Introduction
Tumor formation is a complex process. The initiation
and development of a tumor involves multiple signaling pathways,
including various aspects of cell proliferation, inhibition of
apoptosis, tumor stroma and formation of peritumor blood vessels,
which are all regulated by a variety of factors. Recently, the
effect of cytokines on tumors has been the focus of attention.
Transforming growth factors (TGFs), including TGF-α and -β, are
important factors that regulate cell growth and differentiation
(1).
TGF-β1 inhibits tumor cell proliferation, however,
it also promotes tumor growth and invasion by modulating the tumor
microenvironment, promoting the formation of tumor blood vessels
and matrix, and suppressing the immune response. Therefore, the
present study detected the expression of TGF-β1 in the cytoplasm
and extracellular matrix (ECM) of epithelial ovarian cancer cells,
to investigate the association between its expression, and the
invasion and metastasis of ovarian cancer.
Materials and methods
Materials
A total of 72 paraffin-embedded epithelial ovarian
cancer samples, obtained from the First Affiliated Hospital of
Yangtze University (Jingzhou, China) between March 2001 and 2006,
were included in the present study (Table I). The mean patient age was 45.3
years. The 25 normal ovarian epithelial tissues used in the study
were collected from patients with uterine disorders, who had
received a hysterectomy with concurrent removal of the ovaries, or
from a normal ovarian tissue biopsy; the mean patient age in this
group was 43.5 years. Ten random samples of benign ovarian cysts
(serous [n=6], mucinous [n=3] and endometrial [n=1]) were also
included and the mean age of the patients in this group was 41.7
years. Three lines of epithelial ovarian cancer-associated
fibroblasts (CAFs) and three cultures of normal fibroblasts (NFs;
derived from normal ovarian tissues resected due to early cervical
cancers) were provided by the Laboratory of Gynecologic Oncology of
Union Hospital (Wuhan, China). Ethical approval for the present
study was obtained from the First Affiliated Hospital of Yangtze
University Ethics committee.
 | Table IPathological grade and histological
type of epithelial ovarian cancers. |
Table I
Pathological grade and histological
type of epithelial ovarian cancers.
| Pathological
grade | Serous type | Mucinous type | Endometrioid and
clear cell types |
|---|
| G1 | 9 | 11 | 5 |
| G2 | 10 | 7 | 3 |
| G3 | 18 | 5 | 4 |
| I–II | 15 | 11 | 5 |
| III–IV | 23 | 11 | 7 |
Immunohistochemistry
Immunohistochemical staining was performed using the
Strept Avidin Biotin Peroxidase Complex kit purchased from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
The primary polyclonal rabbit antibodies (1:100) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and
staining was performed according to the manufacturer’s
instructions.
Semi-quantitative polymerase chain
reaction (PCR)
The relative expression of TGF-β1 mRNA was detected
using total RNAs extracted from the epithelial ovarian CAFs and NFs
using TRIzol reagent (ShineGene, Shanghai, China). Total RNAs were
reverse-transcribed to cDNA; 1 μl was used for TGF-β1
amplification. PCR conditions were as follows: Pre-denaturation,
94°C for 4 min; denaturation, 94°C for 45 sec; annealing, 55°C for
1 min; extension, 72°C for 1 min for 29 cycles. Final extension was
at 72°C for 5 min. The specific primers used for the TGF-β1 gene
(199 bp product) amplification were as follows: Forward,
5′-CAACAATTCCTGGCGATACA-3′ and reverse,
5′-GGTAGTGAACCCGTTGATGTCC-3′. The β-actin gene (619 bp product) was
used as an internal reference and the primers used were as follows:
Forward, 5′-AAGAGAGGCATCCTCACCCT-3′ and reverse,
5′-GGAAGGAAGGCTGGAAG-3′. Following the reaction, PCR products were
separated on 2% agarose gel by electrophoresis.
Immunohistochemistry
According to the staining results, cytoplasm or ECM
exhibiting brown granules were considered positive for TGF-β1.
Based on the pigmentation intensity; no pigmentation, light yellow,
yellow or brown, the positivity was scored as 0, 1, 2, or 3,
respectively. Five different regions of each section were selected
to calculate the average percentage of positive cells and the
corresponding scores. Sections with <5% positive cells scored 0;
5–25% positive cells scored 1; 26–50% positive cells scored 2;
51–75% positive cells scored 3; and >75% positive cells scored
4. The two scores were then multiplied to determine the positivity
of a sample, whereby 0–2 corresponds to (−), 3–4 to (+), 5–8 to
(++) and 9–12 to (+++) (2). The
results were examined by two individuals to reduce error and bias.
For semi-quantitative PCR analysis the gel electrophoresis results
were recorded using the Gel Doc XR System [Bio-Rad Laboratories
(Canada) Ltd., Mississauga, ON, Canada] and the densitometry was
analyzed using an automatic image analysis system (Gel Doc XR
System) to calculate the relative contents of TGF-β1 mRNA (β-actin
served as the internal reference). The following formula was used:
TGF-β1 mRNA relative expression level = TGF-β1 band density/β-actin
band density.
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). The
immunohistochemistry results were analyzed using a non-parametric
test and the results of semi-quantitative PCR are presented as the
mean ± standard deviation. The data were compared by t-test and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of TGF-β1 in epithelial
ovarian tumors
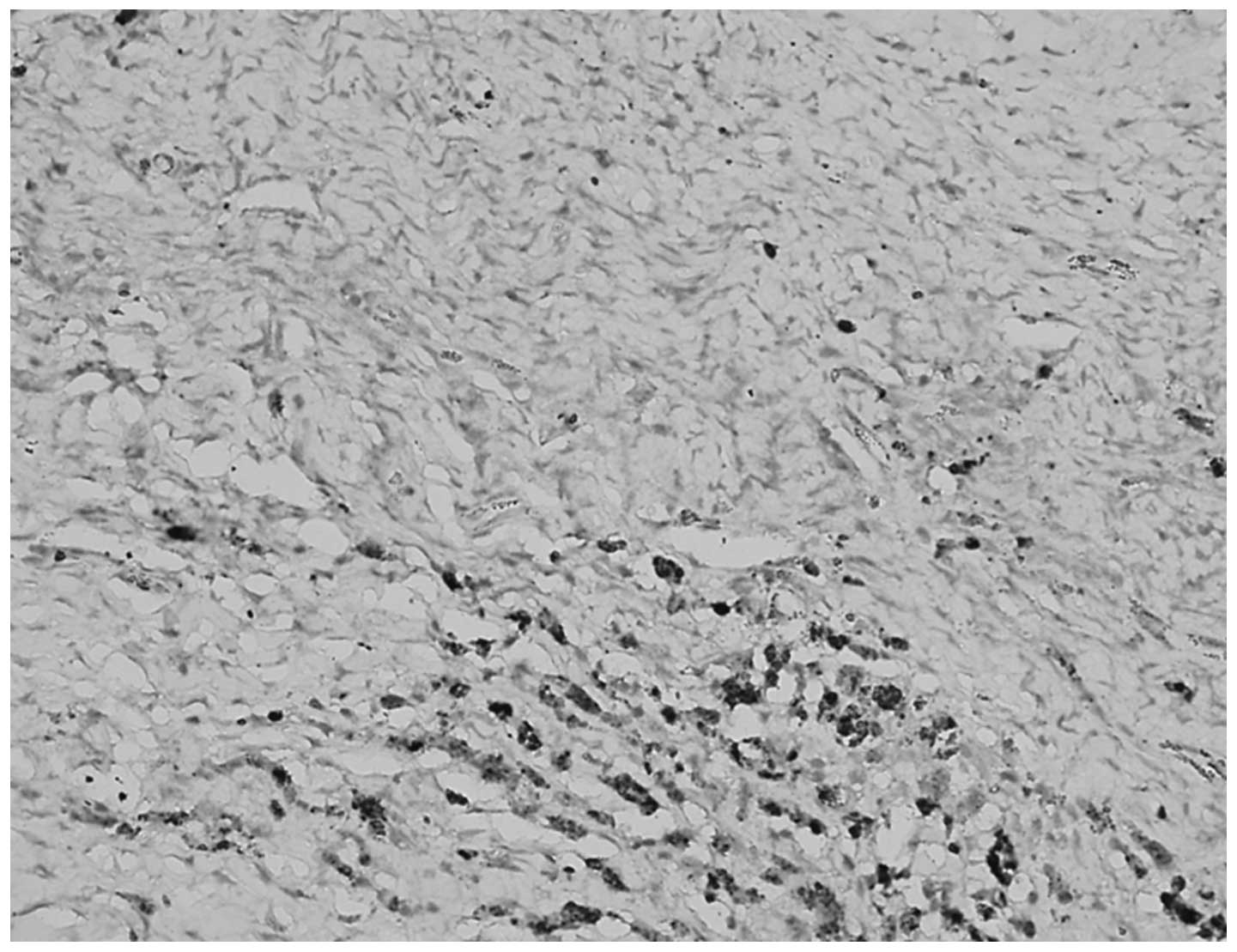
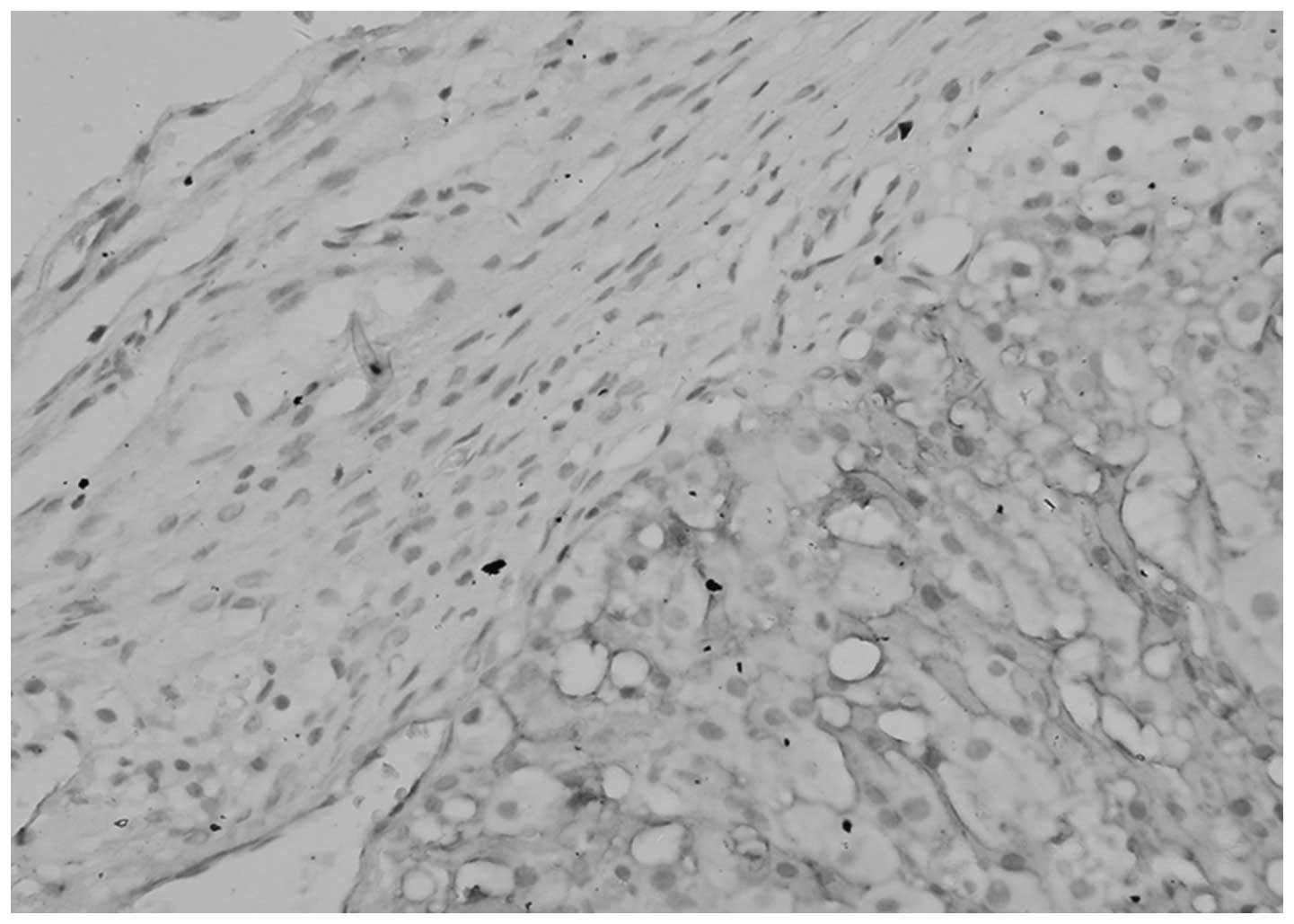
No significant differences were identified between
the expression of TGF-β1 in the cytoplasm and ECM of normal ovarian
tissue and benign tumors (P>0.05), however, the levels of TGF-β1
in the cytoplasm and ECM were significantly different between
epithelial ovarian cancer and the corresponding normal ovarian
tissue (P<0.05; Table II,
Figs. 1 and 2).
 | Table IIExpression of transforming growth
factor-β1 under various pathological conditions. |
Table II
Expression of transforming growth
factor-β1 under various pathological conditions.
| | Cytoplasm [n,
(%)] | Extracellular matrix
[n, (%)] |
|---|
| |
|
|
|---|
| Pathology | Cases | − | + | ++ | +++ | − | + | ++ | +++ |
|---|
| Normal ovarian
tissue | 25 | 6 (24) | 5 (20) | 8 (32) | 6 (24) | 21 (84) | 2 (8) | 2 (8) | 0 |
| Benign ovarian
tumor | 10 | 2 (20) | 2 (20) | 3 (30) | 3 (30) | 6 (60) | 2 (20) | 2 (20) | 0 |
| Epithelial ovarian
cancer | 72 | 42 (58) | 12 (17) | 10 (14) | 8 (11)a | 34 (47) | 15 (21) | 11 (15) | 12 |
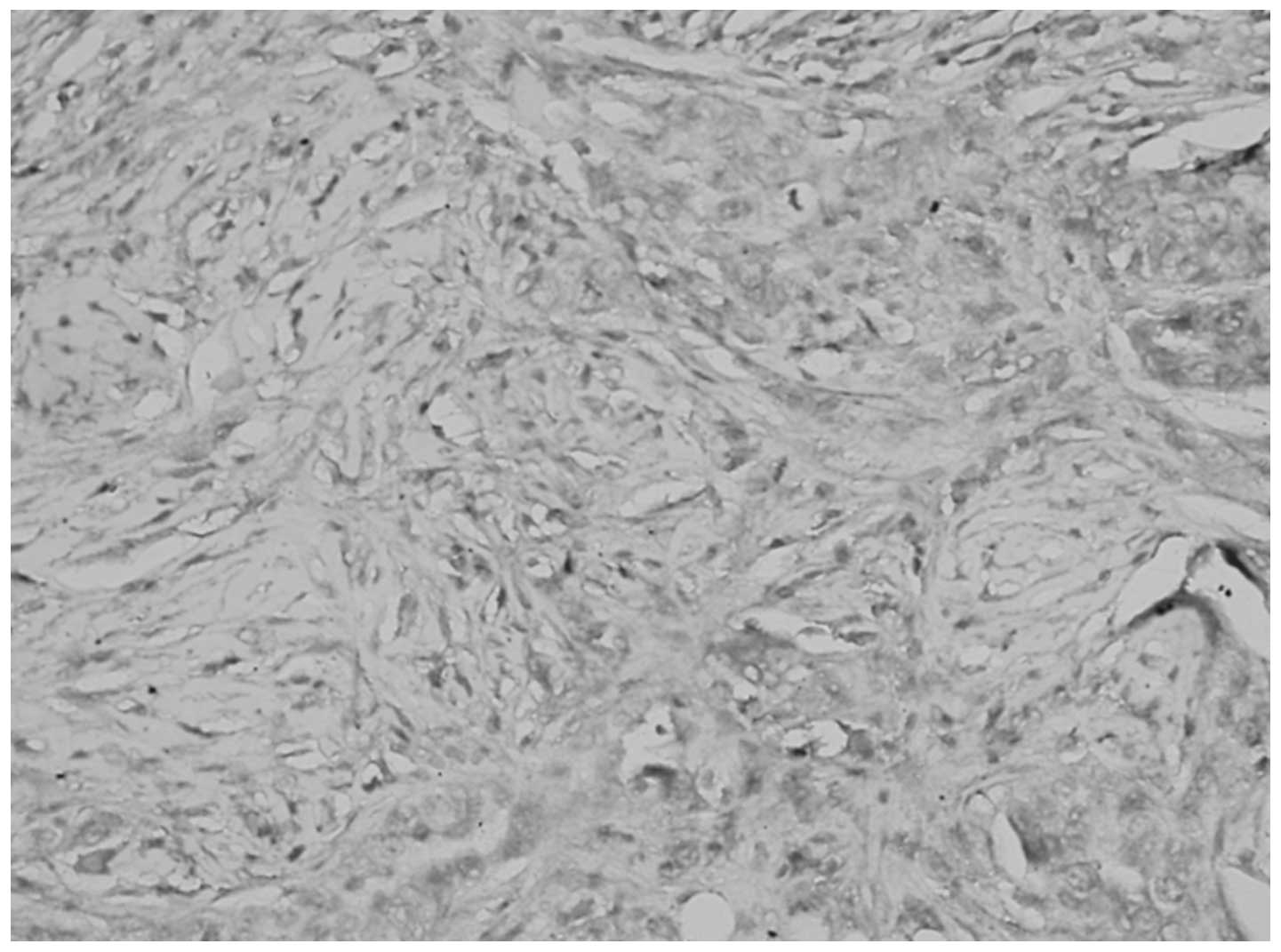
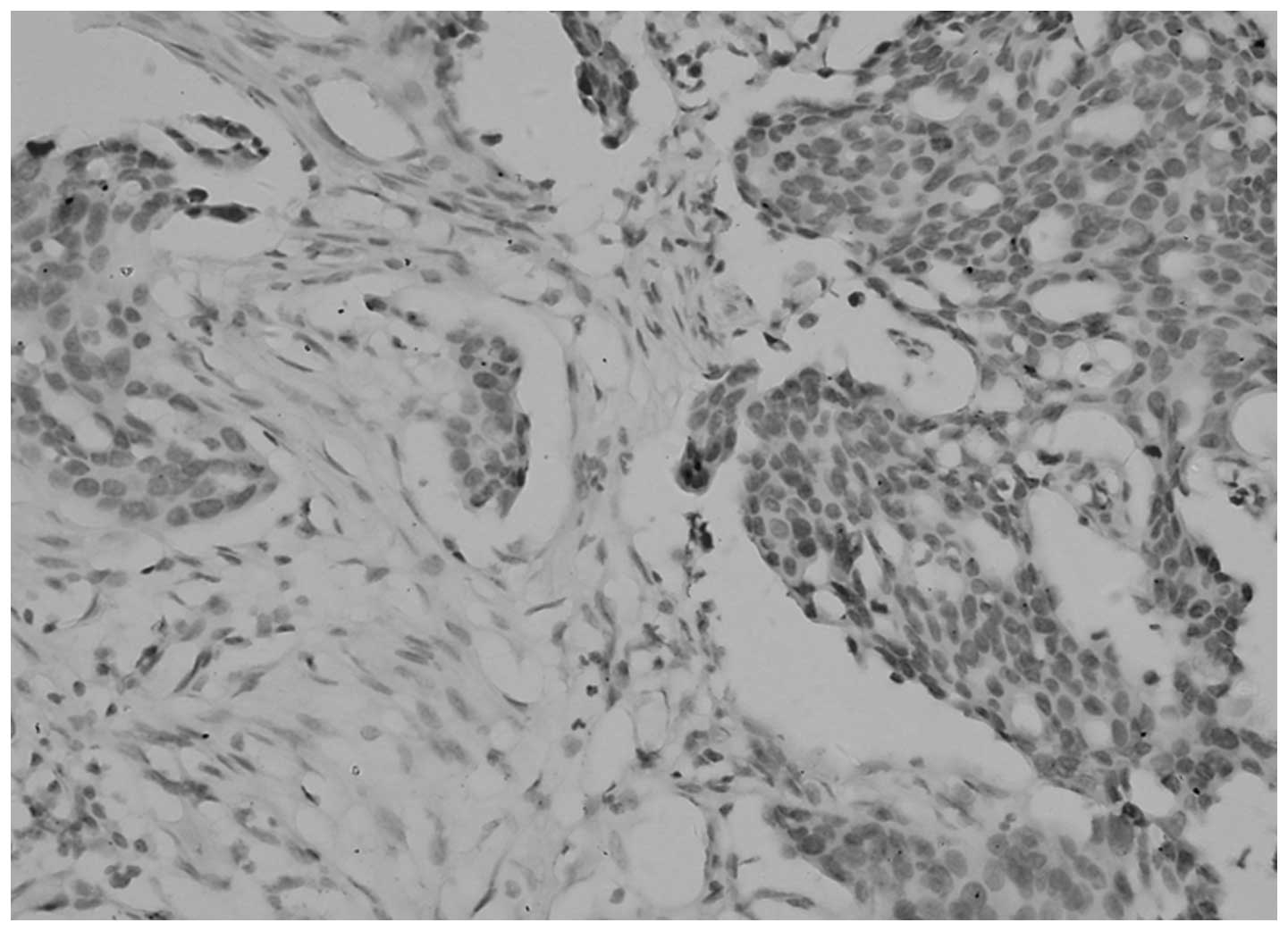
Association between TGF-β1 expression in
epithelial ovarian cancer, and clinical stage and pathological
grade
The differences in TGF-β1 expression in the
cytoplasm and ECM were identified as statistically significant
between the pathological grades and stages (P<0.05; Table III, Figs. 3 and 4).
 | Table IIICorrelation between transforming
growth factor-β1 expression, and the histological grade and
clinical stage of epithelial ovarian cancers. |
Table III
Correlation between transforming
growth factor-β1 expression, and the histological grade and
clinical stage of epithelial ovarian cancers.
| | Cytoplasm [n,
(%)] | Extracellular matrix
[n, (%)] |
|---|
| |
|
|
|---|
| Pathology | Cases | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value |
|---|
| Histological
grade | | | | | | 0.002 | | | | | 0.001 |
|
Well-differentiated | 25 | 8 (32) | 6 (24) | 6 (24) | 5 (20) | | 18 (72) | 3 (12) | 3 (12) | 1 (4) | |
|
Moderately-differentiated | 20 | 12 (60) | 4 (20) | 2 (10) | 2 (10) | | 10 (50) | 4 (20) | 4 (20) | 2 (10) | |
|
Poorly-differentiated | 27 | 22 (82) | 2 (7) | 2 (7) | 1 (4) | | 6 (22) | 8 (30) | 4 (15) | 9 (33) | |
| Total | 72 | 42 (58) | 12 (17) | 10 (14) | 8 (11) | | 34 (47) | 15 (21) | 11 (15) | 12 (17) | |
| Clinical stage | | | | | | 0.001 | | | | | 0.020 |
| Stages I–II | 31 | 11 (35) | 8 (26) | 6 (19) | 6 (19) | | 21 (68) | 5 (16) | 3 (10) | 2 (6) | |
| Stages III–IV | 41 | 31 (76) | 4 (10) | 4 (10) | 2 (5) | | 13 (32) | 10 (24) | 8 (20) | 10 (24) | |
| Total | 72 | 42 (58) | 12 (17) | 10 (14) | 8 (11) | | 34 (47) | 15 (21) | 11 (15) | 12 (17) | |
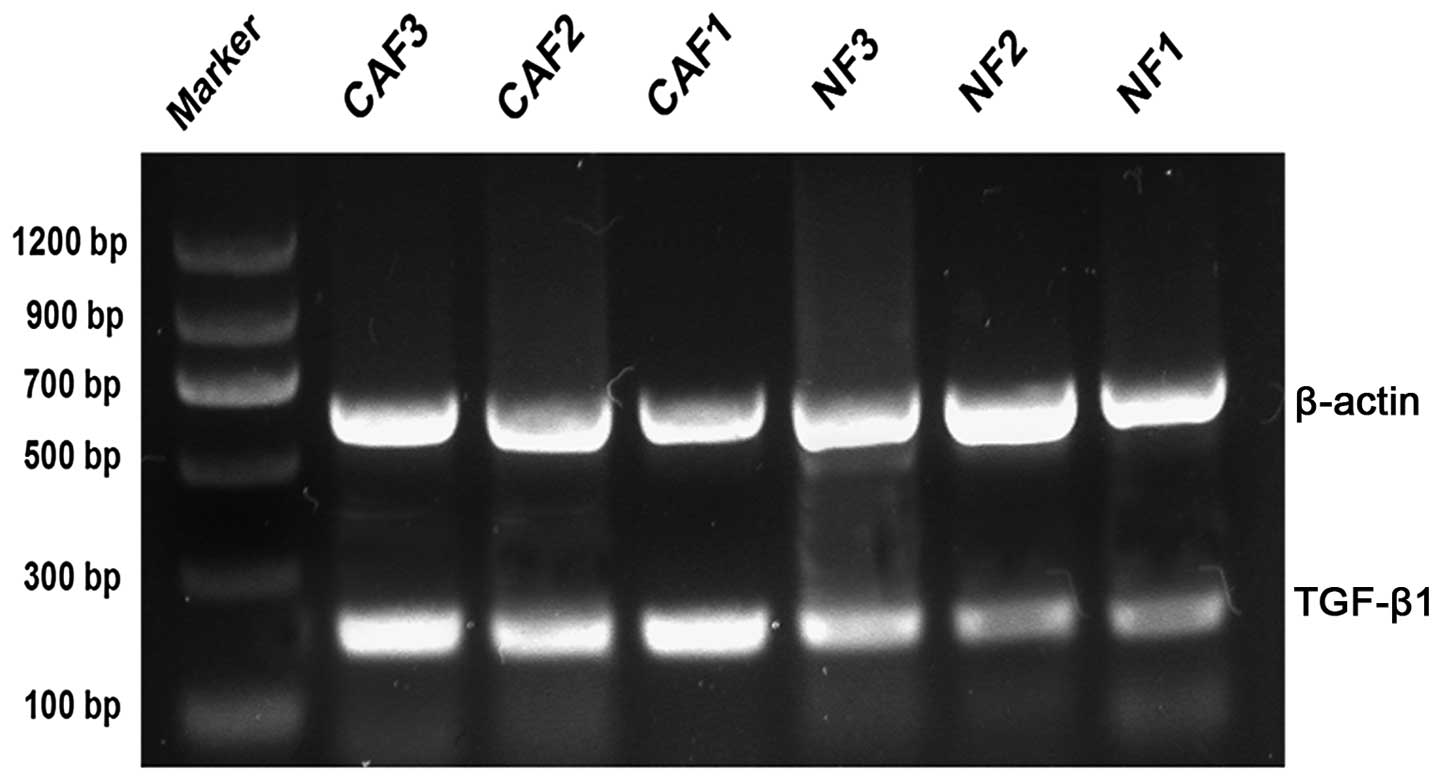
Detection of TGF-β1 mRNA levels using
semi-quantitative PCR
The relative levels of TGF-β1 mRNA in CAFs and NFs
were 1.0271±0.053 and 0.7131±0.0186, respectively. The levels of
TGF-β1 in CAF1, 2 and 3 were 1.1271±0.0642, 0.9886±0.01130 and
0.9654±0.0863, respectively, whereas those in NF1, 2 and 3 were
0.6334±0.0188, 0.608±0.0060 and 0.8980±0.0309, respectively. The
TGF-β1 mRNA level was significantly upregulated in CAFs compared
with that in NFs (P<0.05; Fig.
5).
Discussion
The inhibitory effect of TGF-β1 on the growth of
normal and early stage tumor cells is achieved via inhibition of
the cell cycle progression from the G1 to the S phase. Alexandrow
and Mose (3) reported that the
treatment of numerous cell lines with TGF-β1 led to a rapid
decrease in c-myc mRNA and protein levels, while overexpression of
the c-myc protein antagonized the inhibitory effect of TGF-β1 on
cells entering the S phase. These results indicate that TGF-β1
regulates c-myc expression at the transcriptional and
post-transcriptional levels, thereby inhibiting the c-myc-regulated
cell functions and arresting cell growth in the G1 phase. The
results of the present study indicated that the expression level of
TGF-β1 was relatively high in normal ovarian tissue and benign
ovarian tumors, although the positive expression rate of TGF-β1
decreased gradually from benign ovarian tumors to epithelial
ovarian cancer. Furthermore, the expression of TGF-β1 in advanced
stage and poorly-differentiated epithelial ovarian cancer was
significantly lower than that in early stage and
well-differentiated tumors, indicating that the autocrine loop of
TGF-β1 may be involved in the process of apoptosis in tumor cells
(4), which was suppressed in the
advanced and poorly-differentiated tumors. The suppressed TGF-β1
signaling pathway and the subsequently weakened-inhibition of the
c-myc proto-oncogene accelerated tumor cell proliferation and tumor
progression.
Epithelial ovarian tumor tissues and the majority of
ovarian cancers have a closely associated tumor stroma (5). The occurrence and development of
cancer is not determined by epithelia or stroma alone, but by the
equilibrium of the microenvironment at the tumor-host interface,
which is formed as a result of the interaction between the two
components (6). Among them the
predominant host cells, CAFs, are important for regulating the
equilibrium of this interface system. Numerous studies have
indicated that during the process of tumorigenesis, the stroma
surrounding epithelial cells is activated, which forms a
cancer-associated stroma, subsequently promoting the occurrence and
development of cancer (7). The
predominant feature of an activated stroma is the transformation of
fibroblasts into CAFs. In vitro and in vivo studies
have demonstrated that TGF-β stimulates the conversion of
fibroblasts into the phenotype of CAFs, indicating a critical role
for TGF-β in the formation of a cancer-promoting stromal
environment (8). Rosenthal et
al (9) reported that TGF-β1
upregulates the expression of CAFs, while Xu et al (10) found that the TGF-β-treated SMMC-7721
hepatocellular carcinoma cell line altered significantly, adopting
a spindle-shaped morphology, with reduced expression of E-cadherin
and induction of β-catenin nuclear translocation, enhancing the
cell motility. Previous studies have also shown that TGF-β1
promotes the expression of matrix metalloproteinase-2 (MMP-2) via
the binding of transcription factors c-Jun and c-Fos to the AP1
(Jun/Fos) site in the MMP-2 gene promoter, thereby stimulating the
release of MMP-2 from the tumor and surrounding stromal cells
(11). MMP-2 degrades the
intercellular matrix, as well as the major component of basement
membrane, collagen IV, thereby hydrolyzing the basement membrane,
which allows tumor cells to enter the connective tissue. TGF-β1
affects the ECM in a paracrine manner, exerting its effects to
enhance the interaction between cancer cells and the ECM, which
promotes angiogenesis and the suppression of the immune response,
to provide a suitable microenvironment for cancer cells to
accelerate their growth and metastasis.
In conclusion, the present study demonstrated that
the ability of advanced epithelial ovarian cancer to produce
autocrine TGF-β1 was declined or eliminated. This resulted in a
weakened effect of TGF-β1 with regards to the inhibition of tumor
proliferation and the promotion of tumor cell apoptosis, resulting
in an overall reduction in its tumor suppression effect. However,
in the stroma, the paracrine mechanism of TGF-β1 in cancer cells
remained relatively normal and the released TGF-β1 exerted the
abovementioned effects on the ECM. Recent studies have shown that
the application of a TGF-β1 antibody, TGF-β1 binding protein or
antisense oligos against TGF-β1 may neutralize the effect of
TGF-β1, to achieve antitumor invasion and metastasis. Therefore,
further studies regarding the association between TGF, and the
initiation and development of ovarian cancer may provide novel
insights into the diagnosis and treatment of the disease.
Acknowledgements
This study was supported by the Outstanding Medical
Academic Leader Program of Hubei province.
References
|
1
|
Sporn MB, Roberts AB, Wakefield LM and
Assoian RK: Transforming growth factor-beta: biological function
and chemical structure. Science. 233:532–534. 1986.
|
|
2
|
Wang ST, Liu JJ, Wang CZ, et al:
Expression and correlation of Lewis y antigen and TGF-β1 in ovarian
epithelial carcinoma. Oncol Rep. 27:1065–1071. 2012.
|
|
3
|
Alexandrow MG and Mose HL: Transforming
growth factor beta and cell cycle regulation. Cancer Res.
55:1452–1457. 1995.
|
|
4
|
Zhang Y, Hu YL and Cheng YY: Docetaxel
influences autocrine of transforming growth factors and induces
apoptosis in human ovarian cancer cell line AO. Chin Med Sci J.
21:2042006.
|
|
5
|
Yi C, Li L, Chen K, et al: Expression of
c-Kit and PDGFRα in epithelial ovarian tumors and tumor stroma.
Oncol Lett. 3:369–372. 2012.
|
|
6
|
Liotta LA and Kohn EC: The
microenvironment of the tumor-host interface. Nature. 411:375–379.
2001.
|
|
7
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447. 2003.
|
|
8
|
Micke P and Ostman A: Tumour-stroma
interaction: cancer-associated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45(Suppl 2): S163–S175. 2004.
|
|
9
|
Rosenthal E, McCrory A, Talbert M, et al:
Elevated expression of TGF-beta1 in head and neck cancer-associated
fibroblasts. Mol Carcinog. 40:116–121. 2004.
|
|
10
|
Xu Z, Shen MX, Ma DZ, et al:
TGF-beta1-promoted epithelial-to-mesenchymal transformation and
cell adhesion contribute to TGF-betal-enhanced cell migration in
SMMC-7721 cells. Cell Res. 13:343–350. 2003.
|
|
11
|
Mukai M, Sadahiro S, Tokunaga N, et al:
The expression of MMP-2 and TIMP-2 in patients with colorectal
adenocarcinoma invaded to the submuscosal and proper muscle layer.
Oncol Rep. 5:335–340. 1998.
|