Introduction
The accurate assessment of changes in tumor burden
is critical for anticancer treatment as well as clinical trials of
new drugs. Traditionally, tumor sizes have been measured
bi-dimensionally by obtaining the product of the longest diameter
and the longest perpendicular diameter of each tumor. In the early
1980s, the World Health Organization (WHO) developed the WHO
response criteria in an attempt to standardize the methods for
evaluating the tumor response (1).
However, as the details for selecting and measuring the target
lesions were not clearly described in the WHO guidelines, the
assessment of the tumor response has not been accurately
reproducible between studies (2).
In clinical practice, measuring all of the target lesions using two
dimensions and calculating the sums of their products may result in
errors and is time-consuming.
In 2000, the Response Evaluation Criteria in Solid
Tumors (RECIST) working group presented the RECIST guideline
version 1.0, to clarify and simplify the tumor response criteria
(3). Major features of the RECIST
1.0 guideline included the use of unidimensional measures, rather
than the bi-dimensional measures previously recommended by WHO, for
the evaluation of tumor size and instructions on the number of
target lesions to be evaluated. The RECIST 1.0 recommended
measuring a total of 10 target lesions, with a maximum of five per
organ. Thus, the RECIST 1.0 guideline has been widely accepted as a
standardized method for the assessment of the tumor response.
However, a number of questions and issues regarding the number of
target lesions, the size of lymph nodes (LNs) to be assessed and
the utility of novel imaging technologies have been raised with
regard to the RECIST 1.0 guidelines (4,5).
In 2009, the RECIST Working Group published the
revised RECIST guideline version 1.1, based in part on the
investigation of a database containing data from >6,500 patients
from 16 clinical trials (5,6). The significant modifications to the
RECIST 1.1 included updates concerning the maximum number of target
lesions, the LN measurements and the definition of progressive
disease (PD) (7,8). The maximum number of target lesions to
be assessed was reduced from 10 to five in total, and from five to
two target lesions per organ. While the total of 10 target lesions
proposed in the RECIST 1.0 was an arbitrarily selected number, the
RECIST 1.1 recommended the measurement of a total of five lesions,
based on the analysis of patient data (6) and statistical simulation studies
(9,10). However, the criterion of two target
lesions per organ remains an arbitrary decision and, to the best of
our knowledge, it has not been supported by any objective evidence
(9). Zacharia et al
(11) reported that measuring the
single largest lesion showed approximately the same response
classification as compared with measuring up to five target lesions
in patients with liver metastases of colorectal cancer (CRC). This
finding indicated that the ideal number of target lesions per organ
required to accurately assess the tumor response remains to be
determined in future studies.
The present study proposes the modified RECIST
(mRECIST) 1.1, hypothesizing that measuring the single largest
lesion in each organ into which the cancer had metastasized would
yield approximately the same response classification as measuring
the two target lesions per organ (as recommended by the RECIST 1.1
guidelines). In the present study, computed tomography (CT) was
used to compare the tumor response assessment as obtained using the
mRECIST 1.1 and the RECIST 1.1 guidelines in patients with
metastatic CRC.
Patients and methods
Patients
The present study was performed under the
institutional Review Board’s waiver (IRB no. 2014-02-20), according
to the Korean Ethical Guidelines for Epidemiological Research. The
medical records of patients with metastatic CRC were
retrospectively reviewed. The selected patients had received either
5-flurouracil/leucovorin plus oxaliplatin (FOLFOX) or
5-flurouracil/leucovorin plus irinotecan (FOLFIR1) as a first-line
chemotherapy treatment, between January 2004 and June 2013 at the
Kangnam Sacred Heart Hospital (Seoul, South Korea). The
chemotherapy regimens consisted of biweekly oxaliplatin (85
mg/m2 as a 90-min intravenous [i.v.] infusion on day
one) or irinotecan (150 mg/m2 as a 2-h i.v. infusion on
day one) plus 5-flurouracil/leucovorin (20 mg/m2
leucovorin as a bolus i.v. injection on day one, followed by 3,000
mg/m2 5-flurouracil as a 46-h continuous i.v. infusion).
Patients were considered to be eligible for inclusion in the
present study according to the following criteria: i) A
histologically identified adenocarcinoma of the colon or rectum;
ii) a radiologically or histologically confirmed metastatic disease
with at least two measurable lesions in any one organ according to
the RECIST 1.1; iii) no history of another type of cancer; iv) no
history of previous chemotherapy treatment except for adjuvant
therapy; and v) CT tumor assessment at baseline and following
chemotherapy.
CT examinations
All CT examinations were performed using a 64-MDCT
scanner (SOMATOM Sensation 64; Siemens AG Healthcare, Forchheim,
Germany) with a slice thickness of 5 mm, following which, the
scanned CT images were uploaded onto the Picture Archiving
Communication System (PACS; PiView Star; INFINITT Healthcare Co.
Ltd., Seoul, Korea). The CT scan images that were used for
evaluating tumor response to chemotherapy were performed following
four cycles of FOLFOX or FOLFIRI.
CT tumor measurements
The tumor measurements of each patient were
evaluated from the original CT images. CT tumor measurements were
performed manually on axial CT image planes using the calipers of a
measurement tool on the PACS. The target lesion description, CT
size measurement, sum of the longest diameters of the target
lesions, descriptions of any non-target lesions, and the best tumor
response for each patient were recorded separately by two
oncologists according to the RECIST 1.1 and mRECIST 1.1 guidelines.
Briefly, measurements were taken of the short axis of the LN and
LNs ≥15 mm were considered to be target lesions. LNs that measured
≥10 mm and <15 mm in the short axis were considered to be
non-target lesions, and LNs with a short axis of <10 mm were
regarded as normal. The maximum number of target lesions to be
assessed was five, with a maximum of two per organ, according to
the RECIST 1.1 guidelines or the single largest lesion in each
organ according to the mRECIST 1.1. The diameter of each target
lesion was defined as the mean of the values as measured by two
separate oncologists. In cases where there was a discrepancy in the
tumor measurements between the two medical oncologists, a
board-certified abdomen radiologist re-evaluated the CT
results.
Definitions of tumor response
The definitions of treatment response, used
throughout the present study, were in accordance with the original
RECIST 1.1 guidelines. Complete response (CR) was defined as the
complete disappearance of all tumor lesions. Partial response (PR)
was defined as a reduction in the sum of tumor measurements by
≥30%. PD was defined as ≥20% increase in the sum of the tumor
measurements. In addition, an absolute increase of ≥5 mm to the
lesions was a prerequisite for PD. The appearance of new lesions or
the substantial progression of non-target lesions was considered to
be PD. All other forms of tumor response were classified as stable
disease (SD).
Statistical Analysis
A paired Student’s t-test was used to determine the
statistical significance of changes in the number of target lesions
at baseline between the RECIST 1.1 and mRECIST 1.1 guidelines. The
χ2 test was used to compare the overall response rates
(ORRs) between the two groups. All P-values were based on a
two-sided hypothesis and P<0.05 was considered to indicate a
statistically significant difference. The level of concordance of
the tumor responses between the two criteria was assessed using
kappa statistics; a κ value of >0.75 was interpreted as showing
strong concordance.
Results
Patient characteristics
During the study period, a total of 82 patients with
metastatic CRC received first-line chemotherapy with either the
FOLFOX or FOLFIRI regimen. According to the RECIST 1.1, 18 patients
(21.9%) had no target lesions and four had not been evaluated for
tumor response, therefore, these patients were excluded from the
study. According to the inclusion criteria, 22 patients (26.8%),
who had only one target lesion in each organ that was exhibiting
metastasis, were also excluded from the study. Thus, a total of 38
patients (46.3%), each of which had at least two measurable lesions
in any one organ, were included in the final analyses.
The baseline characteristics of the patients are
summarized in Table I. The patients
consisted of 23 males (60.5%) and 15 females (39.5%) with a median
age of 60 years (range, 42–78 years). A total of 32 patients
(84.2%) had colon cancer, and the remaining six patients (15.8%)
had rectal cancer. A total of 27 patients (71.1%) had well- or
moderately differentiated adenocarcinoma and 11 (28.9%) had poorly
differentiated adenocarcinoma. The most common metastatic site
containing measurable target lesions was the liver (76.3%),
followed by the LNs (34.2%) and the lungs (18.4%). According to the
RECIST 1.1, 24 patients (63.1%) had target lesions in only one
organ, which were most commonly observed in the liver. A total of
11 patients had target lesions in two organs, these were most
commonly found in the liver and the LNs. There were only three
patients who had target lesions in more than three organs. Of the
38 patients included in the study, 33 (86.8%) were treated with the
FOLFOX chemotherapy regimen, and the remaining five (13.2%)
received the FOLFIRI regimen.
 | Table ICharacteristics of the 38 patients
(median age, 60 years; range, 42–78 years). |
Table I
Characteristics of the 38 patients
(median age, 60 years; range, 42–78 years).
| Patients |
|---|
|
|
|---|
| Characteristic | n | % |
|---|
| Gender |
| Male | 23 | 60.5 |
| Female | 15 | 39.5 |
| Site |
| Colon | 32 | 84.2 |
| Rectum | 6 | 15.8 |
| Histology |
| Well- to moderately
differentiated | 27 | 71.1 |
| Poorly
differentiated | 11 | 28.9 |
| Measurable metastatic
lesions |
| Liver | 29 | 76.3 |
| Lungs | 7 | 18.4 |
| Lymph nodes | 13 | 34.2 |
| Peritoneum | 4 | 10.5 |
| Pancreas | 1 | 2.6 |
| Chemotherapy
regimen |
| FOLFOX | 33 | 86.8 |
| FOLFIRI | 5 | 13.2 |
Number of target lesions
The number of target lesions according to the
mRECIST 1.1 was significantly lower as compared with the number
according to the RECIST 1.1 (p<0.0001). The median number of
target lesions was two (range, 2–5) by the RECIST 1.1 and one
(range, 1–3) by the mRECIST 1.1. When the mRECIST 1.1 was adopted,
rather than the RECIST 1.1, no newly defined target lesions were
identified in the metastatic sites of the patients.
Tumor response
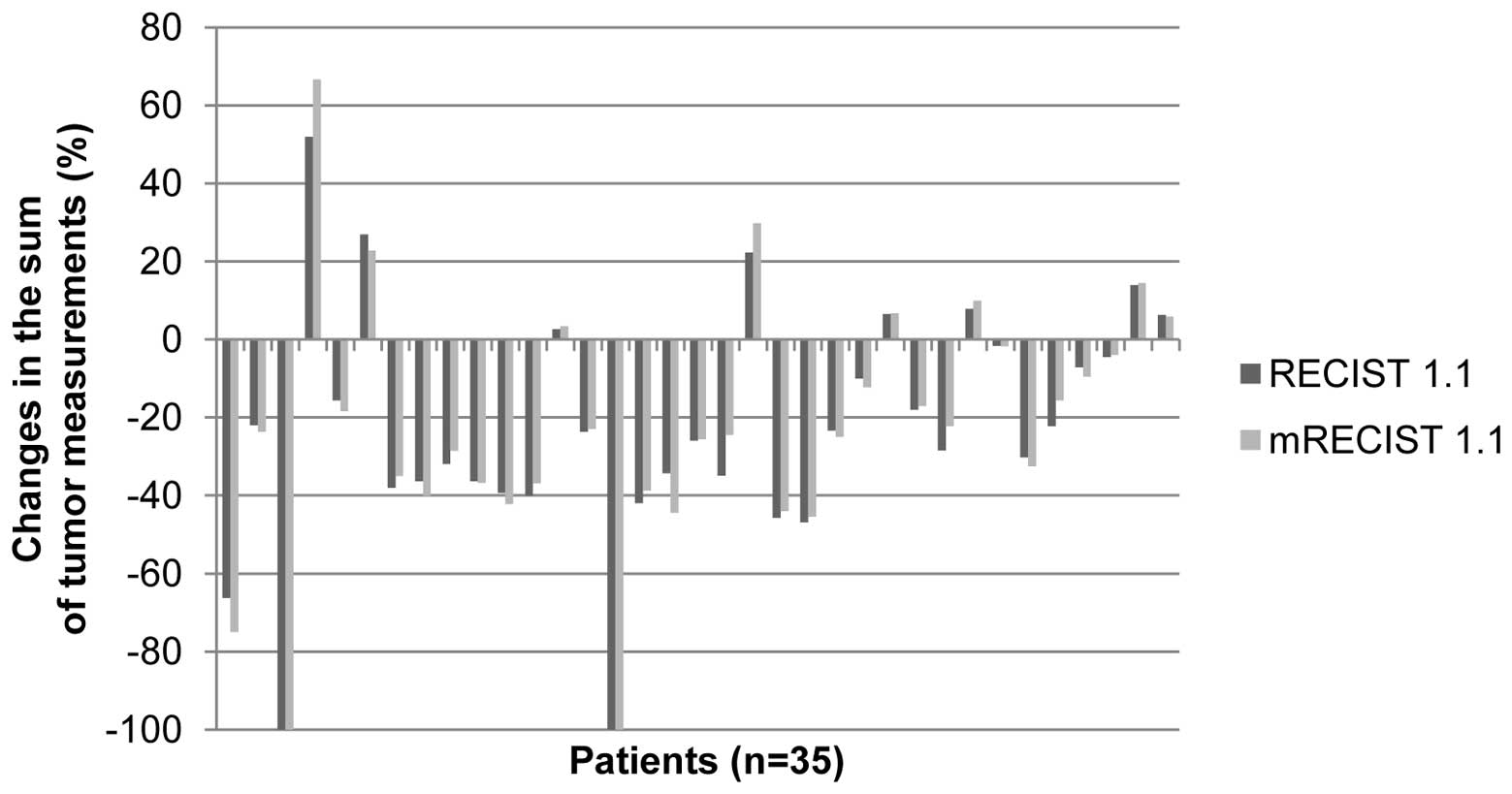
The changes in the sum of tumor measurements,
according to the RECIST 1.1 and mRECIST 1.1 criteria, are presented
as percentages in Fig. 1. Two
patients demonstrated CR and three developed new metastatic lesions
following chemotherapy. Among the remaining 33 patients, 18 (54.5%)
showed an increase in the change rate (range, 0.1–14.7%) of the sum
of the tumor measurements when adopting the mRECIST 1.1, rather
than the RECIST 1.1. The remaining 15 patients (45.5%) demonstrated
a reduction in the change rate (range, 0.4–10.5%) of the sum of the
tumor measurements.
The comparison of the tumor responses between the
RECIST 1.1 and mRECIST 1.1 guidelines is presented in Table II. No significant difference in the
ORRs of FOLFOX or FOLIRI was identified between the two criteria;
39.5% (15/38) according to the RECIST 1.1 and 34.2% (13/38)
according to the mRECIST 1.1 (P=0.226). Almost perfect agreement
between the RECIST 1.1 and the mRECIST 1.1 was observed with regard
to the tumor response assessment, with a κ value of 0.905 (95%
confidence interval, 0.777–1.0). Two patients (5.3%) revealed a
disagreement in the responses between the RECIST 1.1 and mRECIST
1.1 criteria. The two patients demonstrated PR according to the
RECIST 1.1, however, were reclassified as SD according to the
mRECIST 1.1.
 | Table IITumor response assessment by the
RECIST 1.1, as compared with the mRECIST 1.1. |
Table II
Tumor response assessment by the
RECIST 1.1, as compared with the mRECIST 1.1.
| Response by mRECIST
1.1 |
|---|
|
|
|---|
| Rresponse by RECIST
1.1 | CR+PR (n=13) | SD (n=19) | PD (n=6) |
|---|
| CR + PR (n=15) | 13 | 2 | 0 |
| SD (n=17) | 0 | 17 | 0 |
| PD (n=6) | 0 | 0 | 6 |
Discussion
The present study investigated the impact of
measuring the single largest lesion in each organ with metastatic
disease (termed the mRECIST 1.1), compared with measuring two
target lesions per organ, as recommended by the RECIST 1.1, on the
tumor response in patients with metastatic CRC. Single-lesion
measurements significantly decreased the number of target lesions
to be measured at the baseline of first-line chemotherapy. When
compared with the two-lesion measurement, the single-lesion
measurement had a concordant response classification in 94.7% of
patients.
The WHO criteria and the RECIST guidelines depend on
the changes in tumor size as determined using imaging techniques,
therefore, target lesions are the most important radiological
markers in the assessment of the tumor response. However, the
criterion of a total of 10 target lesions, with a maximum of five
lesions per organ, as proposed in the RECIST 1.0 guidelines was
considered to be arbitrary and lacked objective evidence.
Therefore, the RECIST Working Group retrospectively analyzed the
effects of assessing one, two, three or five target lesions, as
opposed to 10, on the tumor response and progression outcome using
their patient database (6). It was
observed that assessing three or five target lesions did not change
the ORR or progression-free survival, when compared with assessing
10 lesions, as recommended by the RECIST 1.0 guidelines. A
statistical simulation study for evaluating the impact of the
number of target lesions also revealed little difference between
five and 10 target lesions in the assessment of the overall tumor
response (9). Based on these
results, the RECIST 1.1 guideline proposed a total of five target
lesions to be measured, with a maximum of two per organ.
Investigators have begun to use the RECIST 1.1 in
clinical trials and in clinical practice anticipating that it will
improve feasibility, as it is a more convenient assessment of tumor
response (12). Almost perfect
agreement between the RECIST 1.1 and the RECIST 1.0 has previously
been demonstrated in the assessment of the tumor response in
patients with NSCLC (12,13), advanced gastric cancer (14,15)
and metastatic CRC (16). However,
the criterion of assessing two target lesions per organ as per the
RECIST 1.1 is considered to be an arbitrary value and, to the best
of our knowledge, has not been supported by any objective evidence
(9). Furthermore, in cases with
more than three metastatic sites, the RECIST 1.1 may not be
representative of all of the involved organs, due to the limited
number of target lesions measured. The present study hypothesized
that measuring the maximal diameter of the single largest lesion in
each organ would be more representative of all of the metastatic
sites. In the present study, of the 38 patients who had two or more
measurable lesions in any organ, 24 patients (63.2%) had target
lesions in only one organ, with the most common site being the
liver. Three patients (7.9%) showed target lesions in three or more
organs. However, no patients were identified to exhibit newly
defined target lesions in the metastatic sites according to the
mRECIST 1.1 guidelines.
There have been few studies investigating the
optimal number of target lesions to be measured in order to assess
the tumor response. Schwartz et al (10) simulated >1.8 million possible
combinations of lesions from unidimensional measurements using a
complex computerized model. The results indicated that the variance
of the response assessment was decreased by 90% if at least four
lesions were measured rather than just one lesion. Darkeh et
al (17) investigated the minimum number of target lesions to
measure, in order to represent the total number of target lesions,
according to the RECIST 1.0 guidelines. In patients with five or
more target lesions, measuring between four and seven lesions did
not lead to a discrepancy; furthermore, the number of discordant
cases was shown to increase gradually from measuring three lesions
(4/53) to measuring one target lesion (8/53). On the basis of these
results, the assessment of at least four lesions was recommended,
when more than four target lesions are present. However, these
studies evaluated the minimum number of target lesions, rather than
considering the optimal number of target lesions, to measure per
organ.
The present study compared the tumor response
assessment between the mRECIST 1.1 (measuring the single largest
lesion in each organ with metastases) and the RECIST 1.1 (measuring
two target lesions per organ) in patients with metastatic CRC.
Patients received either a FOLFOX or FOLFIRI regimen as first-line
chemotherapy in a clinical practice setting, with no confirmation
of tumor response. CT scans were performed following four cycles of
chemotherapy, which translated into intervals of 8–12 weeks. The
ORRs of the first-line chemotherapy according to the RECIST 1.1 and
the mRECIST 1.1 were 39.4% and 34.2%, respectively. A significant
limitation of the present study was the low number of patients that
were assessed, however, the tumor responses showed a high level of
concordance between the two criteria (κ=0.905). Of the 38 patients
that were assessed, only two patients (5.3%) showed disagreement
with regard to the responses during comparison of the two criteria;
these two patients showed a PR according to the RECIST 1.1 and were
reclassified as SD according to the mRECIST 1.1. Traditionally, in
clinical practice, patients who are classified as PR or SD remain
on the same treatment regimen. Therefore, with discordance only
being shown between PR and SD classifications, the present study
determined that the clinical impact of the mRECIST 1.1 on altering
therapeutic decisions appeared to be minimal.
Prior to the release of the RECIST 1.1, Zacharia
et al (11) reported that,
in the majority of CRC patients with hepatic metastases, measuring
the single largest lesion showed the same response classification
as measuring up to five target lesions. Measuring two or more (up
to five) target lesions showed complete concordance in the
evaluation of the best tumor response, and single-lesion
measurements gave a concordant tumor response in 93.3% (28/30) of
patients as compared with the multiple-lesion measurements
(κ=0.88). The above-mentioned findings, which are consistent with
the results from the present study, indicate that it may be
possible to reduce the number of target lesions measured per organ
in clinical practice.
In conclusion, the mRECIST 1.1 demonstrated a high
level of concordance with the original RECIST 1.1 guidelines in the
response assessment of patients with metastatic CRC. As the
clinical impact on therapeutic decisions appeared to be minimal,
the mRECIST 1.1, with the decreased number of target lesions to be
measured, may be more convenient for future use in clinical
practice.
References
|
1
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981.
|
|
2
|
Therasse P: Measuring the clinical
response. What does it mean? Eur J Cancer. 38:1817–1823. 2002.
|
|
3
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
|
|
4
|
Sargent DJ, Rubinstein L, Schwartz L, et
al: Validation of novel imaging methodologies for use as cancer
clinical trial end-points. Eur J Cancer. 45:290–299. 2009.
|
|
5
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version1.1). Eur J Cancer. 45:228–247. 2009.
|
|
6
|
Bogaerts J, Ford R, Sargent D, et al;
RECIST Working Party. Individual patient data analysis to assess
modifications to the RECIST criteria. Eur J Cancer. 45:248–260.
2009.
|
|
7
|
Schwartz LH, Bogaerts J, Ford R, et al:
Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer.
45:261–267. 2009.
|
|
8
|
Dancey JE, Dodd LE, Ford R, et al:
Recommendations for the assessment of progression in randomized
cancer treatment trials. Eur J Cancer. 45:281–289. 2009.
|
|
9
|
Moskowitz CS, Jia X, Schwartz LH and Gönen
M: A simulation study to evaluate the impact of the number of
lesions measured on response assessment. Eur J Cancer. 45:300–310.
2009.
|
|
10
|
Schwartz LH, Mazumdar M, Brown W, et al:
Variability in response assessment in solid tumors: effect of
number of lesions chosen for measurement. Clin Cancer Res.
9:4318–4323. 2003.
|
|
11
|
Zacharia TT, Saini S, Halpern EF and
Sumner JE: CT of colon cancer metastases to the liver using
modified RECIST criteria: determining the ideal number of target
lesions to measure. AJR Am J Roentgenol. 186:1067–1070. 2006.
|
|
12
|
Sun JM, Ahn MJ, Park MJ, et al: Accuracy
of RECIST 1.1 for non-small cell lung cancer treated with EGFR
tyrosine kinase inhibitors. Lung Cancer. 69:105–109. 2010.
|
|
13
|
Nishino M, Jackman DM, Hatabu H, et al:
New Response Evaluation Criteria in Solid Tumors (RECIST)
guidelines for advanced non-small cell lung cancer: comparison with
original RECIST and impact on assessment of tumor response to
targeted therapy. AJR Am J Roentgenol. 195:W221–W228. 2010.
|
|
14
|
Fuse N, Nagahisa-Oku E, Doi T, et al:
Effect of RECIST revision on classification of target lesions and
overall response in advanced gastric cancer patients. Gastric
Cancer. 16:324–328. 2013.
|
|
15
|
Jang GS, Kim MJ, Ha HI, et al: Comparison
of RECIST version 1.0 and 1.1 in assessment of tumor response by
computed tomography in advanced gastric cancer. Chin J Cancer Res.
25:689–694. 2013.
|
|
16
|
Jang HJ, Kim BC, Kim HS, et al: Comparison
of RECIST 1.0 and RECIST 1.1 on computed tomography in patients
with metastatic colorectal cancer. Oncology. 86:117–121. 2014.
|