Introduction
Cervical cancer is a leading cause of morbidity and
mortality in females worldwide (1,2), and
remains the most common type of gynecological malignant tumor in
China (3). Infection by an
oncogenic human papillomavirus (HPV), in particular high-risk HPV
(hrHPV) types 16 and 18, is a risk factor for developing cervical
cancer (4). HPV is a
double-stranded DNA virus, which infects cutaneous and mucosal
epithelial cells of the human body. HPV genomes consist of early,
late, upstream regulatory and non-coding regions. These viruses
usually clear in 70–90% of HPV infected individuals. Although HPV
is the major causative agent of cervical cancer, the viral
infection alone is not sufficient for cancer progression (5). Investigating the underlying cellular
and molecular mechanisms in cervical carcinogenesis is a key aim of
studies worldwide in the vaccine era.
Autophagy is a self-degradation mechanism, which is
associated with tumor progression, including cervical cancer.
Autophagy is a highly conserved intercellular process in all
eukaryotes, from yeast to humans (6). Autophagy occurs as a result of certain
stresses or inductions, including nutrient starvation, radiation or
cytotoxic compounds. Subsequently, cytoplasm components or
organelles are delivered to a double-membrane vesicle
(autophagosome), which are then fused with lysosomes for protein
degradation (7). To date, autophagy
has been found to be involved in a variety of physiological and
pathophysiological processes, including cell survival, cell death,
tumor suppression and metastasis, antigen presentation, pathogen
clearance, anti-aging and neurodegeneration (8–11).
Beclin-1 and microtubule-associated protein 1A/1B
light chain 3 (LC3) genes exhibit a pivotal role in mammalian
autophagy (12). Beclin-1, a
mammalian orthologue of yeast Atg6, is involved in the signaling
pathway which activates autophagy and in the initial step of
autophagosome formation (13). LC3
comprises a soluble form, LC3A, and a lipidated form, termed LC3B.
LC3B has been found to correlate with autophagy, and is recruited
into autophagosomes. In addition, LC3B protein is widely used as a
marker of autophagy in diverse cell types (14,15).
Hence, in this study, Beclin-1 and LC3B expression in cervical
cancer and precancerous lesions was evaluated. Targeted
manipulation of complex autophagic signaling may present an
innovative strategy for the identification of clinically relevant
biomarkers in cervical cancer in the future.
We hypothesized that autophagy reduction may promote
the development of cervical squamous cell carcinoma (SCC),
particularly following hrHPV infection. Using the established
quantum dot (QD)-based immunofluorescence histochemistry (IHC)
technique, the expression of Beclin-1 and LC3B in cervical cancer
and the correlation with clinicopathological parameters of cervical
SCC was investigated. hrHPV infection was also detected using
QD-based fluorescence in situ hybridization (FISH) to
investigate the association between autophagy and hrHPV
infection.
Materials and methods
Patients and tissue samples
A total of 124 formalin-fixed, paraffin-embedded
(FFPE) cervical lesion samples, including 80 cases of cervical SCC
and 24 cases of cervical adenocarcinoma, obtained from patients who
underwent total hysterectomy due to complaints other than uterine
cervical lesions, including uterine fibroids and endometriosis, and
20 cases of normal cervical tissues were used in this study. All
study samples were obtained from patients between 2007 and 2010 who
were treated at Zhongnan Hospital of Wuhan University (Wuhan,
China). Two pathologists (Yang GF and Chen HL) reconfirmed the
histopathological features of these samples. The histological
diagnosis and grading of cervical cancers was established according
to the International Federation of Gynecology and Obstetrics (FIGO)
guidelines (Table I) (16). Approval for this study was obtained
from the Ethics committee of Wuhan University. Written informed
consent was obtained from the patient.
 | Table ICorrelation between Beclin-1 and LC3B
protein expression and clinicopathological parameters of cervical
squamous cell carcinomas. |
Table I
Correlation between Beclin-1 and LC3B
protein expression and clinicopathological parameters of cervical
squamous cell carcinomas.
| | Beclin-1, n (%) | | LC3B, n (%) | |
|---|
| |
| |
| |
|---|
| Group | Cases, n | − | + | P-value | − | + | P-value |
|---|
| Age | | | | 0.463 | | | 0.715 |
| ≥45 | 38 | 23 (61) | 15 (39) | | 22 (58) | 16 (42) | |
| <45 | 42 | 22 (52) | 20 (48) | | 26 (62) | 16 (38) | |
| Grade | | | | 0.723 | | | 0.180 |
| I + II | 28 | 15 (54) | 13 (46) | | 14 (50) | 14 (50) | |
| III | 52 | 30 (58) | 22 (42) | | 34 (65) | 18 (35) | |
| Tumor stage | | | | 0.159 | | | 0.079 |
| T1 | 65 | 39 (60) | 26 (40) | | 42 (65) | 23 (35) | |
| T2 + T3 | 15 | 6 (40) | 9 (60) | | 6 (40) | 9 (60) | |
| TNM stage | | | | 0.219 | | | 0.232 |
| I + II | 56 | 29 (52) | 27 (48) | | 36 (64) | 20 (36) | |
| III + IV | 24 | 16 (67) | 8 (33) | | 12 (50) | 12 (50) | |
| Lymph node
status | | | | 0.127 | | | 0.364 |
| N0 | 57 | 29 (51) | 28 (49) | | 36 (63) | 21 (37) | |
| N1–3 | 23 | 16 (70) | 7 (30) | | 12 (52) | 11(48) | |
| hrHPV infection | | | | 0.008 | | | 0.019 |
| Negative | 13 | 3 (23) | 10 (77) | r = −0.295 | 4 (30) | 9 (70) | r = −0.263 |
| Positive | 67 | 42 (63) | 25 (37) | | 44 (66) | 23 (34) | |
| Beclin-1 | | | | - | | | 0.005 |
| Negative | 45 | - | - | | 33 (73) | 12 (27) | r = 0.309 |
| Positive | 35 | - | - | | 15 (43) | 20 (57) | |
Tissue microarray (TMA) construction
Hematoxylin and eosin-stained sections of all 124
cases cervical lesion specimens were reviewed and the most
representative areas were selected for TMA construction. The TMAs
were constructed using a tissue-arraying instrument (Beecher
Instruments, Silver Spring, MD, USA), as described in our previous
study (17). Briefly, two cores
(diameter, 1.5 mm) were removed from the selected area of each
donor FFPE specimen and precisely arrayed in a recipient paraffin
block. Next, 4-μm thick sections were consecutively incised from
the recipient block and transferred to poly-L-lysine-coated glass
slides. Hematoxylin and eosin staining was performed on TMA for the
confirmation of tumor samples.
QD IHC
The expression of Beclin-1 and LC3B protein was
detected by QD-IHC, using primary rabbit anti-human Beclin-1
polyclonal antibody (1:50; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and murine anti-human LC3B monoclonal antibody
(1:150; Cell Signaling Technology, Inc., Danvers, MA, USA). QD-IHC
was performed according to the manufacturer’s instructions (Wuhan
Jiayuan Quantum Dot Co., Ltd., Wuhan, China), as described in our
previous study (18). The TMAs were
observed using an Olympus BX53 fluorescence microscope (Olympus
Corporation, Tokyo, Japan) equipped with an Olympus CCD DP73. A
positive signal in the cytoplasm or cell membrane is bright red,
target-specific and photostable, and the background
autofluorescence is green. Negative controls of Beclin-1 and LC3B
were carried out by replacing the primary antibodies with
Tris-buffered saline.
All immunostained sections were evaluated by two
pathologists (Yang GF and Chen HL) with no prior knowledge of the
patients’ clinical statuses. Expression was semiquantitatively
scored by assessing the intensity of the staining and the
percentage of stained cells. The sections were scanned using a
high-power field, for calculating the percentage of Beclin-1- and
LC3B-positive areas (PAs). PAs were graded as follows: 0, PA ≤20%;
1, PA 20–40%; 2, PA 40–60%; and 3, PA >60%. Subsequently, the
intensity of staining (IS) was evaluated in high-power hot-spots
and scored as: 0, negative; 1, weak; and 2, strong. Beclin-1 and
LC3-B intensity distribution (ID) scores for each case were
calculated by the following equation: ID = PA × IS. If the ID was
>2, the samples were considered positive; while if the ID was
<2, the samples were considered negative, based on the findings
of previous reports (19).
QD FISH
Breifly, 4-μm thick sections of cervical TMAs were
deparaffinized, and hydrated sections were hybridized using
biotin-labeled HPV 16/18 DNA probes (PanPath B.V., Budel, the
Netherlands) and a QD-FISH detection kit (Wuhan Jiayuan Quantum Dot
Co., Ltd.). Detection and staining was performed according to the
manufacturer’s instructions, and other reagents and steps were the
same as those in our previous study involving QD-FISH imaging of
Epstein-Barr virus in gastric cancer (11). Red punctate or diffuse nuclear
staining was regarded as being positive for HPV16/18 and the
background autofluorescence was green. Assessment of HPV16/18
QD-FISH signals was carried out using the Olympus BX53 microscope
(CCD DP73; Olympus Corporation). A tissue block from a confirmed
HPV-positive cervical carcinoma was used as positive control.
Statistical analysis
All data were analyzed using SPSS, version 17.0
(SPSS, Inc., Chicago, IL, USA). χ2 and Fisher’s exact
tests were used to compare different rates of expression.
Correlations were calculated using the Spearman’s rank correlation
test. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of Beclin-1 and LC3B in normal
and cervical cancer tissues
Fluorescent semiconductor nanocrystal QDs are an
important class of fluorescent labels used for biological and
biomedical imaging (20). In our
previous studies, the QD-IHC technique achieved levels of
sensitivity and specificity that were sufficient for detecting
known expression signals in biopsy and FFPE specimens, enabling
high-throughput application (18,19).
In the present study, the subcellular localization
and expression of Beclin-1 and LC3B proteins in normal cervical
squamous epithelial and cancer tissues was detected via QD-IHC.
Specific Beclin-1 staining was predominantly observed at the
cytoplasm membrane in normal cervical epithelial and cancer tissues
(Fig. 1A–C). In normal cervical
squamous epithelial tissues, the positive rate of Beclin-1 of the
20 samples was 90%. By contrast, Beclin-1 was detected in 44%
(35/80) of SCCs and 42% (10/24) of adenocarcinomas. The expression
of Beclin-1 was significantly decreased in cancer tissues when
compared with that in normal cervical tissues (Table II). LC3B was located at the cell
membrane and cytoplasm in normal cervical squamous epithelial and
cancer tissues (Fig. 1D–F), and no
stone-like expression pattern was identified as previously
described in breast carcinoma and lung cancer (20,21).
In normal cervical tissues, the positive rate of LC3B in the 20
samples was 80%. However, LC3B was detected in 32 (40%) of the 80
SCCs, and 46% (11/24) of adenocarcinomas. A significant decrease in
LC3B expression was identified in cancer tissues when compared with
that in normal cervical tissues (Table
II).
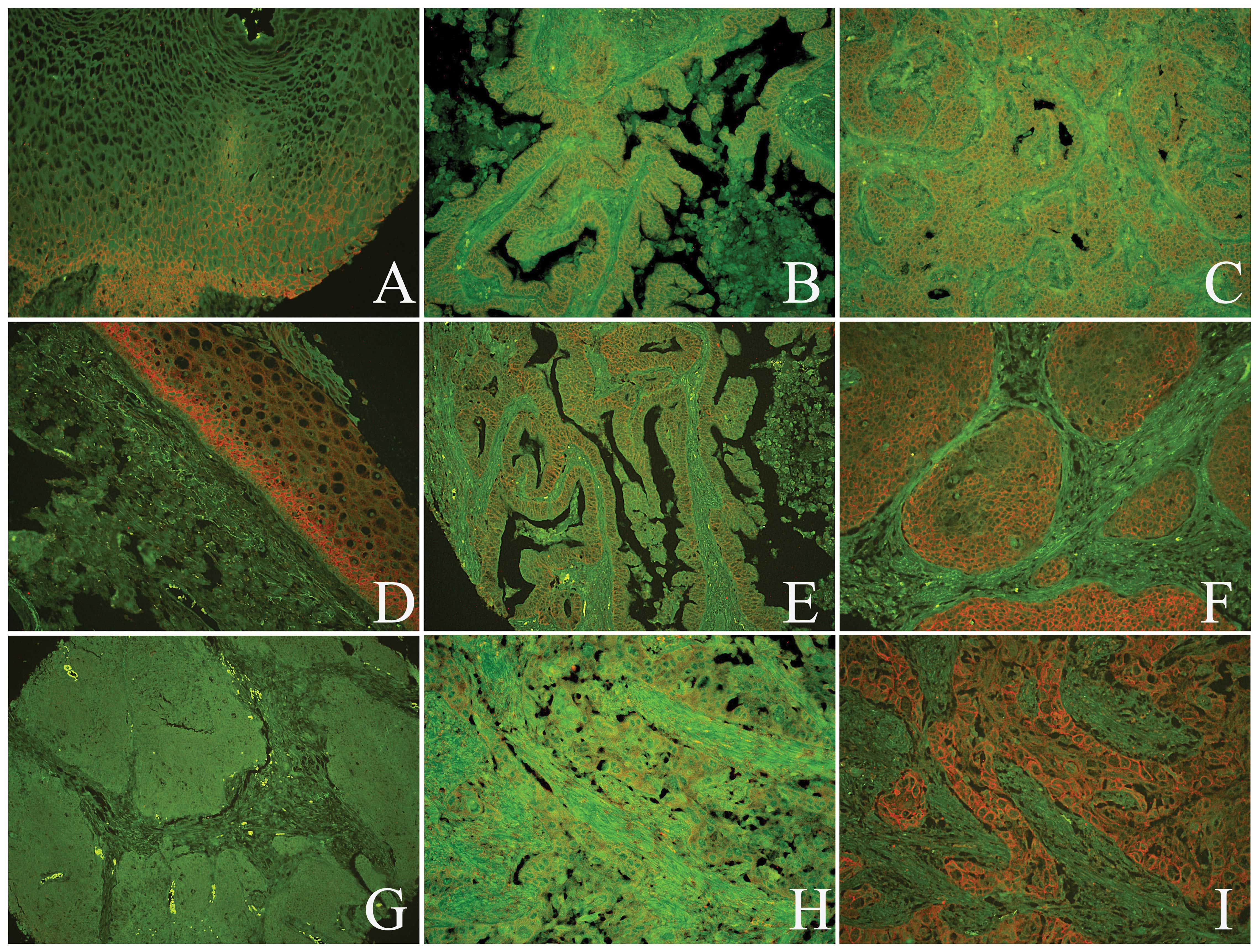 | Figure 1Expression of Beclin-1 and LC3B
proteins in the normal cervical and cancerous tissues was analyzed
by quantum dot immunofluorescence histochemistry. A positive signal
for Beclin-1 was located (A) at the cytoplasm membrane of normal
cervical squamous epithelial cells, (B) in the cervical
adenocarcinoma cells and (C) in the cervical squamous cell cancer
cells. A positive signal for LC3B was observed in the (D) normal
cervical squamous epithelial cells, (E) cervical adenocarcinoma
cells and (F) cervical squamous cell cancer cells; however, a
negative signal for LC3B was detected in the stroma. (G) Beclin-1
and LC3B protein expression was negative in the same cervical SCC
case. The expression of (H) Beclin-1 and (I) LC3B proteins were
positive at the same cervical SCC case (A–F, H and I,
magnification, ×200; G, magnification, ×100). LC3B,
microtubule-associated proteins 1A/1B light chain 3B; SCC, squamous
cell carcinoma. |
 | Table IIExpression of Beclin-1 and LC3B
proteins, and hrHPV infection in the different cervical lesion
tissues. |
Table II
Expression of Beclin-1 and LC3B
proteins, and hrHPV infection in the different cervical lesion
tissues.
| | Beclin-1, n(%) | | LC3B, n(%) | | hrHPV, n(%) | |
|---|
| |
| |
| |
| |
|---|
| Different
groups | Cases, n | − | + | P-value | − | + | P-value | − | + | P-value |
|---|
| Normal cervical
epithilium | 20 | 2 (10) | 18 (90) | 0.000a | 4 (20) | 16 (80) | 0.001a | 18 (90) | 2 (10) | 0.001a |
| SCC | 80 | 45 (56) | 35 (44) | 0.001b | 48 (60) | 32 (40) | 0.020b | 13 (16) | 67 (84) | 0.001b |
| Adenocarcinoma | 24 | 14 (58) | 10 (42) | 0.857c | 13 (54) | 11 (46) | 0.611c | 9 (37) | 15 (63) | 0.025c |
Clinicopathological significance of
Beclin-1 and LC3B in cervical SCC
To investigate the effect of Beclin-1 and LC3B
protein expression on malignant progression, the correlation
between Beclin-1 and LC3B expression and the clinicopathological
features, including age and differentiation grade, were
respectively examined. However, as shown in Table I, no significant association was
identified between Beclin-1, LC3B and the various
clinicopathological features.
Correlation between Beclin-1 and LC3B in
cervical SCCs
The association between Beclin-1 and LC3B (Table I) was further investigated. In 45
cases of Beclin-1-negative expression, 33 (73.3%) cases exhibited
negative expression of LC3B (Fig.
1G), and in 35 cases of Beclin-1-positive expression, 20
(57.1%) cases were LC3B-positive (Fig.
1H and I), whereby a positive correlation was identified
(r=0.309; P=0.005).
Clinicopathological significance of
negative Beclin-1 and LC3B expression in the cervical SCCs with
hrHPV infection
The hrHPV infection signal was detected using
QD-FISH with biotin-labeled DNA probes. In cervical cancer, the
positive hrHPV signal was located in the nuclei of tumor cells
(Fig. 2); however, the majority of
normal cervical tissues exhibited a negative signal. Positive rates
of hrHPV were 83.75% (67/80) for cervical SCC and 62.5% (15/24) for
cervical adenocarcinoma, and a significant difference was
identified when compared with normal cervical tissues (Table II). In addition, the result of
hrHPV infection was evaluated, and Beclin-1 and LC3B were found to
negatively correlate with hrHPV infection (Fig. 2E and F; Table II). Simultaneously, the
clinicopathological significance of negative Beclin-1 and LC3B
expression in cervical SCC with hrHPV infection was investigated.
As shown in Table III, the
negative expression of Beclin-1 and LC3B in cervical SCC with hrHPV
infection was found to promote a higher clinical tumor node
metastasis (TNM) stage and lymph node metastasis.
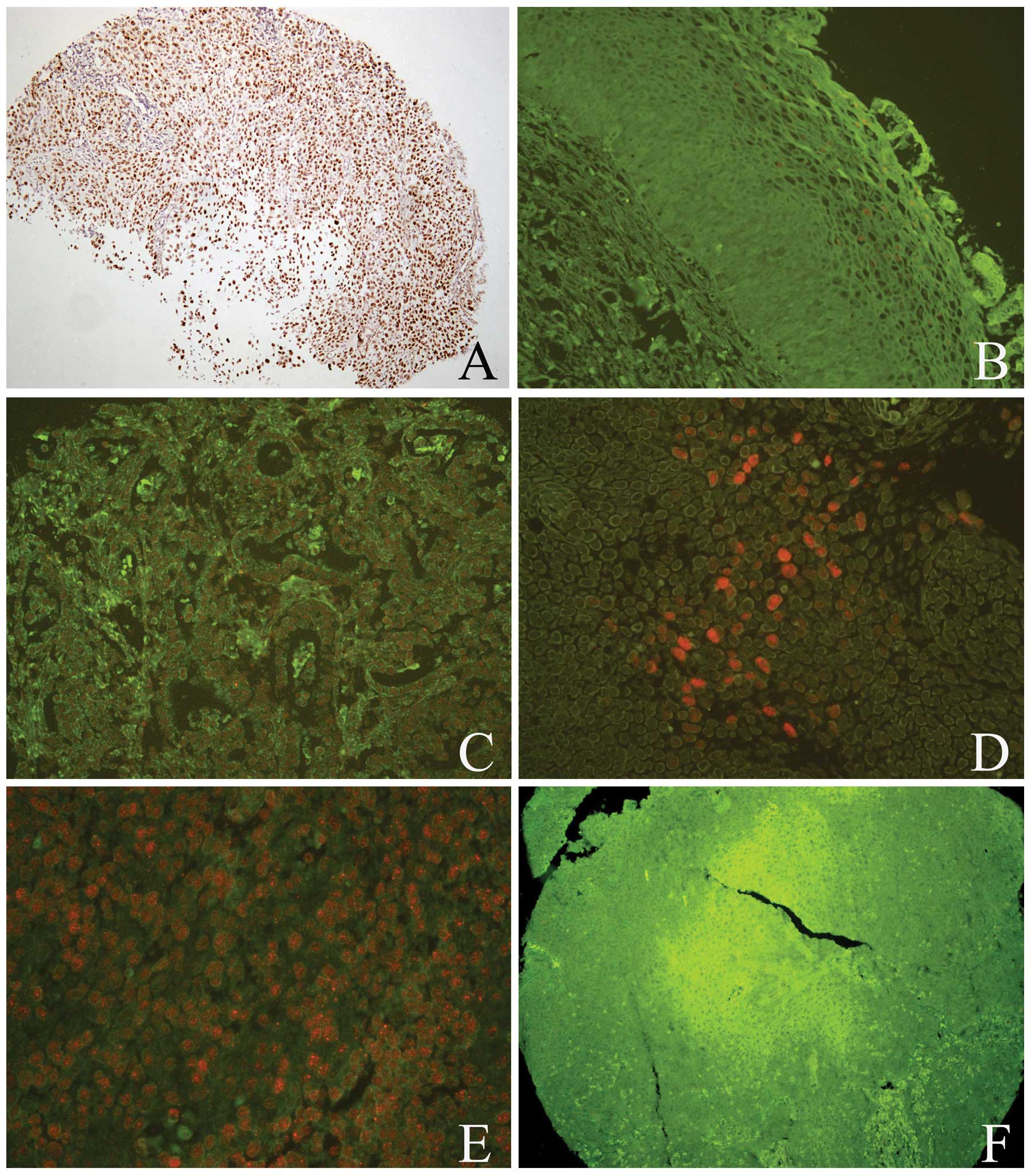 | Figure 2hrHPV infection was detected by
quantum dot fluorescence in situ hybridization in the normal
cervical and cancerous tissues. (A) Positive controls revealed
hrHPV located in the nuclei, which was detected by chromogenic in
situ hybridization. hrHPV exhibited a positive signal in the (B)
normal cervical tissues, (C) adenocarcinoma and (D) SCC. (E and F)
In the same SCC case, hrHPV infection exhibited a positive signal
(E); however, Beclin-1 exhibited a negative signal (F). (A, B and
F, magnification, ×100; C–E, magnification, ×200). hrHPV, high risk
human papillomavirus; SCC, squamous cell carcinoma. |
 | Table IIIClinicopathological significance of
negative Beclin-1 and LC3B expression in cervical squamous cell
carcinomas with hrHPV infection. |
Table III
Clinicopathological significance of
negative Beclin-1 and LC3B expression in cervical squamous cell
carcinomas with hrHPV infection.
| | Beclin-1, n(%) | | LC3B, n(%) | |
|---|
| |
| |
| |
|---|
| Group | Cases, n | − | + | P-value | − | + | P-value |
|---|
| Grade | | | | 0.451 | | | 0.254 |
| I + II | 23 | 13 (57) | 10 (43) | | 13 (57) | 10 (43) | |
| III | 44 | 29 (66) | 15 (34) | | 31 (70) | 13 (30) | |
| Tumor stage | | | | 0.482 | | | 0.293 |
| T1 | 57 | 37 (65) | 20 (35) | | 39 (68) | 18 (32) | |
| T2 + T3 | 10 | 5 (50) | 5 (50) | | 5 (50) | 5 (50) | |
| TNM stage | | | | 0.083 | | | 0.044 |
| I + II | 48 | 27 (56) | 21 (44) | | 28 (58) | 20 (42) | |
| III + IV | 19 | 15 (79) | 4 (21) | | 16 (84) | 3 (16) | |
| LN status | | | | 0.034 | | | 0.065 |
| N0 | 49 | 27 (55) | 22 (45) | | 29 (59) | 20 (41) | |
| N1–3 | 18 | 15 (83) | 3 (17) | | 15 (83) | 3 (17) | |
Discussion
The current study revealed that the expression
levels of Beclin-1 and LC3B are significantly lower in cervical
cancer tissues than in normal cervical squamous epithelial tissues,
and were found to negatively correlate with hrHPV infection. In
addition, a positive correlation was identified between Beclin-1
and LC3B protein expression. The results also revealed that the
absence of autophagy in combination with hrHPV infection may
accelerate cervical SCC progression. Therefore, reduced expression
of Beclin-1 and LC3B may be associated with cervical
carcinogenesis. Furthermore, the hrHPV-host cell interaction may
inhibit autophagy, which may aid virus duplication and infection,
and cervical cancer development.
Autophagy is a self-degradation mechanism, which is
associated with tumor progression. Recently, the role of autophagy
in cancer development and treatment has been investigated in
vitro and in vivo (23).
Previous studies have indicated that autophagy is involved in tumor
suppressor pathways. Inactivation of autophagy-specific genes,
including Beclin-1, results in increased tumorigenesis in mice, and
enforcement of the expression of such genes inhibits the formation
of human breast tumors in mouse models (24). In the present study, the expression
of Beclin-1 and LC3B proteins was significantly decreased in cancer
tissues when compared with that of normal cervical tissues, and a
positive association was identified between Beclin-1 and LC3B
protein expression in cervical SCC, which demonstrated that
reduction of autophagy aids cervical carcinogenesis. mRNA and
protein levels of Beclin-1 and LC3B are also significantly
decreased in lung cancer tissues (25). The positive expression of Beclin-1
in non-Hodgkin lymphomas correlates with the presence of
LC3-positive autophagic vacuoles (26). In melanocytic neoplasms, similar
results have also been observed, whereby Beclin-1 cytoplasmic
protein and mRNA, as well as LC3 mRNA and LC3B protein,
significantly decreased with tumor progression (13). However, these results differ from
those reported in gastric cancer cells, which revealed that
Beclin-1 protein and mRNA expression was significantly higher than
that of the corresponding normal tissues (27). In various gastrointestinal cancers,
LC3B expression has been found to positively correlate with Ki-67
index in early cancers, indicating that LC3B expression is
advantageous for cancer development in the early phases of
carcinogenesis (23); however, the
mechanism remains unclear.
The association between Beclin-1 and LC3B expression
and the clinical characteristics of cervical SCC patients was also
analyzed. No significant correlation was identified between the
expression of Beclin-1 and LC3B and age, pathological grade, tumor
depth, lymph node metastasis, or TNM stage of patients with
cervical SCC. Although Beclin-1 and LC3 were not found to be
associated with age, FIGO stage, pathological differentiation or
lymph node metastasis, they may exhibit prognostic significance in
early stage cervical SCC (28).
Similar results from previous studies have revealed that the
expression of Beclin-1 and LC3B in lung cancer tissues was not
affected by patient age, gender, smoking, histological type, lymph
node metastasis or TNM stage (25).
Furthermore, in esophageal carcinomas, LC3 expression was not found
to correlate with various clinicopathological factors, including
survival (23). By contrast,
significant correlations have been identified between the
peripheral intensity level of LC3 expression and tumor size and
tumor necrosis of pancreatic cancer (29). These observations highlight the
different roles of Beclin-1 and LC3B in the progression of various
cancers. In order to utilize the modulation of autophagy for cancer
therapy, further investigation with regards to the role of
autophagy in human cancers is required.
Cell autophagic machinery is known to capture and
degrade intracellular various pathogens, which is an important
component of the host response against viral infections (30). Therefore, numerous viruses have
developed methods to block autophagy or subvert this mechanism
(31). HPV infection induces an
autophagic response, including upregulation of marker proteins for
autophagy, in host keratinocytes (32). In HeLa cells infected by HPV16
pseudovirions in vitro, autophagy was observed to be induced
during the HPV16 entry process, which implies that autophagosomes
are generated from the plasma membrane as a result of HPV infection
(33). The specificity of genetic
knockdowns of mTOR, Beclin-1 and Atg7 have confirmed that
HPV-induced restraint of autophagy is important for early infection
events (31,34).
In the present study, the hrHPV infection signal was
detected by QD-FISH using biotin-labeled DNA probes. The positive
signal for hrHPV was 83.75% (67/80) in cervical SCC tissues and
62.5% (15/24) in cervical adenocarcinoma, which is significantly
higher than that of normal cervical squamous epithelial cells,
indicating that hrHPV infection is involved in the development of
cervical cancer (35). In addition,
the result of hrHPV infection was investigated, and significant
negative correlations were detected between Beclin-1, LC3B and
hrHPV infection, indicating that hrHPV infection may inhibit
autophagy. It is hypothesized that the HPV-host cell interaction
stimulates the PI3K/Akt/mTOR pathway and inhibits autophagy, and in
combination these events aid virus infection (36). In addition, persistent HPV infection
may stabilize the ATPase family AAA domain containing 3A (an
anti-autophagy factor) expression to inhibit cell autophagy and
apoptosis, as well as increasing drug resistance in uterine
cervical cancer (37).
Simultaneously, the clinicopathological significance of negative
Beclin-1 and LC3B expression in cervical SCC with hrHPV infection
was investigated, and the results revealed that negative expression
of Beclin-1 and LC3B in cervical SCC with hrHPV infection promoted
a higher clinical TNM stage and lymph node metastasis, indicating
that the absence of autophagy combined with hrHPV infection may
accelerate cervical SCC progression. In addition, the mTOR pathway
is important in cervical carcinogenesis and thus, targeted
therapies may be developed for SCC as well as its precursor lesion,
high-grade squamous intraepithelial lesion (38).
In conclusion, this study revealed that the
decreased expression of Beclin-1 and LC3B may be involved in
cervical carcinogenesis. The absence of autophagy combined with
hrHPV infection may accelerate cervical SCC progression. The
results highlight the clinical importance of the hrHPV-host cell
interaction, which may inhibit autophagy, aiding virus duplication
and infection, as well as cervical cancer development. However,
further studies are required to clarify the mechanism with regards
to hrHPV regulation of the autophagy signaling pathway and its
involvement in the development and progression of cervical
cancer.
Acknowledgements
The authors would like to thank Wuhan Jiayuan
Quantum Dot Co., Ltd (Wuhan, China) for providing QD IHC technical
support.
References
|
1
|
Pandey S, Mishra M and Chandrawati: Human
papillomavirus screening in north Indian women. Asian Pac J Cancer
Prev. 13:2643–2646. 2012.
|
|
2
|
Walboomers JM, Jacobs MV, Manos MM, et al:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999.
|
|
3
|
Li J, Kang LN and Qiao YL: Review of the
cervical cancer disease burden in mainland China. Asian Pac J
Cancer Prev. 12:1149–1153. 2011.
|
|
4
|
Muñoz N: Human papillomavirus and cancer:
the epidemiological evidence. J Clin Virol. 19:1–5. 2000.
|
|
5
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.
|
|
6
|
Nakatogawa H, Suzuki K, Kamada Y and
Ohsumi Y: Dynamics and diversity in autophagy mechanisms: lessons
from yeast. Nat Rev Mol Cell Biol. 10:458–467. 2009.
|
|
7
|
Liu JJ, Lin M, Yu JY, Liu B and Bao JK:
Targeting apoptotic and autophagic pathways for cancer
therapeutics. Cancer Lett. 300:105–114. 2011.
|
|
8
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011.
|
|
9
|
Mathew R and White E: Autophagy in
tumorigenesis and energy metabolism: friend by day, foe by night.
Curr Opin Genet Dev. 21:113–119. 2011.
|
|
10
|
Mizushima N: The pleiotropic role of
autophagy: from protein metabolism to bactericide. Cell Death
Differ. 12(Suppl 2): S1535–S1541. 2005.
|
|
11
|
Chen HL, Peng J, Zhu XB, et al: Detection
of EBV in nasopharyngeal carcinoma by quantum dot fluorescent in
situ hybridization. Exp Mol Pathol. 89:367–371. 2010.
|
|
12
|
Eskelinen EL and Saftig P: Autophagy: a
lysosomal degradation pathway with a central role in health and
disease. Biochim Biophys Acta. 1793:664–673. 2009.
|
|
13
|
Miracco C, Cevenini G, Franchi A, et al:
Beclin 1 and LC3 autophagic gene expression in cutaneous
melanocytic lesions. Hum Pathol. 41:503–512. 2010.
|
|
14
|
Zhang YH, Wu YL, Tashiro S, Onodera S and
Ikejima T: Reactive oxygen species contribute to oridonin-induced
apoptosis and autophagy in human cervical carcinoma HeLa cells.
Acta Pharmacol Sin. 32:1266–1275. 2011.
|
|
15
|
Wang ZH, Xu L, Wang Y, Cao MQ, Li L and
Bai T: Clinicopathologic correlations between human papillomavirus
16 infection and Beclin 1 expression in human cervical cancer. Int
J Gynecol Pathol. 30:400–406. 2011.
|
|
16
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009.
|
|
17
|
Li M, Chen H, Diao L, Zhang Y, Xia C and
Yang F: Caveolin-1 and VEGF-C promote lymph node metastasis in the
absence of intratumoral lymphangiogenesis in non-small cell lung
cancer. Tumori. 96:734–743. 2010.
|
|
18
|
Chen H, Xue J, Zhang Y, Zhu X, Gao J and
Yu B: Comparison of quantum dots immunofluorescence histochemistry
and conventional immunohistochemistry for the detection of
caveolin-1 and PCNA in the lung cancer tissue microarray. J Mol
Histol. 40:261–268. 2009.
|
|
19
|
He Y, Zhao X, Gao J, et al: Quantum
dots-based immunofluorescent imaging of stromal fibroblasts
caveolin-1 and light chain 3B expression and identification of
their clinical significance in human gastric cancer. Int J Mol Sci.
13:13764–13780. 2012.
|
|
20
|
Li ZH, Peng J and Chen HL: Bioconjugated
quantum dots as fluorescent probes for biomedical imaging. J
Nanosci Nanotechnol. 11:7521–7536. 2011.
|
|
21
|
Karpathiou G, Sivridis E, Koukourakis MI,
et al: Light-chain 3A autophagic activity and prognostic
significance in non-small cell lung carcinomas. Chest. 140:127–134.
2011.
|
|
22
|
Sivridis E, Koukourakis MI, Zois CE, et
al: LC3A-positive light microscopy detected patterns of autophagy
and prognosis in operable breast carcinomas. Am J Pathol.
176:2477–2489. 2010.
|
|
23
|
Yoshioka A, Miyata H, Doki Y, et al: LC3,
an autophagosome marker, is highly expressed in gastrointestinal
cancers. Int J Oncol. 33:461–468. 2008.
|
|
24
|
Liang XH, Jackson S, Seaman M, et al:
Induction of autophagy and inhibition of tumorigenesis by beclin 1.
Nature. 402:672–676. 1999.
|
|
25
|
Jiang ZF, Shao LJ, Wang WM, Yan XB and Liu
RY: Decreased expression of Beclin-1 and LC3 in human lung cancer.
Mol Biol Rep. 39:259–267. 2012.
|
|
26
|
Nicotra G, Mercalli F, Peracchio C, et al:
Autophagy-active beclin-1 correlates with favourable clinical
outcome in non-Hodgkin lymphomas. Mod Pathol. 23:937–950. 2010.
|
|
27
|
Geng QR, Xu DZ, He LJ, et al: Beclin-1
expression is a significant predictor of survival in patients with
lymph node-positive gastric cancer. PLoS One. 7:e459682012.
|
|
28
|
Zhu W, Pan X, Li F, Zhang Y and Lu X:
Expression of Beclin 1 and LC3 in FIGO stage I–II cervical squamous
cell carcinoma and relationship to survival. Tumour Biol.
33:1653–1659. 2012.
|
|
29
|
Fujii S, Mitsunaga S, Yamazaki M, et al:
Autophagy is activated in pancreatic cancer cells and correlates
with poor patient outcome. Cancer Sci. 99:1813–1819. 2008.
|
|
30
|
Deretic V and Levine B: Autophagy,
immunity, and microbial adaptations. Cell Host Microbe. 5:527–549.
2009.
|
|
31
|
Jordan TX and Randall G: Manipulation or
capitulation: virus interactions with autophagy. Microbes Infect.
14:126–139. 2012.
|
|
32
|
Griffin LM, Cicchini L and Pyeon D: Human
papillomavirus infection is inhibited by host autophagy in primary
human keratinocytes. Virology. 437:12–19. 2013.
|
|
33
|
Ishii Y: Electron microscopic
visualization of autophagosomes induced by infection of human
papillomavirus pseudovirions. Biochem Biophys Res Commun.
433:385–389. 2013.
|
|
34
|
Spangle JM and Münger K: The human
papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling
and increases protein synthesis. J Virol. 84:9398–9407. 2010.
|
|
35
|
Clarke MA, Wentzensen N, Mirabello L, et
al: Human papillomavirus DNA methylation as a potential biomarker
for cervical cancer. Cancer Epidemiol Biomarkers Prev.
21:2125–2137. 2012.
|
|
36
|
Surviladze Z, Sterk RT, DeHaro SA and
Ozbun MA: Cellular entry of human papillomavirus type 16 involves
activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway
and inhibition of autophagy. J Virol. 87:2508–2517. 2013.
|
|
37
|
Chen TC, Hung YC, Lin TY, et al: Human
papillomavirus infection and expression of ATPase family AAA domain
containing 3A, a novel anti-autophagy factor, in uterine cervical
cancer. Int J Mol Med. 28:689–696. 2011.
|
|
38
|
Feng W, Duan X, Liu J, Xiao J and Brown
RE: Morphoproteomic evidence of constitutively activated and
overexpressed mTOR pathway in cervical squamous carcinoma and high
grade squamous intraepithelial lesions. Int J Clin Exp Pathol.
2:249–260. 2009.
|
















