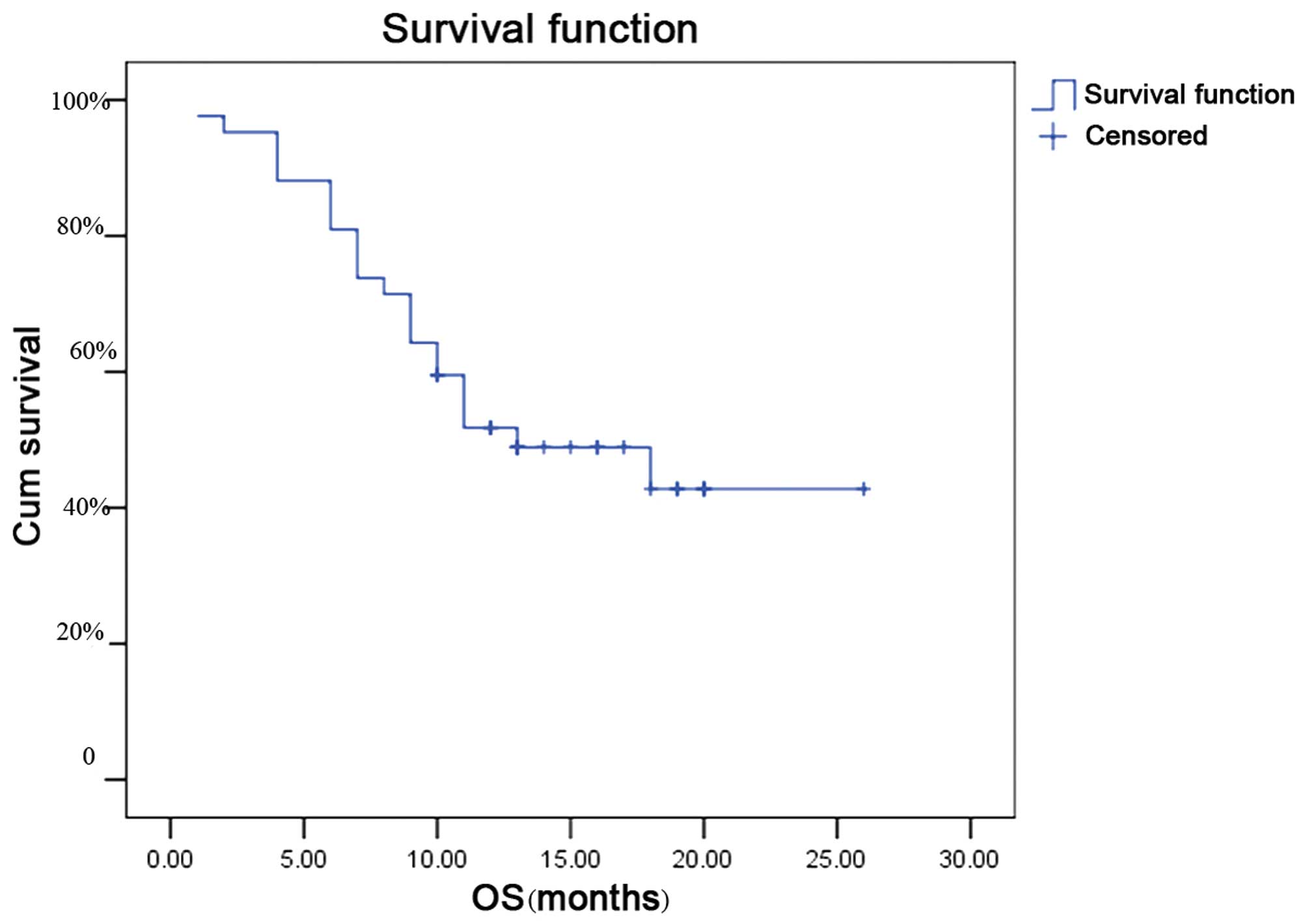
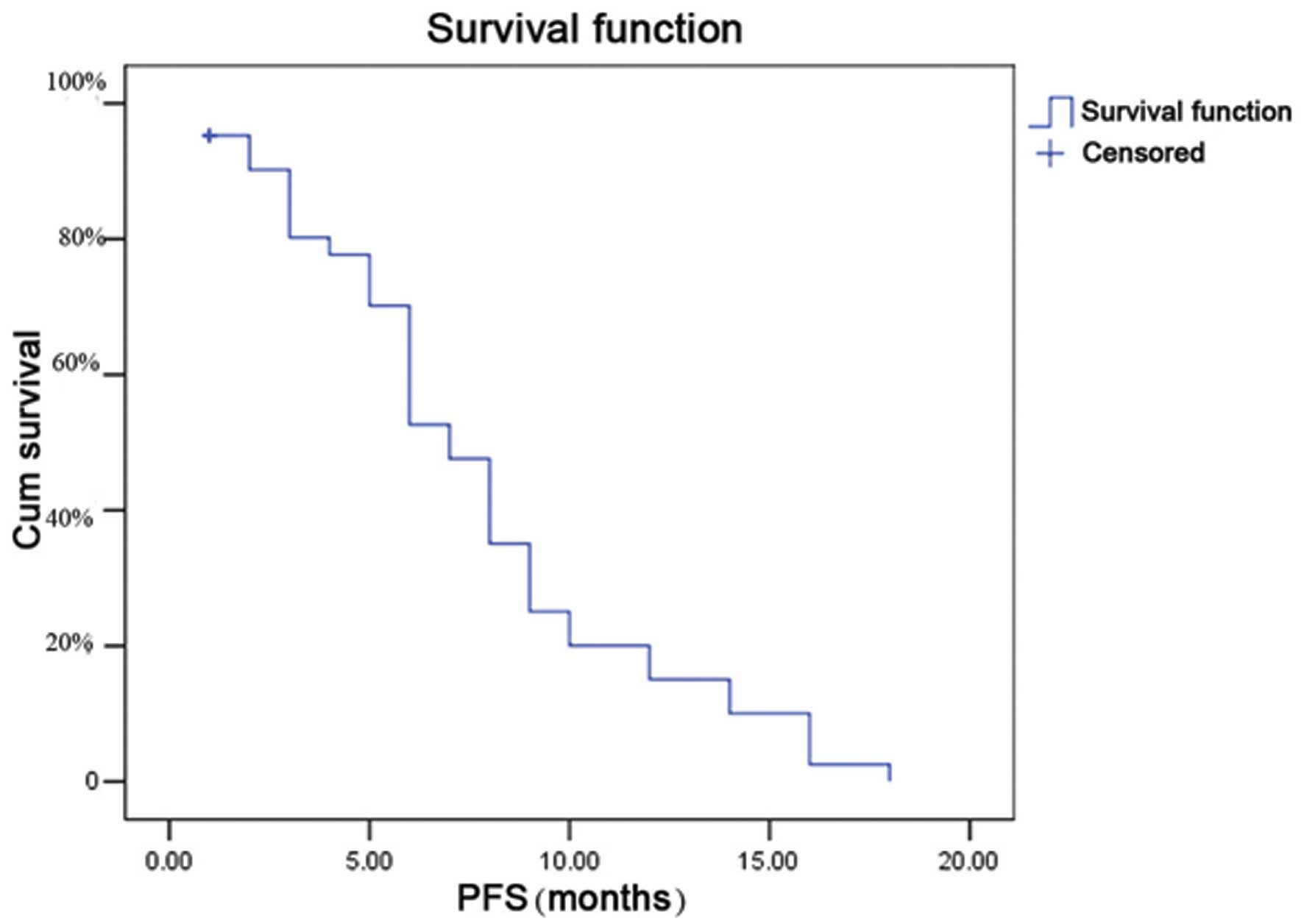
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
|
|
2
|
Fang M, Wang S, Zheng Y, et al:
Maintenance therapy with oral etoposide following first-line
docetaxel-cisplatin chemotherapy in metastatic non-small cell lung
cancer patients. Bangladesh J Pharmacol. 7:192–198. 2012.
|
|
3
|
Sharma S, Bell D, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007.
|
|
4
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial). J Clin Oncol. 21:2237–2246. 2003.
|
|
5
|
Kris MG, Natale RB, Herbst RS, et al:
Efficacy of gefitinib, an inhibitor of the epidermal growth factor
receptor tyrosine kinase, in symptomatic patients with non-small
cell lung cancer: a randomized trial. JAMA. 290:2149–2158.
2003.
|
|
6
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010.
|
|
7
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010.
|
|
8
|
Zhao Q, Shentu J, Xu N, et al: Phase I
study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine
kinase inhibitor, in patients with advanced NSCLC and other solid
tumors. Lung Cancer. 73:195–202. 2011.
|
|
9
|
Tan F, Shen X, Wang D, et al: Icotinib
(BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays
potent efficacy in preclinical studies. Lung Cancer. 76:177–182.
2012.
|
|
10
|
Liu D, Jiang J, Hu P, et al: Quantitative
determination of icotinib in human plasma and urine using liquid
chromatography coupled to tandem mass spectrometry. J Chromatogr B
Analyt Technol Biomed Life Sci. 877:3781–3786. 2009.
|
|
11
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
|
|
12
|
National Cancer Institute. National Cancer
Institute Common Toxicity Criteria. http://ctep.cancer.gov/protocolDevelopment/electronic.applications/docs/ctcaev3.pdf.
Accessed June 22, 2014
|
|
13
|
Thatcher N, Chang A, Parikh P, et al:
Gefitinib plus best supportive care in previously treated patients
with refractory advanced non-small-cell lung cancer: results from a
randomised, placebo-controlled, multicentre study (Iressa Survival
Evaluation in Lung Cancer). Lancet. 366:1527–1537. 2005.
|
|
14
|
Lee DH, Han JY, Yu SY, et al: The role of
gefitinib treatment for Korean never-smokers with advanced or
metastatic adenocarcinoma of the lung: a prospective study. J
Thorac Oncol. 1:965–971. 2006.
|
|
15
|
Inoue A, Suzuki T, Fukuhara T, et al:
Prospective phase II study of gefitinib for chemotherapy-naive
patients with advanced non-small-cell lung cancer with epidermal
growth factor receptor gene mutations. J Clin Oncol. 24:3340–3346.
2006.
|
|
16
|
Asahina H, Yamazaki K, Kinoshita I, et al:
A phase II trial of gefitinib as first-line therapy for advanced
non-small cell lung cancer with epidermal growth factor receptor
mutations. Br J Cancer. 95:998–1004. 2006.
|
|
17
|
Sunaga N, Tomizawa Y, Yanagitani N, et al:
Phase II prospective study of the efficacy of gefitinib for the
treatment of stage III/IV non-small cell lung cancer with EGFR
mutations, irrespective of previous chemotherapy. Lung Cancer.
56:383–389. 2007.
|
|
18
|
Sutani A, Nagai Y, Udagawa K, et al:
Gefitinib for non-small-cell lung cancer patients with epidermal
growth factor receptor gene mutations screened by peptide nucleic
acid-locked nucleic acid PCR clamp. Br J Cancer. 95:1483–1489.
2006.
|
|
19
|
Paz-Ares L, Sanchez JM, García-Velasco A,
et al: A prospective phase II trial of erlotinib in advanced
non-small cell lung cancer (NSCLC) patients (p) with mutations in
the tyrosine kinase (TK)domain of the epidermal growth factor
receptor (EGFR). J Clin Oncol. 24:369s2006.
|
|
20
|
Sequist LV, Martins RG, Spigel D, et al:
iTARGET: A phase II trial to assess the response to gefitinib in
epidermal growth factor receptor (EGFR)-mutated non-small
cell lung cancer (NSCLC) tumors. J Clin Oncol. 25:386s2007.
|
|
21
|
O’Connell J, Kris MG, Gralla RJ, et al:
Frequency and prognostic importance of pretreatment clinical
characteristics in patients with advanced non-small cell lung
cancer treated with combination chemotherapy. J Clin Oncol.
4:1604–1614. 1986.
|
|
22
|
Radzikowska E, Glaz P and Roszkowski K:
Lung cancer in women: age, smoking, histology, performance status,
stage, initial treatment, and survival. Population-based study of
20 561 cases. Ann Oncol. 13:1087–1093. 2002.
|