SWOG-directed intergroup study 0116: a
phase III trial of adjuvant radiochemotherapy versus observation
after curative gastric cancer resection. J Clin Oncol.
30:2327–2333. 2012.
|
|
6
|
Sasako M, Inoue M, Lin JT, et al: Gastric
Cancer Working Group report. Jpn J Clin Oncol. 40(Suppl 1):
i28–i37. 2010.
|
|
7
|
Kappas AM, Fatouros M and Roukos DH: Is it
time to change surgical strategy for gastric cancer in the United
States? Ann Surg Oncol. 11:727–730. 2004.
|
|
8
|
Songun I, Putter H, Kranenbarg EM, Sasako
M and van de Velde CJ: Surgical treatment of gastric cancer:
15-year follow-up results of the randomised nationwide Dutch D1D2
trial. Lancet Oncol. 11:439–449. 2010.
|
|
9
|
Degiuli M, Sasako M and Ponti A; Italian
Gastric Cancer Study Group. Morbidity and mortality in the Italian
Gastric Cancer Study Group randomized clinical trial of D1 versus
D2 resection for gastric cancer. Br J Surg. 97:643–649. 2010.
|
|
10
|
Wu CW, Hsiung CA, Lo SS, et al: Nodal
dissection for patients with gastric cancer: a randomized
controlled trial. Lancet Oncol. 7:309–315. 2006.
|
|
11
|
Oh DY and Bang YJ: Adjuvant and
neoadjuvant therapy for gastric cancer. Curr Treat Options Oncol.
14:311–320. 2013.
|
|
12
|
Mullaney PJ, Wadley MS, Hyde C, Wyatt J,
et al: Appraisal of compliance with the UICC/AJCC staging system in
the staging of gastric cancer. Union Internacional Contra la
Cancrum/American Joint Committee on Cancer. Br J Surg.
89:1405–1408. 2002.
|
|
13
|
Japanese Gastric Cancer Association.
Japanese Classification of Gastric Carcinoma - 2nd English edition.
Gastric Cancer. 1:10–24. 1998.
|
|
14
|
Ajani JA, Welch SR, Raber MN, Fields WS
and Krakoff IH: Comprehensive criteria for assessing
therapy-induced toxicity. Cancer Invest. 8:147–159. 1990.
|
|
15
|
Vasilescu C, Popa M, Tudor S, Manuc M and
Diculescu M: Robotic surgery of locally advanced gastric cancer -
an initial experience. Acta Chir Belg. 112:209–212. 2012.
|
|
16
|
Cunningham D, Allum WH, Stenning SP, et
al; MAGIC Trial Participants. Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006.
|
|
17
|
Nakashima S, Hatanaka N, Yoshikawa Y, et
al: Long-term survival of a case of advanced cancer of the
esophagogastric junction with complete response to low-dose
5-fluorouracil and cisplatin chemotherapy. Gan To Kagaku Ryoho.
39:1559–1561. 2012.(In Japanese).
|
|
18
|
Gomceli I, Demiriz B and Tez M: Gastric
carcinogenesis. World J Gastroenterol. 18:5164–5170. 2012.
|
|
19
|
Hallissey MT, Dunn JA, Ward LC and Allum
WH: The second British Stomach Cancer Group trial of adjuvant
radiotherapy or chemotherapy in resectable gastric cancer:
five-year follow-up. Lancet. 343:1309–1312. 1994.
|
|
20
|
Roviello F, Marrelli D, Morgagni P, et al;
Italian Research Group for Gastric Cancer. Survival benefit of
extended D2 lymphadenectomy in gastric cancer with involvement of
second level lymph nodes: a longitudinalmulticenter study. Ann Surg
Oncol. 9:894–900. 2002.
|
|
21
|
Lim DH, Kim DY, Kang MK, et al: Patterns
of failure in gastric carcinoma after D2 gastrectomy and
chemoradiotherapy: a radiation oncologist’s view. Br J Cancer.
91:11–17. 2004.
|
|
22
|
Leong CN, Chung HT, Lee KM, et al:
Outcomes of adjuvant chemoradiotherapy after a radical gastrectomy
and a D2 node dissection for gastric adenocarcinoma. Cancer J.
14:269–275. 2008.
|
|
23
|
Song S, Chie EK, Kim K, et al:
Postoperative chemoradiotherapy in high risk locally advanced
gastric cancer. Radiat Oncol J. 30:213–217. 2012.
|
|
24
|
Lee J, Lim do H, Kim S, et al: Phase III
trial comparing capecitabine plus cisplatin versus capecitabine
plus cisplatin with concurrent capecitabine radiotherapy in
completely resected gastric cancer with D2 lymph node dissection:
the ARTIST trial. J Clin Oncol. 30:268–273. 2012.
|