Introduction
The Wnt pathway is involved in cell proliferation
and differentiation (1). This
pathway is divided into the canonical (β-catenin-dependent) and
non-canonical (β-catenin-independent) pathways. The canonical
pathway is activated when Wnt proteins bind to their receptor,
frizzled (Fz), and co-receptor, low-density lipoprotein
receptor-related protein 5 and 6, to form a complex (2,3). In
the absence of Wnt signals, cytoplasmic β-catenin is maintained at
low levels through destruction by the glycogen synthase kinase-3 β
complex (4). β-catenin acts as a
co-factor of T-cell factor/lymphoid enhancer factor and activates
target genes. Constitutive activation of the Wnt pathway leads to
abnormal cell growth and the development of cancer (5).
One of the non-canonical Wnt pathways is the planar
cell polarity (PCP) pathway, whereby Fz activates a downstream
pathway that includes small guanosine 5′-triphosphatases,
ras-related C3 botulinum toxin substrate 1 and Ras homologue gene
family member A (1). The PCP
pathway is involved in cell polarity during morphogenesis (6). Another Wnt-Ca2+ pathway is
one in which Wnt binds to Fz to activate heterotrimeric G proteins,
leading to the activation of phospholipase C. The
Wnt-Ca2+ pathway is involved in cancer (7–9). Wnt5a
mediates the metastasis of malignant melanoma via the
Wnt-Ca2+ pathway (10).
Structural and functional homology studies have
identified 10 members of the Fz family of genes (11). Fz9 is involved in the canonical
pathway (12) and is not expressed
in the human fetal or adult liver (13). The short interfering RNA of Fz9
suppresses the cell proliferation and motility of hepatocellular
carcinoma (HCC) and hepatoblastoma (HB) cell lines (13). Fz2 is involved in the non-canonical
pathway, as it induces intracellular Ca2+ release
(14). Reverse transcriptase
polymerase chain reaction (PCR) results have shown that Fz2 is
slightly expressed in the human fetal liver, but not in the adult
liver (13). Fz2 is expressed in
all HCC and HB cell lines. The role of Fz2 in liver carcinogenesis
is, however, not completely understood.
The levels of Fz2 expression in HCC cell lines were
therefore analyzed in the present study. The expression of Fz2 was
suppressed with short hairpin (sh)RNA to clarify its role in the
proliferation of HCC cell lines.
Materials and methods
Cell culture
The HCC cell lines, HLE, HLF, PLC/PRL/5, Huh-7,
Hep3B, Huh-6, and HepG2, were purchased from RIKEN Cell Bank
(Tsukuba, Japan). The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum (FBS; Life Technologies,
Grand Island, NY, USA). The cell lines were cultured with 5% carbon
dioxide at 37°C in a humidified chamber. The study was approved by
the ethics committee of the National Hospital Organization,
Shimoshizu Hospital (Yotsukaido, Japan).
Quantitative PCR
The cells were spread in 6-well plates (Asahi Techno
Glass, Co., Ltd., Tokyo, Japan) and cultured. When they reached 80%
confluency, the cells were further cultured for 48 h following
transfection. Total RNA (5 μg), isolated with Isogen (Nippon Gene,
Tokyo, Japan), was used to generate cDNA with Super Script III and
oligo(dT) primers, following the manufacturer’s instructions (Life
Technologies). Human fetal and adult liver RNA was purchased from
Clontech Laboratories, Inc., (Mountain View, CA, USA). Quantitative
PCR was performed using the Fast SYBR Green Master Mix (Life
Technologies) and analyzed with the MiniOpticon Detection System
(Bio-Rad, Hercules, CA, USA). The primer pairs for quantitative PCR
and the resultant product sizes were as follows: Fz2 (NM_001466)
forward, 5′-TCCTCAAGGTGCCATCCTATCTC-3′ and reverse,
5′-TGGTGACAGTGAAGAAGGTGGAAG-3′ (183 bp); cyclin D1 (NM_053056)
forward, 5′-AGAGGCGGAGGAGAACAAACAG-3′ and reverse,
5′-AGGCGGTAGTAGGACAGGAAGTTG-3′ (180 bp); and ribosomal protein L
(RPL)19 (BC095445) forward, 5′-CGAATGCCAGAGAAGGTCAC-3′ and reverse,
5′-CCATGAGAATCCGCTTGTTT-3′ (157 bp). Quantitative PCR was performed
for 40 cycles of 5 sec of denaturation and 5 sec of
annealing/extension. RPL19 was used as an internal control.
Immunostaining
Sections of 38-week-old female human fetal liver,
64-year-old male adult liver and 60-year-old female HCC tissue
(BioChain, Hayward, CA, USA) were deparaffinized, autoclaved and
incubated, first with hydrogen peroxide and then for 30 min with 2%
normal goat serum in phosphate buffered saline (PBS; washing
buffer). Following overnight incubation with a rabbit polyclonal
anti-Fz2 antibody (1:5,000; Sigma-Aldrich), specimens were rinsed
with PBS and subsequently incubated with horseradish
peroxidase-labeled anti-rabbit antibody (1:500) for 2 h (GE
Healthcare, Pittsburgh, PA, USA). Next, diaminobenzidine (Dako,
Glostrup, Denmark) was applied to the tissue sections as a
chromogen and the nuclei were stained with hematoxylin (Muto Pure
Chemicals Co., Ltd., Tokyo, Japan) for 15 sec. The specimens were
observed and images were captured under an AX80 microscope
(Olympus, Tokyo, Japan).
Cell proliferation analysis
The cells were trypsinized, harvested, spread onto
96-well flat-bottom plates (Asahi Techno Glass Co., Ltd.) at a
density of 1,000 cells per well, and then incubated for 24 h in
DMEM supplemented with 10% FBS. Subsequent to being cultured, the
cells were transfected with the shRNA of Fz2 (shRNA-Fz2) for 72 h.
Cell cultures were subjected to
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt (MTS) assays, according to the manufacturer’s
instructions (Promega Corporation, Madison, WI, USA). MTS is
bio-reduced by cells into a colored formazan product that reduces
absorbance at 490 nm. Absorbance was analyzed at a wavelength of
490 nm with an iMark Microplate Absorbance Reader (Bio-Rad).
shRNA transfection
The shRNA-Fz2 (OriGene Technologies, Rockville, MD,
USA) was transfected into the cells using Lipofectamine LTX (Life
Technologies), according to the manufacturer’s instructions.
Briefly, the shRNA was incubated with PLUS reagent for 5 min,
following which, LTX reagent was added. A 30-min incubation at room
temperature ensued, and the complex was subsequently applied to the
cell culture medium. A negative control for shRNA was purchased
from OriGene Technologies.
Statistical analysis
Cell proliferation and quantitative PCR data were
analyzed by a one-factor analysis of variance. Statistical analysis
was performed using JMP 5.0 software (SAS Institute Inc., Cary, NC,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Quantitative PCR was performed to determine the
levels of Fz2 expression in the HCC cell lines compared with those
in the fetal and adult liver cells (Fig. 1). All cell lines had elevated levels
of Fz2 compared with the adult liver cells. The highest and lowest
expression levels of Fz2 were 246.9±15.7 in the HLF cells and
5.8±1.4 in the Hep3B cells, respectively. These data suggest that
Fz2 is upregulated in HCC cells.
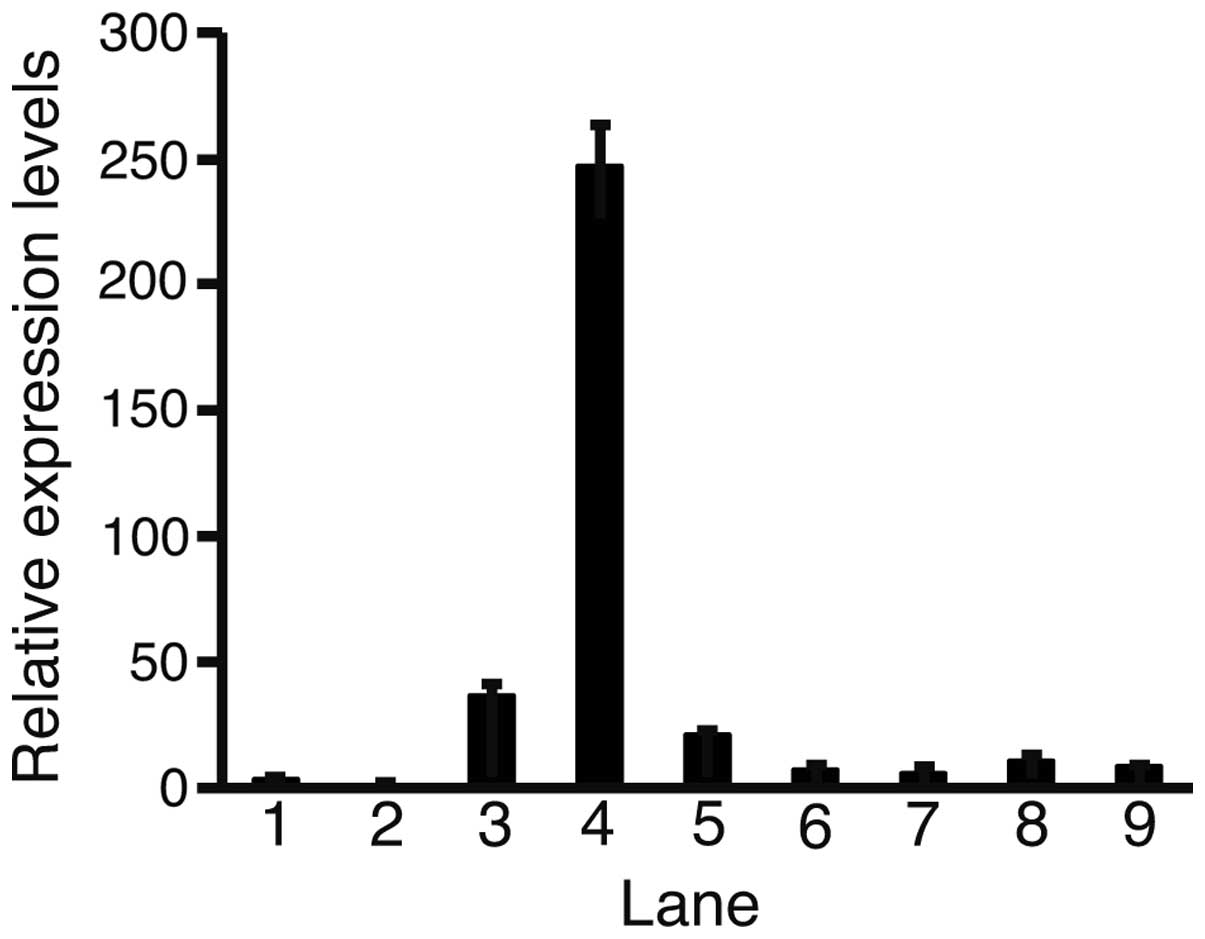 | Figure 1Quantitative polymerase chain reaction
(PCR). Relative expression levels of frizzled (Fz)-2 were analyzed
with quantitative PCR. Levels of Fz2 expression were normalized
against those of the ribosomal protein L19. Each relative
expression level of Fz2 was calculated as: Fz2 value/adult liver
value. Lane 1, fetal liver; 2, adult liver; 3, HLE; 4, HLF; 5,
PLC/PRL/5; 6, Huh-7; 7, Hep3B; 8, Huh-6; 9, HepG2; error bar,
standard error. |
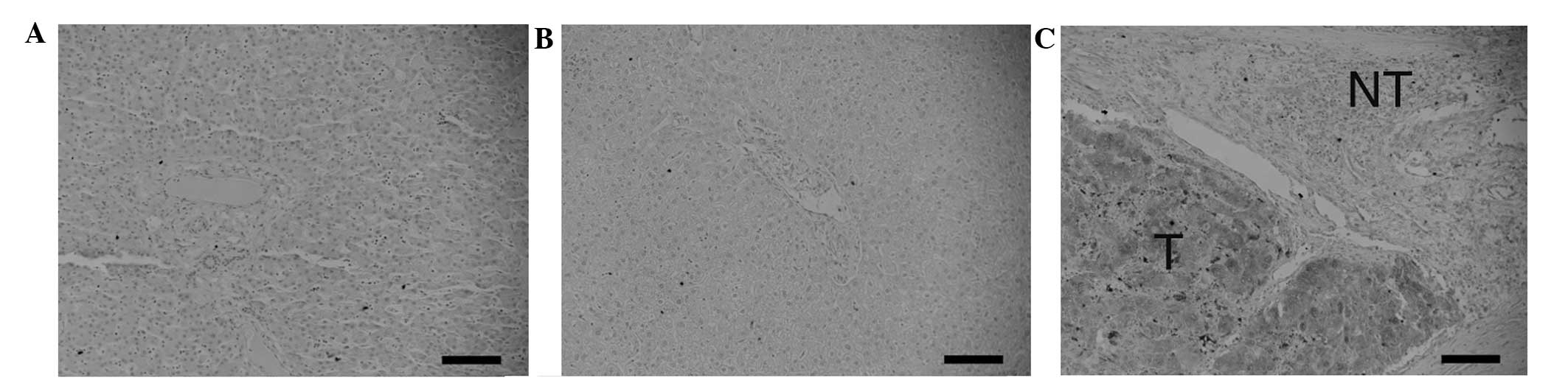
Immunostaining was performed to clarify the
expression levels of Fz2 at the protein level (Fig. 2). The fetal (Fig. 2A) and adult liver cells (Fig. 2B) did not express Fz2. By contrast,
Fz2 was expressed in the tumorous HCC tissue (Fig. 2C). Fz2 was not expressed in the
surrounding non-tumorous tissues. These data clearly indicate that
Fz2 is upregulated at the RNA and protein levels.
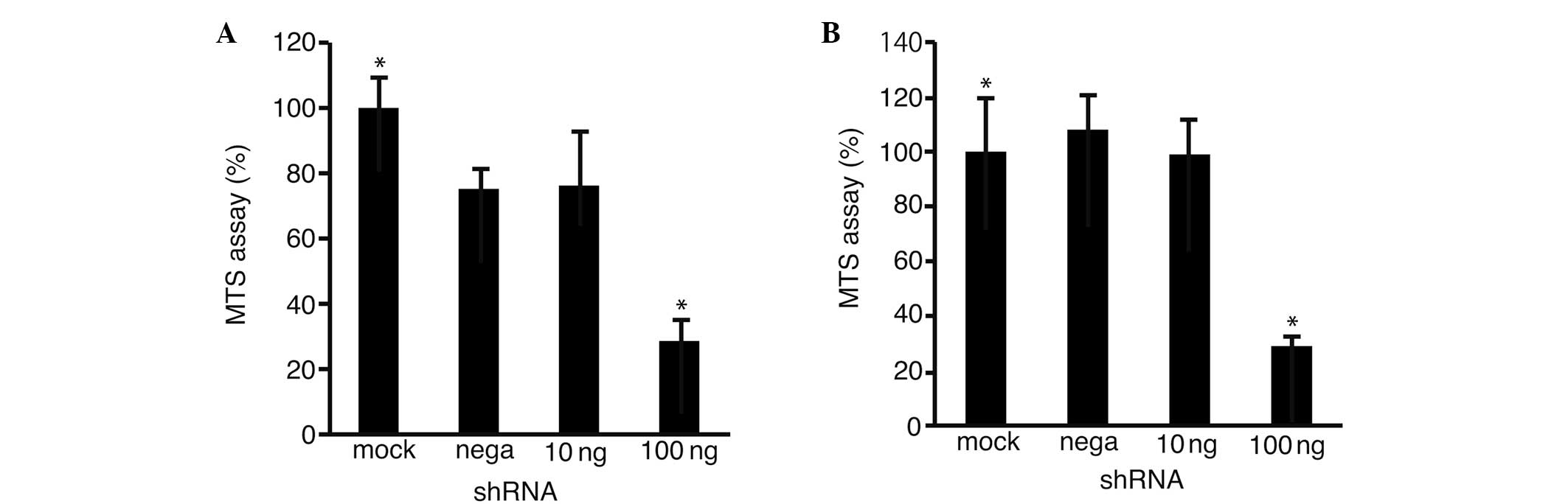
To clarify the possibility that Fz2 was involved in
cell proliferation, an MTS assay was performed on the HLF (Fig. 3A) and Hep3B (Fig. 3B) cells transfected with the
shRNA-Fz2. Cell proliferation was suppressed to 28.6±6.4%
(P<0.05 vs. mock transfection) in the HLF cells and to 29.8±4.3%
(P<0.05) in the Hep3B cells at 100 ng shRNA-Fz2 per well.
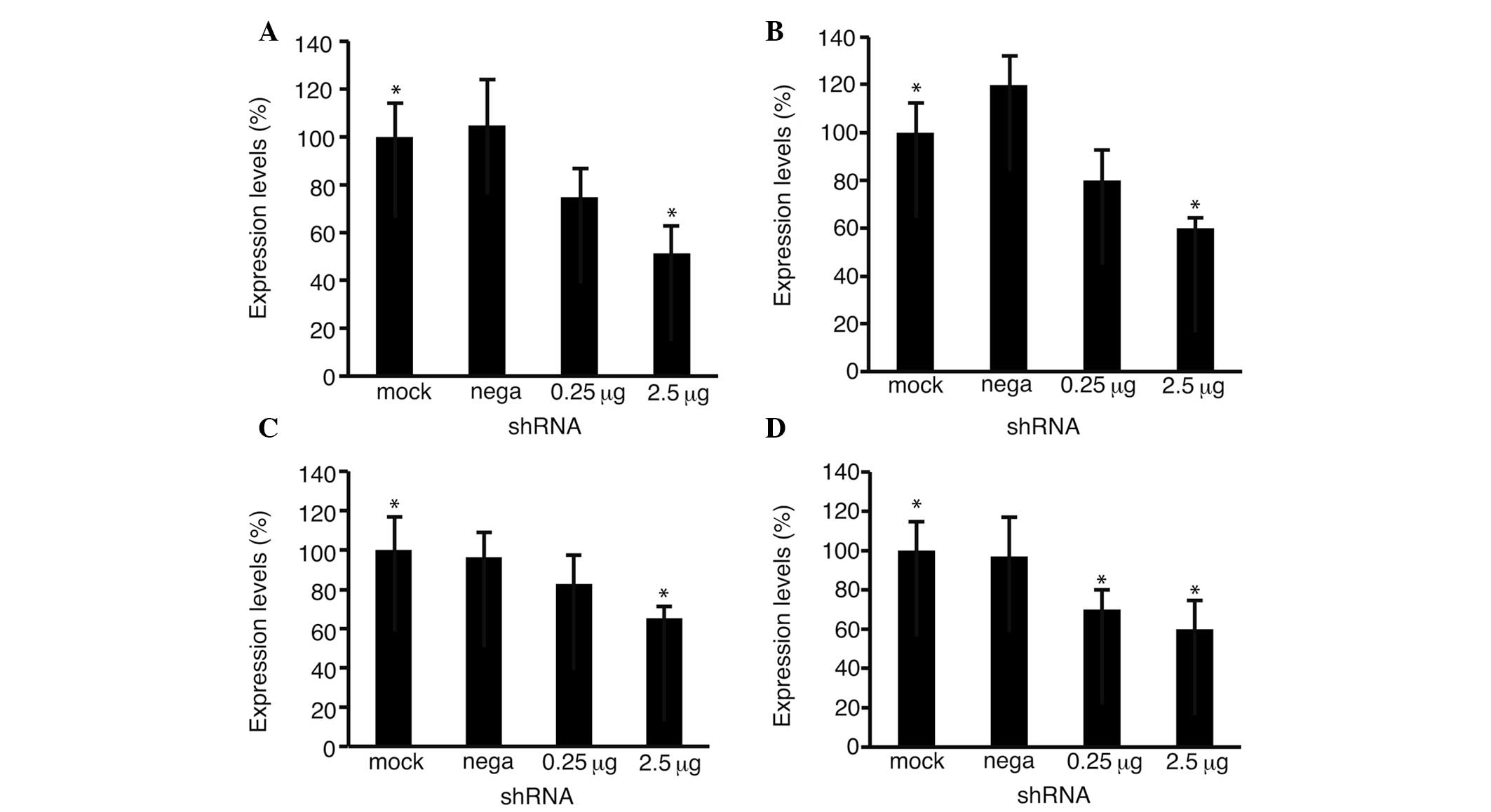
Quantitative PCR was performed to analyze the levels
of Fz2 (Fig. 4A and B) and cyclin
D1 (Fig. 4C and D) expression. The
expression levels of Fz2 decreased to 51.3±10.7% (P<0.05 vs.
mock transfection) in the HLF cells and 58.9±4.4% (P<0.05) in
the Hep3B cells at 2.5 μg per well, confirming the effectiveness of
shRNA-Fz2. The expression levels of cyclin D1 decreased to
65.2±5.9% (P<0.05) in the HLF cells and 60.8±14.6% (P<0.05)
in the Hep3B cells at 2.5 μg per well.
Discussion
Certain types of Fzs are upregulated in HCC. Fz3, 6,
and 7 are upregulated in the tumorous tissues of HCC (15) and Fz2 is expressed in Hep3B
(16). In the present study,
quantitative PCR demonstrated that Fz2 was expressed to a greater
degree in the HCC and HB cell lines than in the adult liver. The
finding that Fz2 was more frequently upregulated in the tumorous
tissues than in the non-tumorous tissues was expected.
Immunostaining confirmed this hypothesis, showing that Fz2 was
upregulated in the tumorous tissue more so than in the non-tumorous
tissue from a surgical specimen of HCC. Systematic analysis of
surgical specimens would be necessary to confirm this hypothesis.
Immunostaining and western blot analysis have shown that Fz2 is
upregulated in pancreatic cancer (17,18).
The possible involvement of Fz2 in pancreatic cancer and HCC was
expected.
Fz2 may be involved in cell proliferation. shRNA-Fz2
has been shown to suppress the proliferation of MIA-PaCa2, a
pancreatic cancer cell line (17).
In the present study, shRNA-Fz2 suppressed the proliferation of the
HCC cell lines. shRNA-Fz2 suppressed the proliferation of the HLF
cells, which had a 246.9±15.7-times higher expression level of Fz2
compared with the adult liver cells. The level of cyclin D1
expression is downregulated in MIA-PaCa2 cells (17). The present data clearly show that
cyclin D1 was downregulated in the HLF and Hep3B cells. The
downregulation of cyclin D1 may be one cause of the suppression of
cell proliferation. The present data indicate that Fz2 may be a
suitable molecular therapeutic target for HCC. One of the merits of
Fz2 is that it was not expressed in the tumorous tissue of
pancreatic cancer or HCC (17). The
suppression of Fz2 expression was expected to have no effect on the
surrounding non-tumorous tissues.
The next step would be to show apoptosis in the
suppression of HCC cell proliferation with shRNA-Fz2. Another
option would be to combine the shRNA-Fz2 treatment with other
anticancer reagents (19,20).
Acknowledgements
This study was supported by a Research Grant-in-Aid
for Scientific Research (C) (grant no. 23591002) from the Japan
Society for the Promotion of Science.
References
|
1
|
Gómez-Orte E, Sáenz-Narciso B, Moreno S
and Cabello J: Multiple functions of the noncanonical Wnt pathway.
Trends Genet. 29:545–553. 2013.
|
|
2
|
Tanaka SS, Kojima Y, Yamaguchi YL,
Nishinakamura R and Tam PP: Impact of WNT signaling on tissue
lineage differentiation in the early mouse embryo. Dev Growth
Differ. 53:843–856. 2011.
|
|
3
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009.
|
|
4
|
Takahashi-Yanaga F: Activator or
inhibitor? GSK-3 as a new drug target. Biochem Pharmacol.
86:191–199. 2013.
|
|
5
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007.
|
|
6
|
Axelrod JD: Progress and challenges in
understanding planar cell polarity signaling. Semin Cell Dev Biol.
20:964–971. 2009.
|
|
7
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: New insights into the mechanism of Wnt signaling
pathway activation. Int Rev Cell Mol Biol. 291:21–71. 2011.
|
|
8
|
Jessen JR: Noncanonical Wnt signaling in
tumor progression and metastasis. Zebrafish. 6:21–28. 2009.
|
|
9
|
Wang Y: Wnt/Planar cell polarity
signaling: a new paradigm for cancer therapy. Mol Cancer Ther.
8:2103–2109. 2009.
|
|
10
|
Dissanayake SK, Wade M, Johnson CE, et al:
The Wnt5A/protein kinase C pathway mediates motility in melanoma
cells via the inhibition of metastasis suppressors and initiation
of an epithelial to mesenchymal transition. J Biol Chem.
282:17259–17271. 2007.
|
|
11
|
Wang HY, Liu T and Malbon CC:
Structure-function analysis of Frizzleds. Cell Signal. 18:934–941.
2006.
|
|
12
|
Zeng G, Awan F, Otruba W, et al: Wnt’er in
liver: expression of Wnt and frizzled genes in mouse. Hepatology.
45:195–204. 2007.
|
|
13
|
Fujimoto T, Tomizawa M and Yokosuka O:
SiRNA of frizzled-9 suppresses proliferation and motility of
hepatoma cells. Int J Oncol. 35:861–866. 2009.
|
|
14
|
Kühl M, Sheldahl LC, Park M, Miller JR and
Moon RT: The Wnt/Ca2+ pathway: a new vertebrate Wnt
signaling pathway takes shape. Trends Genet. 16:279–283. 2000.
|
|
15
|
Bengochea A, de Souza MM, Lefrançois L, et
al: Common dysregulation of Wnt/Frizzled receptor elements in human
hepatocellular carcinoma. Br J Cancer. 99:143–150. 2008.
|
|
16
|
Yuzugullu H, Benhaj K, Ozturk N, et al:
Canonical Wnt signaling is antagonized by noncanonical Wnt5a in
hepatocellular carcinoma cells. Mol Cancer. 8:902009.
|
|
17
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Frizzled-2: A potential novel
target for molecular pancreatic cancer therapy. Oncol Lett.
7:74–78. 2014.
|
|
18
|
Zeng G, Germinaro M, Micsenyi A, et al:
Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma.
Neoplasia. 8:279–289. 2006.
|
|
19
|
Tomizawa M and Yokosuka O:
Picropodophyllin suppresses the proliferation and invasion of
hepatocellular carcinoma under serum starvation. Mol Med Rep.
1:685–688. 2008.
|
|
20
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Sorafenib suppresses the cell
cycle and induces the apoptosis of hepatocellular carcinoma cell
lines in serum-free media. Exp Ther Med. 1:863–866. 2010.
|