Introduction
Gastric cancer (GC) is one of the most frequently
diagnosed malignancies in East Asia, particularly in China
(1,2). Detection of the disease in the
preclinical or pre-symptomatic phases is the key to successful
treatment and patient outcome. In China, gastroscopy is
increasingly used as a primary detection tool due to its diagnostic
accuracy. However, less than half of eligible individuals (>50
years of age) undergo GC screening. Numerous patients are diagnosed
at an advanced stage, leading to a high mortality rate of GC
(3). The identification of more
reliable and noninvasive screening tests may increase compliance
with GC screening guidelines by individuals who are reluctant to
undergo invasive tests, or when gastroscopy detection is not
feasible or readily available.
A number of studies have demonstrated that cancer
patients exhibit significantly higher serum levels of
tumor-specific DNA mutations, with >90% of the total circulating
cell-free DNA (cfDNA) derived from malignant tissue, compared with
those with non-malignant diseases (4–8). The
mechanism of cfDNA release into the circulation is poorly
understood; however, it is hypothesized that methylation of gene
promoter regions is crucial for tumor carcinogenesis (6). These alterations have been shown to
occur early in the development of cancer (9), suggesting that the detection of free
methylated circulating DNA presents a promising approach for the
development of plasma-based screening methods for non-invasive
monitoring of GC progression.
The aim of the present study was to evaluate the
potential of using the promoter methylation status of a panel of
apoptosis-related genes, including the ring finger protein 180
(RNF180) (10), death-associated
protein kinase 1 (DAPK1) (11,12)
and secreted frizzled-related protein 2 (SFRP2) (11,13)
genes, in circulating plasma DNA for the detection of GC. The three
genes included in this study were selected as they are known to be
functional tumor suppressor genes in GC, and have previously been
reported to be highly methylated in tumors and the serum/plasma of
GC patients (11–15). The methylation-specific polymerase
chain reaction (PCR) (MSP) technique was used to analyze the
specificity and sensitivity of this method for GC detection and to
evaluate the clinical diagnostic significance of these DNA
methylation-based plasma markers.
Materials and methods
Patients and samples
A total of 42 controls and 57 GC patients diagnosed
at Li Huili Hospital (Ningbo, China) between July 2011 and July
2012 were enrolled in this study. None of the enrolled patients had
received preoperative chemotherapy or radiation therapy. This study
was approved by the ethics committee of Li Huili Hospital (Ningbo,
China) and informed consent was obtained from all participants. All
patients were diagnosed with GC based on pathological and/or
cytological evidence. The clinicopathological features are shown in
Table I. Tumor stage was determined
according to the tumor node metastasis (TNM) criteria of the Union
for International Cancer Control/American Joint Committee on
Cancer, 2010 (16). The plasma
samples were acquired prior to treatment in the patient and control
groups. The plasma samples were immediately isolated by
centrifugation at 1,000 × g for 10 min and stored at −80°C until
DNA was extracted.
 | Table IClinicopathological features and
RNF180, DAPK1 and SFRP2 DNA methylation status in plasma samples of
57 patients with gastric cancer. |
Table I
Clinicopathological features and
RNF180, DAPK1 and SFRP2 DNA methylation status in plasma samples of
57 patients with gastric cancer.
| | RNF180 | DAPK1 | SFRP2 |
|---|
| |
|
|
|
|---|
| Parameters | n | M | U | P-value | M | U | P-value | M | U | P-value |
|---|
| Gender | | | | 0.137 | | | 0.928 | | | 0.446 |
| Male | 39 | 20 | 15 | | 19 | 20 | | 27 | 12 | |
| Female | 18 | 13 | 9 | | 9 | 9 | | 14 | 4 | |
| Age, years | | | | 0.627 | | | 0.910 | | | 0.569 |
| <60 | 24 | 13 | 11 | | 12 | 12 | | 16 | 8 | |
| ≥60 | 33 | 20 | 13 | | 16 | 17 | | 25 | 8 | |
| Tumor size,
cm3 | | | | 0.018 | | | 0.705 | | | 0.784 |
| <6 | 23 | 9 | 14 | | 12 | 11 | | 17 | 6 | |
| ≥6 | 34 | 24 | 10 | | 16 | 18 | | 24 | 10 | |
| Histological
type | | | | 0.025 | | | 0.940 | | | 0.769 |
| Differentiated | 14 | 4 | 10 | | 7 | 7 | | 10 | 4 | |
|
Undifferentiated | 43 | 29 | 14 | | 21 | 22 | | 31 | 12 | |
| TNM stage | | | | 0.002 | | | 0.647 | | | 0.683 |
| I–II | 20 | 6 | 14 | | 9 | 11 | | 15 | 5 | |
| III–IV | 37 | 27 | 10 | | 19 | 18 | | 26 | 11 | |
| Lymph node
metastasis | | | | 0.008 | | | 0.672 | | | 0.660 |
| N0 | 24 | 9 | 15 | | 11 | 13 | | 18 | 6 | |
| N1–3 | 33 | 24 | 9 | | 17 | 16 | | 23 | 10 | |
| Distant
metastasis | | | | 0.018 | | | 0.961 | | | 0.882 |
| M0 | 50 | 26 | 24 | | 24 | 26 | | 35 | 15 | |
| M1 | 7 | 7 | 0 | | 4 | 3 | | 6 | 1 | |
DNA extraction and bisulfite
modification
A total of 400 μl DNA from each plasma sample was
extracted using the QIAamp DNA Blood mini kit (Qiagen, Hilden,
Germany)according to the manufacturer’s instructions. Plasma DNA
was eluted in a total volume of 80 μl elution buffer (EB) and
stored at −20°C.
Sodium bisulfite modification was conducted using
the Qiagen Epitect Plus DNA bisulfite kit (Qiagen), according to
the manufacturer’s instructions. DNA was then resuspended in 30 μl
EB and stored at −20°C.
MSP
Methylated and unmethylated primers specific for the
promoter sequences of the target genes, RNF180, DAPK1 and SFRP2,
were designed to amplify the bisulfite-modified DNA. The primer
sequences used are shown in Table
II. The 50-μl reaction mixture contained 2 μl of DNA template,
10 μl of KAPA2G buffer (Kapa Biosystems, Woburn, MA, USA), 1 μl of
10 mM dNTP mix (Kapa Biosystems), 1 μl of each primer at 50 mM, and
0.5 units of KAPA2GTM Robust Hotstart DNA polymerase
(Kapa Biosystems).
 | Table IIPrimer sequences and annealing
temperatures used for the methylation-specific polymerase chain
reaction. |
Table II
Primer sequences and annealing
temperatures used for the methylation-specific polymerase chain
reaction.
| Primer | Sequence,
5′-3′ | Annealing
temperature, °C | Product size,
bp | Reference |
|---|
| RNF180 MF |
GGAGAAAAATTTTTTTACGGTTTC | 50 | 109 | |
| RNF180 MR |
CACGTCTACGAATTCCCAC | | | |
| RNF180 UF |
AGGGAGAAAAATTTTTTTATGGTTTT | 46 | 109 | |
| RNF180 UR |
CACATCTACAAATTCCCACCC | | | |
| DAPK1 MF |
GGATAGTCGGATCGAGTTAACGTC | 52 | 98 | (14) |
| DAPK1 MR |
CCCTCCCAAACGCCGA | | | |
| DAPK1 UF |
GGAGGATAGTTGGATTGAGTTAATGTT | 56 | 106 | |
| DAPK1 UR |
CAAATCCCTCCCAAACACCAA | | | |
| SFRP2 MF |
GGGTCGGAGTTTTTCGGAGTTGCGC | 58 | 138 | (13) |
| SFRP2 MR |
CCGCTCTCTTCGCTAAATACGACTCG | | | |
| SFRP2 UF |
TTTTGGGTTGGAGTTTTTTGGAGTTGTGT | 54 | 145 | |
| SFRP2 UR |
AACCCACTCTCTTCACTAAATACAACTCA | | | |
The conditions for amplification included a single
cycle at 95°C for 5 min and a subsequent 10 cycles at 95°C for 30
sec, melting temperature 8°C (Tm; 0.8°C, descending by 0.8°C for
each cycle) for 60 sec and 72°C for 30 sec; then 38 cycles at 95°C
for 30 sec, Tm for 60 sec and 72°C for 30 sec; followed by a final
extension step of 10 min at 72°C. The PCR products were then
electrophoresed on a 2.5% agarose gel and visualized under
ultraviolet illumination (ChemiDoc XRS; Bio-Rad, Hercules, CA,
USA). Each experiment was repeated at least three times. The
operator who performed all assays was blinded to all clinical
information.
Statistical analysis
SPSS, version 13.0 (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses. The mean of variables was
compared between two groups using Student’s t-test. The association
between the methylation status of various genes and the
clinicopathological characteristics of patients was evaluated using
the χ2 test or the Fisher’s exact test. To estimate the
predictive power of clinical and plasma markers for the presence of
GC, multivariate logistic regression analyses were performed. Odds
ratios (ORs) with 95% confidence intervals (CIs) were used as a
measure of association. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 57 GC patients and 42 healthy controls
donated blood within a 12-month time period prior to receiving any
treatment. The mean age ± standard deviation of patients with GC
was 61.49±12.02 years, while that of the controls was 57.21±8.45
years. The ratio of male to female patients was 39:18 in the cancer
group and 27:15 in the control group. No significant differences in
age and gender were identified between the two groups (data not
shown). The clinicopathological characteristics of the 57 GC
patients are shown in Table I.
Detection of aberrant RNF180, DAPK1 and
SFRP2 promoter methylation in plasma
To determine whether the DNA methylation status of
the RNF180, DAPK1 and SFRP2 genes in plasma samples had diagnostic
value for GC, MSP analysis was used to investigate the frequency of
DNA methylation of these genes in the plasma samples of 42 control
and 57 GC patients. In the peripheral blood plasma, RNF180
methylation was detected in 57.89% (33/57) of GC patients, while
methylation of this gene was observed in 23.81% (10/42) of
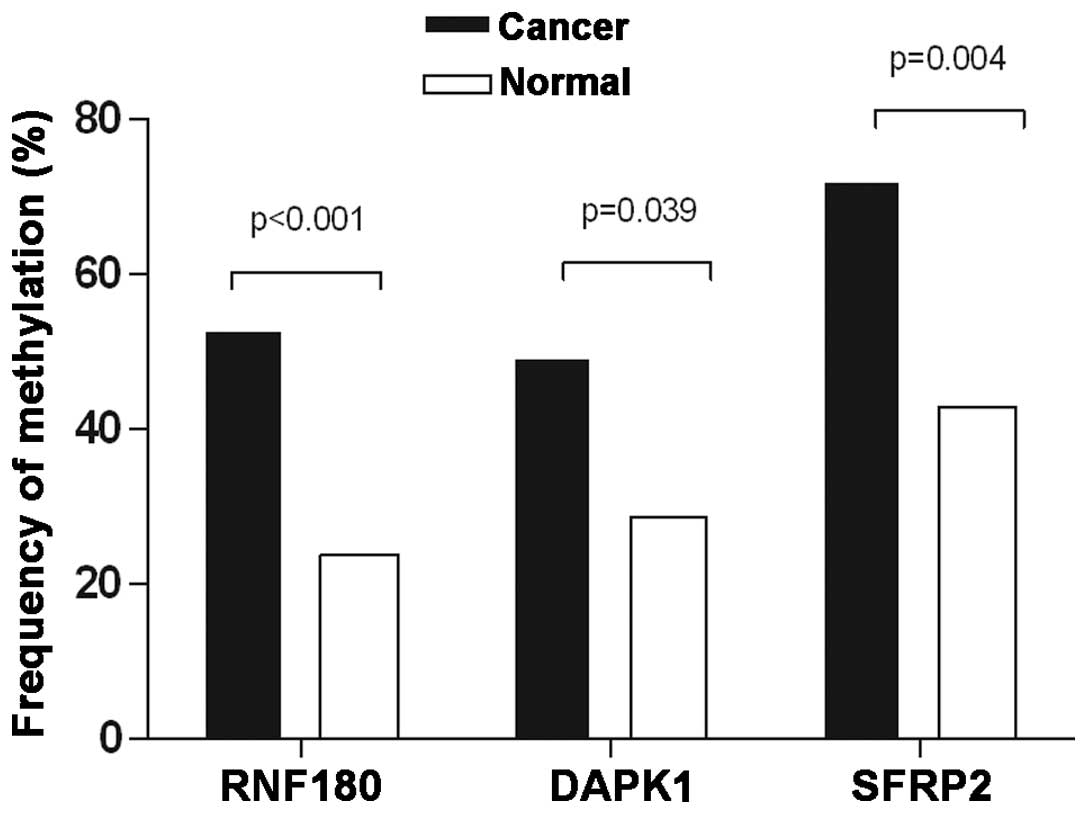
noncancerous control patients (P=0.0007; Fig. 1). The methylation frequencies of
DAPK1 were 49.12% (28/57) in GC patients and 28.57% (12/42) in
noncancerous controls (P=0.0394; Fig.
1). Regarding SFRP2, methylation was detected in 71.93% (41/57)
of GC patients and 42.86% (18/42) of control patients (P=0.0036;
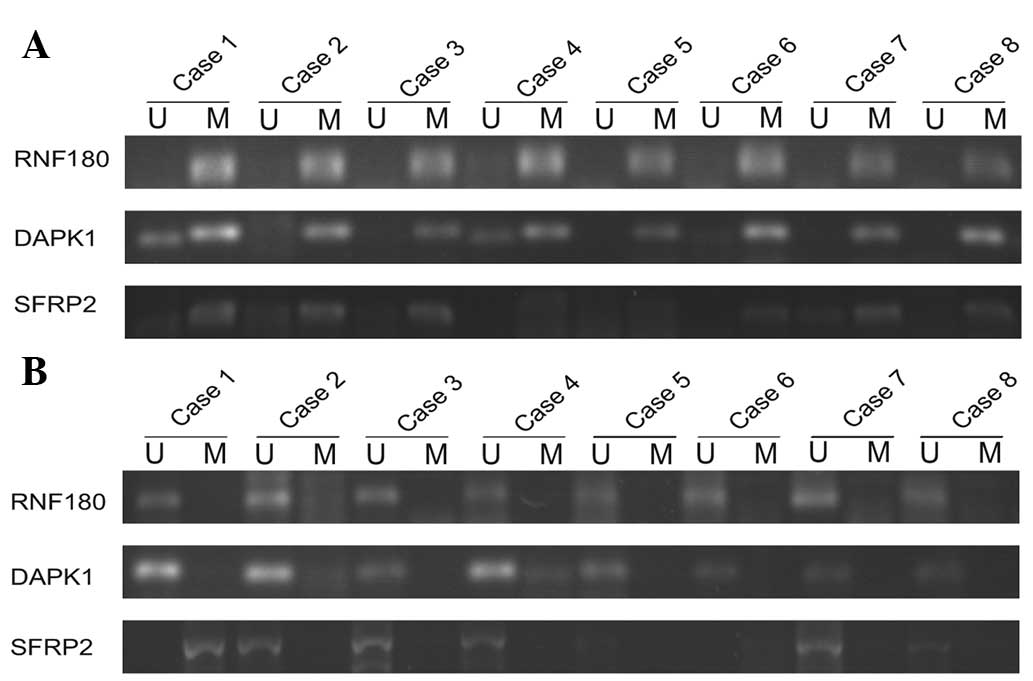
Fig. 1). Representative agarose gel
electrophoresis results of the MSP for the three genes are shown in
Fig. 2.
Association between promoter methylation
in plasma DNA and clinicopathological parameters of GC
patients
The clinicopathological characteristics of the GC
patients and the methylation status of RNF180, DAPK1 and SFRP2 are
shown in Table I. No association
between DAPK1 and SFRP2 methylation in the plasma DNA, and gender,
age, tumor size, differentiation status, TNM stage, lymph node
metastasis or distant metastasis were identified. However, the
methylation levels of the RNF180 gene were found to positively
correlate with tumor size (P=0.018), histological type (P=0.025),
TNM stage (P=0.002), lymph node metastasis (P=0.008) and distant
metastasis (P=0.018).
Comparison of the predictive power of
RNF180, DAPK1 and SFRP2 methylation and their combination for GC
detection
Multivariate regression analyses revealed a
significant correlation between GC and RNF180 methylation (OR,
3.528; 95% CI, 0.542–0.861; P=0.007) and SFRP2 methylation (OR,
2.647; 95% CI, 1.080–6.487; P=0.033), but not for DAPK1 methylation
(OR, 1.540; 95% CI, 0.610–3.890; P=0.361), in GC patients and
controls (Table III).
 | Table IIIMultivariate regression analysis for
methylation in gastric cancer patients and controls. |
Table III
Multivariate regression analysis for
methylation in gastric cancer patients and controls.
| Gene
methylation | Odds ratio (95%
confidence interval) | P-value |
|---|
| RNF180 | 3.528
(0.542–0.861) | 0.007 |
| DAPK1 | 1.540
(0.610–3.890) | 0.361 |
| SFRP2 | 2.647
(1.080–6.487) | 0.033 |
The ORs (95% CI) for predicting the presence of GC
using the RNF180 (P=0.0007), DAPK1 (P=0.0394) and SFRP2 methylation
statuses (P=0.0036) were 4.40 (1.82–10.65), 2.41 (1.03–5.63) and
3.42 (1.47–7.92), respectively (Table
IV). Furthermore, if RNF180 and DAPK1 methylation were
combined, the OR (95% CI) for cancer prediction [OR, 4.86
(2.03–11.66); P=0.0003] was similar to that of the RNF180
methylation [OR, 4.40 (1.82–10.65); P=0.0007] alone and to that of
the methylation of the three genes combined [OR, 4.40 (1.61–12.03);
P=0.0026]. When RNF180 and SFRP2 methylation were combined, the OR
(95% CI) for cancer prediction [OR, 5.57 (2.13–14.57); P=0.0002]
was superior to that for RNF180 methylation alone [OR, 4.40
(1.82–10.65); P=0.0007]; however, the specificity (47.62%) for
cancer prediction was markedly lower than that of RNF180
methylation alone (76.19%).
 | Table IVComparison of the predictive powers
for gastric cancer between RNF180, DAPK1 and SFRP2 methylation
alone and in combination. |
Table IV
Comparison of the predictive powers
for gastric cancer between RNF180, DAPK1 and SFRP2 methylation
alone and in combination.
| Gene
methylation | Sensitivity, % (95%
CI) | Specificity, % (95%
CI) | Odds ratio (95%
CI) | P-value |
|---|
| RNF180 | 57.89
(44.08–70.86) | 76.19
(60.55–87.95) | 4.40
(1.82–10.65) | 0.0007 |
| DAPK1 | 49.12
(35.63–62.71) | 71.43
(55.42–84.28) | 2.41
(1.03–5.633) | 0.0394 |
| SFRP2 | 71.93
(58.46–83.03) | 57.14
(40.96–72.28) | 3.42
(1.47–7.92) | 0.0036 |
| RNF180 + DAPK1 | 76.79
(63.58–87.02) | 59.52
(43.28–74.37) | 4.86
(2.03–11.66) | 0.0003 |
| RNF180 + SFRP2 | 85.96
(74.21–93.74) | 47.62
(32.00–63.58) | 5.57
(2.13–14.57) | 0.0002 |
| DAPK1 + SFRP2 | 82.46
(70.09–91.25) | 42.86
(27.72–59.04) | 3.53
(1.41–8.81) | 0.0057 |
| RNF180 + DAPK1 +
SFRP2 | 87.72
(76.32–94.92) | 38.10
(23.57–54.36) | 4.40
(1.61–12.03) | 0.0026 |
Overall, the combination of RNF180 and SFRP2
methylation appeared to be the most effective predictor of GC, with
regard to predictive power and cost-performance.
Discussion
Aberrant promoter methylation is the predominant
mechanism which inactivates tumor-associated genes, particularly
tumor suppressor genes, along with genetic silencing, which
ultimately leads to gastric carcinogenesis. Numerous genes have
been found to be methylated in GC, including hMLH1, p16, RUNX3,
DAPK1, SFRP2 and RNF180. hMLH1 encodes DNA repair proteins and is
closely associated with poor prognosis of GC patients; the
frequency and specificity were found to be 8.6–80 and 4.1–80%,
respectively (17–19). P16 inhibits cell cycle progression,
and has been found to correlate with poor tumor differentiation,
lymph node metastasis and poor survival. The frequency and
specificity of P16 were found to be 30.4–44.2 and 76–100%,
respectively (15,18,20).
RUNX3 belongs to the RUNX family of transcriptional factors, and
has been found to correlate with depth of tumor invasion, lymph
node and distant metastasis. The frequency and specificity of RUNX3
were 56–75.2 and 92.6%, respectively (21–23).
DAPK1 is a positive regulator of cell apoptosis, and has been found
to correlate with poorly differentiated tumors and lymph node
metastasis. The frequency and specificity of DAPK1 were 30.9–83.2
and 57.8–100%, respectively (12,15,24–26).
No significant correlation has been identified between SFRP2, a
candidate tumor-suppressor gene, and clinical outcomes. The
frequency and specificity of SFRP2 were 80.0–90.0 and 31.0–73.3%,
respectively (13,27). RNF180 is a novel potential tumor
suppressor in GC; however, no correlation with clinical outcomes
was identified. The frequency and specificity of RNF180 were 76%
and 100%, respectively (10).
However, our preliminary study revealed that the
methylation of hMLH1, P16 and RUNX3 plasma biomarkers was extremely
low in GC patients (data not shown). Of note, in the current study,
the methylation levels of the apoptosis-related genes, RNF180,
DAPK1 and SFRP2, were observed to be significantly higher in the
plasma DNA of GC patients when compared with controls.
The methylation of a core functional region of the
promoter of RNF180, a novel ring finger-encoded product, has been
suggested to significantly correlate with human GC development and
pre-cancerous lesions. RNF180 acts as a potential tumor suppressor,
exhibiting a critical role in the suppression of cell proliferation
and induction of apoptosis (27).
Cheung et al (10)
demonstrated that methylation of RNF180 was detected in 56.25%
(18/32) of plasma samples from cancer patients, whereas RNF180
methylation was not detected in the plasma of 64 normal controls.
In the present study, the results revealed that the methylation of
RNF180 was detected in 57.89% (33/57) of GC patient plasma samples
and in 23.81% (10/42) of the controls. The frequency of RNF180
methylation was 23.81% (10/42) in controls. Although the
specificity was lower, the GC patient population tested in the
current study was larger than that of the previous report (10). Additionally, in the present study,
the association of plasma DNA methylation of RNF180 in each sample
with clinical outcomes was investigated.
DAPK1 is a calcium/calmodulin-dependent
serine/threonine protein kinase involved in apoptosis and tumor
suppression (14,15). Reduced expression and aberrant
methylation of DAPK1 has been reported in numerous human cancers,
including GC (28,29). In previous studies, the sensitivity
and specificity of predicting GC using the serum DNA methylation
status of DAPK1 were found to be 48.1% (26/54) and 100% (0/30),
respectively (12). However, in the
present study, DAPK1 plasma methylation was detected in 49.12%
(28/57) of GC patient, and in 28.57% (12/42) of the controls.
Although the sensitivity was higher than previously reported, the
specificity remained low.
SFRP2 has been identified as a modulator of the Wnt
signaling pathway, which is associated with multiple tumor types,
including GC (13,31). Recent epigenetic studies have
demonstrated that silencing of the SFRP2 by promoter methylation at
CpG islands enhanced tumor growth and expansion in GC (13,32,33).
Cheng et al (13) revealed
that serum SFRP2 methylation was a potential biomarker for GC, as
SFRP2 methylation was detected in a total of 66.7% (12/18) GC
patients, however, no SFRP2 methylation was detected in the sera of
18 normal subjects. Similarly, in the current study, SFRP2
methylation was detected in 71.93% of GC patient plasma samples,
however, the specificity of this single biomarker was relatively
low (57.14%).
Considering that aberrant DNA methylation of plasma
exhibits a significant role in GC development and progression, the
clinical significance of DNA methylation was investigated to
evaluate the diagnostic power of the RNF180, DAPK1 and SFRP2
markers in plasma. Firstly, the association between the plasma DNA
methylation status of RNF180 in each sample and clinical data was
evaluated. Of note, tumor size (P=0.018), histological type
(P=0.025), TNM stage (P=0.002), lymph node metastasis (P=0.008) and
distant metastasis (P=0.018) were found to be significantly
associated with RNF180 methylation, indicating that this epigenetic
alteration may be a valuable marker for the prognosis of GC
patients. However, no significant correlation was identified
between DAPK1 and SFRP2 methylation in the plasma DNA and the
clinicopathological characteristics evaluated, indicating that
these epigenetic events are involved in the multistep process of
gastric carcinogenesis, and may present potential biomarkers for
early diagnosis in GC. Further studies may be required to
characterize the source of cfDNA and the mechanisms involved in its
release into the blood to provide an improved explanation for this
observation.
One strategy to improve GC diagnosis is to combine
multiple methylation biomarkers in plasma that have potential
clinical applications. In particular, the high specificity of
RNF180 methylation is an attractive candidate as a marker for such
a panel, which would exhibit increased sensitivity without major
impacts on specificity. The combination of RNF180, DAPK1 and SFRP2
methylation in this study did increase sensitivity, but at a cost
to specificity. Thus, a pilot study investigating the performance
of RNF180 methylation in combination with SFRP2 was carried out. As
expected, the combination of RNF180 and SFRP2 methylation exhibited
a significantly higher OR and a marginal reduction in diagnostic
sensitivity, as well as a higher specificity, when compared with
the combination of RNF180, DAPK1 and SFRP2 methylation. Therefore,
the combination of RNF180 and SFRP2 methylation may be optimal for
GC diagnosis.
In conclusion, using an MSP approach, RNF180 was
identified as a novel plasma hypermethylated gene in GC. Although
the functional effect of RNF180 methylation in GC was not
identified, its high potential as a biomarker in plasma-based DNA
testing was demonstrated. Furthermore, this study suggests that
combining RNF180 and SFRP2 methylation may be particularly
promising. The full potential of this marker or its combination
with SFRP2 requires validation in a larger, well-controlled cohort
study to verify its performance in detecting GC, and to investigate
the potential clinical application for monitoring GC treatment and
predicting responses to chemotherapy and radiotherapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of Ningbo (grant no. 2012A610212) and the
Scientific Innovation Team Project of Ningbo (grant no.
2013B82010).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.
|
|
2
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.
|
|
3
|
Shi Y and Zhou Y: The role of surgery in
the treatment of gastric cancer. J Surg Oncol. 101:687–692.
2010.
|
|
4
|
Leon SA, Shapiro B, Sklaroff DM and Yaros
MJ: Free DNA in the serum of cancer patients and the effect of
therapy. Cancer Res. 37:646–650. 1977.
|
|
5
|
Hanash SM, Baik CS and Kallioniemi O:
Emerging molecular biomarkers - blood-based strategies to detect
and monitor cancer. Nat Rev Clin Oncol. 8:142–150. 2011.
|
|
6
|
Kohler C, Barekati Z, Radpour R and Zhong
XY: Cell-free DNA in the circulation as a potential cancer
biomarker. Anticancer Res. 31:2623–2628. 2011.
|
|
7
|
Sharma VK, Vouros P and Glick J: Mass
spectrometric based analysis, characterization and applications of
circulating cell free DNA isolated from human body fluids. Int J
Mass Spectrom. 304:172–183. 2011.
|
|
8
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011.
|
|
9
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007.
|
|
10
|
Cheung KF, Lam CN, Wu K, et al:
Characterization of the gene structure, functional significance,
and clinical application of RNF180, a novel gene in gastric cancer.
Cancer. 118:947–959. 2012.
|
|
11
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013.
|
|
12
|
Lee TL, Leung WK, Chan MW, et al:
Detection of gene promoter hypermethylation in the tumor and serum
of patients with gastric carcinoma. Clin Cancer Res. 8:1761–1766.
2002.
|
|
13
|
Cheng YY, Yu J, Wong YP, et al: Frequent
epigenetic inactivation of secreted frizzled-related protein 2
(SFRP2) by promoter methylation in human gastric cancer. Br J
Cancer. 97:895–901. 2007.
|
|
14
|
Martinez-Glez V, Franco-Hernandez C,
Gonzalez-Gomez P, et al: DAPK1 promoter hypermethylation in brain
metastases and peripheral blood. Neoplasma. 54:123–126. 2007.
|
|
15
|
Ben Ayed-Guerfali D, Benhaj K, Khabir A,
et al: Hypermethylation of tumor-related genes in Tunisian patients
with gastric carcinoma: clinical and biological significance. J
Surg Oncol. 103:687–694. 2011.
|
|
16
|
Washington K: 7th edition of the AJCC
cancer staging manual: stomach. Ann Surg Oncol. 17:3077–3079.
2010.
|
|
17
|
Xiong HL, Liu XQ, Sun AH, He Y, Li J and
Xia Y: Aberrant DNA methylation of P16, MGMT, hMLH1 and hMSH2 genes
in combination with the MTHFR C677T genetic polymorphism in gastric
cancer. Asian Pac J Cancer Prev. 14:3139–3142. 2013.
|
|
18
|
Wani M, Afroze D, Makhdoomi M, et al:
Promoter methylation status of DNA repair gene (hMLH1) in gastric
carcinoma patients of the Kashmir valley. Asian Pac J Cancer Prev.
13:4177–4181. 2012.
|
|
19
|
Mir MR, Shabir N, Wani KA, et al:
Association between p16, hMLH1 and E-cadherin promoter
hypermethylation and intake of local hot salted tea and sun-dried
foods in Kashmiris with gastric tumors. Asian Pac J Cancer Prev.
13:181–186. 2012.
|
|
20
|
Abbaszadegan MR, Moaven O, Sima HR, et al:
p16 promoter hypermethylation: a useful serum marker for early
detection of gastric cancer. World J Gastroenterol. 14:2055–2060.
2008.
|
|
21
|
Lu XX, Yu JL, Ying LS, et al: Stepwise
cumulation of RUNX3 methylation mediated by Helicobacter pylori
infection contributes to gastric carcinoma progression. Cancer.
118:5507–5017. 2012.
|
|
22
|
Kang GH, Lee S, Cho NY, et al: DNA
methylation profiles of gastric carcinoma characterized by
quantitative DNA methylation analysis. Lab Invest. 88:161–170.
2008.
|
|
23
|
Li WQ, Pan KF, Zhang Y, et al: RUNX3
methylation and expression associated with advanced precancerous
gastric lesions in a Chinese population. Carcinogenesis.
32:406–410. 2011.
|
|
24
|
Sugita H, Iida S, Inokuchi M, et al:
Methylation of BNIP3 and DAPK indicates lower response to
chemotherapy and poor prognosis in gastric cancer. Oncol Rep.
25:513–518. 2011.
|
|
25
|
Yao D, Shi J, Shi B, et al: Quantitative
assessment of gene methylation and their impact on clinical outcome
in gastric cancer. Clin Chim Acta. 413:787–794. 2012.
|
|
26
|
Ksiaa F, Ziadi S, Amara K, Korbi S and
Trimeche M: Biological significance of promoter hypermethylation of
tumor-related genes in patients with gastric carcinoma. Clin Chim
Acta. 404:128–133. 2009.
|
|
27
|
Hiraki M, Kitajima Y, Koga Y, et al:
Aberrant gene methylation is a biomarker for the detection of
cancer cells in peritoneal wash samples from advanced gastric
cancer patients. Ann Surg Oncol. 18:3013–3019. 2011.
|
|
28
|
Banzai C, Nishino K, Quan J, Yoshihara K,
Sekine M, Yahata T and Tanaka K; Gynecological Cancer Registry of
Niigata. Promoter methylation of DAPK1, FHIT, MGMT, and CDKN2A
genes in cervical carcinoma. Int J Clin Oncol. 19:127–132.
2014.
|
|
29
|
Kilinc D, Ozdemir O, Ozdemir S, Korgali E,
Koksal B, Uslu A and Gultekin YE: Alterations in promoter
methylation status of tumor suppressor HIC1, SFRP2, and DAPK1 genes
in prostate carcinomas. DNA Cell Biol. 31:826–832. 2012.
|
|
30
|
Ogawa M, Mizugishi K, Ishiguro A, et al:
Rines/RNF180, a novel RING finger gene-encoded product, is a
membrane-bound ubiquitin ligase. Genes Cells. 13:397–409. 2008.
|
|
31
|
Tamura G: Alterations of tumor suppressor
and tumor-related genes in the development and progression of
gastric cancer. World J Gastroenterol. 12:192–198. 2006.
|
|
32
|
Kinoshita T, Nomoto S, Kodera Y, Koike M,
Fujiwara M and Nakao A: Decreased expression and aberrant
hypermethylation of the SFRP genes in human gastric cancer.
Hepatogastroenterology. 58:1051–1056. 2011.
|
|
33
|
Nojima M, Suzuki H, Toyota M, et al:
Frequent epigenetic inactivation of SFRP genes and constitutive
activation of Wnt signaling in gastric cancer. Oncogene.
26:4699–4713. 2007.
|