Introduction
Osteosarcoma is the most frequent type of malignant
primary bone tumor in childhood (1). The prognosis for localized extremity
osteosarcoma treated with surgery alone is poor, with a 2-year
survival rate of <20%, as the tumor exhibits a high propensity
to metastasize to the lungs (2).
Although there have been developments in treatment with the
administration of large doses of adjuvant/neoadjuvant chemotherapy,
30% of patients with localized disease and 80% of patients with
metastatic disease at diagnosis suffer relapse (3,4). In
addition, the failure of standard multimodal therapy in
osteosarcoma is common. However, surgical resection has been
demonstrated to prolong survival times in osteosarcoma patients,
particularly among those with pulmonary metastases (5–8).
Furthermore, stereotactic radiosurgery has been effective in
certain osteosarcoma patients with pulmonary metastases (9). However, neither the value of
second-line chemotherapy nor the best treatment strategy for
relapsed patients with high-grade, advanced or metastatic disease
has been well-defined (10). This
is further complicated by the fact that there are a limited number
of studies concerning the management of patients with relapsed
osteosarcoma, due to the relative rarity of the disease.
Consequently, outcomes such as tumor response rate following
second-line treatment are not well-established; therefore,
metastatic osteosarcoma remains difficult to treat. Since a lack of
consensus has been reached regarding the choice of second-line
therapy, the efficacy of novel agents is being continuously
evaluated. Although several novel agents, including pirarubicin
(11), topotecan (12), irinotecan (13), imatinib mesylate (14) and temozolamide (15) have been investigated, the response
rates remain low and survival times short.
Gemcitabine, a difluorinated deoxycytidine analog,
is taken up by cells and is converted to the active diphosphate and
triphosphate forms by deoxycytidine kinase. The active forms reduce
deoxynucleotide reserves and alter DNA chain elongation, thus
gemcitabine induces cell death (16). Docetaxel is a semisynthetic taxane
analog of paclitaxel. This molecule induces cytotoxicity by
stabilizing microtubules, preventing depolymerization, which
results in cell cycle arrest and subsequent apoptosis (17). The gemcitabine plus docetaxel
combination has been observed to exert additive and synergistic
antitumor effects in patients with recurrent osteosarcoma (18–25).
Pemetrexed is a newly developed antifolate drug that
targets multiple enzymes involved in DNA synthesis and folate
metabolism. When compared with methotrexate, a drug widely used in
the treatment of osteosarcoma, pemetrexed is polyglutamated by
folylpolyglutamate synthase at 90 to 200 times greater efficiency
(26). A phase II trial has
previously demonstrated the efficacy of pemetrexed in soft tissue
sarcoma (27), and pemetrexed has
also been shown to exert broad-spectrum effects in numerous types
of solid tumor (28–30). Pemetrexed has a wider range of
activity than methotrexate, the corresponding antifolate
predecessor, as pemetrexed exerts effects on multiple targets, and
has the ability to affect purine and pyrimidine synthesis (31). Therefore, pemetrexed appears to have
potential for osteosarcoma treatment. A phase II study has
demonstrated that approximately one-third of patients with relapsed
osteosarcoma survived for at least 1 year after pemetrexed
treatment (32). Furthermore,
several studies have demonstrated that in head/neck cancer and
malignant pleural mesothelioma, pemetrexed plus cisplatin
combination treatment may prolong survival times in certain
patients compared with treatment with pemetrexed or cisplatin alone
(33–35). Since cisplatin is one of the most
active and widely used drugs in the treatment of high-grade
osteosarcoma, the pemetrexed plus cisplatin combination may have
improved efficacy compared with cisplatin alone. To investigate the
efficacy and safety of the pemetrexed plus cisplatin combination in
patients with refractory/metastatic osteosarcoma the present
retrospective study was conducted and the results obtained were
compared with those of patients receiving the gemcitabine plus
docetaxel combination. To the best of our knowledge, no study has
been conducted regarding the use of the pemetrexed plus cisplatin
combination in osteosarcoma patients, nor any comparison conducted
between the pemetrexed plus cisplatin combination and the
gemcitabine plus docetaxel combination.
Materials and methods
Patient eligibility
Between January 2005 and May 2011, patients with
refractory/metastatic osteosarcoma who received the gemcitabine
plus docetaxel or pemetrexed plus cisplatin combination as
second-line chemotherapy at Shanghai Jiaotong University (Shanghai,
China) were selected for this retrospective case series study in
accordance with the following criteria: i) diagnosis confirmed
histologically; ii) resistance to prior treatment consisting of
standard high-grade osteosarcoma chemotherapy agents, including
doxorubicin, cisplatin, high-dose methotrexate and ifosfamide
(completed >3 weeks prior to trial entry); iii) metastatic and
unresectable progressive disease (PD); iv) Karnofsky performance
status >70 with life expectancy >3 months; and v) adequate
renal, hepatic and hemopoietic function. All enrolled patients
exhibited radiological evidence of disease progression prior to the
initiation of treatment. Clinical characteristics, including age,
gender, pathological subtype and performance status were collected
for statistical analysis. Ethical approval for the study was
provided by the ethics committee of the Affiliated Sixth People’s
Hospital (Shanghai, China) and informed consent was obtained from
each patient or patient’s guardian. Information regarding toxicity
associated with the administration of chemotherapy was recorded
according to the National Cancer Institute (NCI) Common Terminology
Criteria for Adverse Events (version 3.0).
Treatment
The gemcitabine plus docetaxel regimen was
administered as follows: Patients initially received gemcitabine at
a dose of 675 mg/m2 intravenously over 90 min on days 1
and 8 of each 21-day course. The patients also received ondansetron
prior to initiation of chemotherapy on days 1 and 8. Gemcitabine
administered on day 8 was followed by 75 mg/m2 docetaxel
administered intravenously over 60 min. To minimize the severity
and incidence of hypersensitivity and fluid retention associated
with docetaxel, dexamethasone treatment was initiated either the
day prior to or the same day as when docetaxel was administered,
and was continued for 2 days thereafter.
The pemetrexed plus cisplatin combination was
administered as follows: 500 mg/m2 pemetrexed was
intravenously administered over 10 min on day 1 of a 21-day cycle,
followed by 100 mg/m2 cisplatin administered
intravenously. A preparation of 4 mg dexamethasone was taken orally
twice daily on the day prior to, the day of and the day after each
administration of pemetrexed. Supplementation with 400 μg folic
acid, taken orally, was administered daily 1 week prior to the
first pemetrexed treatment and was continued until 3 weeks after
experimental therapy was discontinued. At 1 week prior to day 1 of
cycle 1, 1,000 μg vitamin B12 was intramuscularly injected and this
was repeated every 9 weeks until the study was terminated.
If a patient experienced unacceptable toxicity,
treatment was postponed for up to 42 days, initiated at day 1 of
any cycle to allow recovery from toxicity until the Common Toxicity
Criteria (36) grade 3/4 symptoms
had resolved. Subsequently, therapy was resumed at 75% of the
previous dosage. Any patient requiring >42 days recovery time or
>2 reductions due to toxicity was to be withdrawn from the
study.
Efficacy assessment
Tumor response was usually evaluated in between
every two chemotherapy cycles by CT/MRI scan according to the
Response Evaluation Criteria in Solid Tumors from the NCI.
Treatment responses were classified accordingly as complete
response (CR), partial response (PR), stable disease (SD) or PD.
The endpoints were to evaluate overall response rate (CR + PR),
disease control rate (overall response rate + SD), progression-free
survival time (PFS) and overall survival time (OS). PFS and OS
times were defined as the intervals between the initiation of
treatment and progression of the disease or when the patient
succumbed to the disease, respectively. Toxicity was recorded for
each cycle of chemotherapy according to the NCI Common Toxicity
Criteria grading system (36).
Toxicity was classified into four levels, with the following
associated factors recorded: i) white blood cell count, ii)
platelet count, iii) hemoglobin, iv) gastrointestinal toxicities
(nausea and vomiting), v) fatigue, vi) impaired liver function and
vii) impaired renal function. The Kaplan-Meier method was employed
to compare the PFS and OS results.
Results
Patient characteristics
A total of 39 patients with metastatic high-grade
osteosarcoma, treated between January 2005 and May 2011, were
included in the present study. Of these patients, 21 were provided
the gemcitabine plus docetaxel combination with the remaining 18
administered the pemetrexed plus cisplatin combination. The
characteristics of patients in the two groups are listed in
Table I. Factors such as age,
gender, initial tumor site, histotype of tumor, method of surgery
for initial tumor and stage were well-balanced between the two
groups (all P>0.05).
 | Table IPatient characteristics in the
gemcitabine/docetaxel and pemetrexed/cisplatin treatment
groups. |
Table I
Patient characteristics in the
gemcitabine/docetaxel and pemetrexed/cisplatin treatment
groups.
| Clinicopathological
parameter |
Gemcitabine/docetaxel |
Pemetrexed/cisplatin | P-value |
|---|
| Gender | | | >0.05 |
| Male | 15 | 14 | |
| Female | 6 | 4 | |
| Age (years) | | | >0.05 |
| <18 | 11 | 11 | |
| ≥18 | 10 | 7 | |
| Location | | | >0.05 |
| Limbs | 18 | 16 | |
| Nonextremities | 3 | 2 | |
| Histotype | | | >0.05 |
| Conventional | 18 | 17 | |
| Others | 3 | 1 | |
| Surgical | | | >0.05 |
| Amputation | 7 | 6 | |
| Limb salvage | 14 | 12 | |
| Metastasis at
diagnosis | | | >0.05 |
| Yes | 11 | 8 | |
| No | 10 | 10 | |
Response and outcome
In the gemcitabine plus docetaxel group, 21 patients
received up to 56 courses of treatment and at least two cycles of
chemotherapy (median, two cycles per patient; range, 2–6). No CR
was observed thereafter. The overall response rate and disease
control rate were 9.5% (2 out of 21) and 28.5% (6 out of 21),
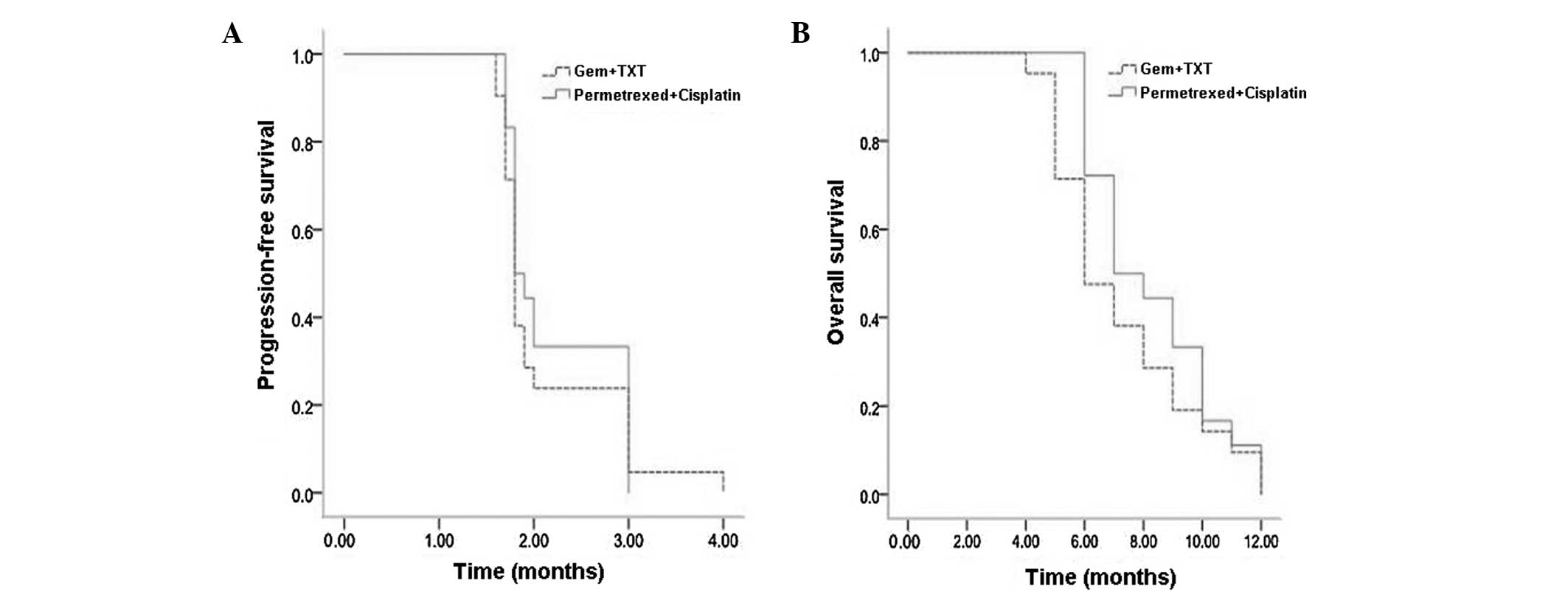
respectively. The median PFS time was 1.8 months and the median OS
time was 6 months.
The 18 patients in the pemetrexed plus cisplatin
group received up to 48 courses of treatment with at least two
cycles of chemotherapy (median, two cycles per patient; range,
2–6). No CR was observed thereafter. The overall response rate and
disease control rate were 5.5% (1 out of 18) and 33.3% (6 out of
18), respectively. The median PFS time was 1.8 months and the
median OS time was 7 months.
Subsequent to statistical analysis, no significant
differences were identified between overall response rates, disease
control rates, median PFS times or median OS times in the two
groups (Table II, Fig. 1A and B).
 | Table IITumor responses in the
gemcitabine/docetaxel and pemetrexed/cisplatin groups. |
Table II
Tumor responses in the
gemcitabine/docetaxel and pemetrexed/cisplatin groups.
| Group | No. patients | CR | PR | SD | PD |
|---|
|
Gemcitabine/docetaxel | 21 | 0 | 2 | 4 | 15 |
|
Pemetrexed/cisplatin | 18 | 0 | 1 | 5 | 12 |
Toxicity
Table III
summarizes the main side effects and all grades of adverse events
in the two groups. In general, no drug-related mortality occurred
in either group, and chemotherapy-related adverse events were
predominantly associated with grade 1 or 2. In 56 courses of
gemcitabine plus docetaxel treatment, the observed grade 3 and 4
toxic effects were as follows: 17 cases of leucopenia (30.3%), four
cases of anemia (7.1%), nine cases of thrombocytopenia (16.1%),
three cases of nausea and vomiting (5.4%) and eight cases of
fatigue (14.3%). Conversely, in 48 courses of pemetrexed plus
cisplatin therapy, the observed grade 3 and 4 toxic effects were as
follows: Two cases of leucopenia (4.2%), one case of anemia (2%),
two cases of nausea and vomiting (4.2%) and three cases of fatigue
(6.3%). Statistical analysis revealed a significant difference
(P<0.05) in the incidence of grade 3/4 thrombocytopenia and
leucopenia between the two groups.
 | Table IIIHematological and non-hematological
toxicity in patients treated with gemcitabine/docetaxel or
pemetrexed/cisplatin. |
Table III
Hematological and non-hematological
toxicity in patients treated with gemcitabine/docetaxel or
pemetrexed/cisplatin.
| Gemcitabine/docetaxel
group (56 courses), n (%) | Pemetrexed/cisplatin
group (48 courses), n (%) |
|---|
|
|
|
|---|
| Toxic event | Grade 1/2 | Grade 3 | Grade 4 | Grade 1/2 | Grade 3 | Grade 4 |
|---|
| Anemia | 32 (57.1) | 3 (5.3) | 1 (1.8) | 21 (43.7) | 1 (2.0) | 0 (0.0) |
| Leucopenia | 30 (53.5) | 12 (21.4) | 5 (8.9) | 11 (22.9) | 2 (4.1) | 0 (0.0) |
| Thrombocytopenia | 26 (46.4) | 7 (12.5) | 2 (3.6) | 8 (16.7) | 0 (0.0) | 0 (0.0) |
| Nausea and
vomiting | 18 (32.1) | 3 (5.3) | 0 (0.0) | 20 (41.7) | 2 (4.1) | 0 (0.0) |
| Fatigue | 22 (39.2) | 6 (10.7) | 2 (3.6) | 21 (43.7) | 3 (6.3) | 0 (0.0) |
| Impaired liver
function | 5 (8.9) | 0 (0.0) | 0 (0.0) | 12 (25.0) | 0 (0.0) | 0 (0.0) |
| Impaired kidney
function | 2 (3.5) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| Alopecia | 12 (21.4) | 0 (0.0) | 0 (0.0) | 13 (27) | 0 (0.0) | 0 (0.0) |
Treatment postponement on day 8 occurred in three
patients during three of the 56 gemcitabine plus docetaxel
combination chemotherapy courses due to a consistent platelet count
of <10,000/μl (in one patient) and a consistent WBC count of
<1,500/μl (in two patients). No treatment in patients with the
pemetrexed plus cisplatin combination had to be postponed due to
toxicity.
Discussion
Patients with refractory/metastatic osteosarcoma
have a poor prognosis and novel strategies are therefore required
to improve outcomes in this subgroup of patients. In the present
retrospective study, treatment of refractory/metastatic
osteosarcoma patients with a combination of gemcitabine and
docetaxel resulted in a 28.5% disease control rate, a median PFS
time of 1.8 months and a median OS time of 6 months. The result was
consistent with those of previous studies (24,25).
The main adverse reaction of gemcitabine plus docetaxel treatment
was grade 3/4 myelosuppression, as detected by leucopenia and
thrombocytopenia.
The pemetrexed plus cisplatin combination
demonstrated a promising potential in treating
refractory/metastatic osteosarcoma. This treatment achieved an
efficacy that was parallel to that of the gemcitabine plus
docetaxel combination. The adverse reactions following pemetrexed
plus cisplatin treatment were well-tolerated with fewer incidences
of grade 3/4 toxic events compared with gemcitabine plus docetaxel
therapy. Considering that the majority of refractory/metastatic
osteosarcoma patients have received large quantities of multi-drug
and high-dose chemotherapies (38,39),
the potential reserves of bone marrow function in the patients are
likely to be poor. Therefore, pemetrexed plus cisplatin treatment
may be better tolerated in these patients.
However, the results of the present study are, to
some extent, different from those of a recent phase II trial
designed to evaluate the efficacy of pemetrexed alone as a
second-line chemotherapy in advanced/metastatic osteosarcoma
(28). The phase II trial indicated
that although it was tolerated well by patients, pemetrexed did not
exhibit a good response in refractory/metastatic osteosarcoma
(28). This may be the result of
certain shortcomings in the present study, such as the
retrospective nature and possible patient selection bias, which may
affect the prognosis. Such differences may have been due to the
supra-additive effect of the pemetrexed plus cisplatin combination.
Previous studies have reported that compared with pemetrexed plus
cisplatin alone, pemetrexed plus cisplatin treatment achieves an
improved prognosis in certain types of cancer (33–35).
This ‘synergistic effect’ may be associated with the
cisplatin-reinforcement effect of pemetrexed; the patients who
benefited from the pemetrexed plus cisplatin combination in the
present study had shown drug-resistance during prior treatment,
including resistance to cisplatin. Cisplatin enhancement of
pemetrexed efficacy is also a possibility. Further studies using
the basic model are required to elucidate the underlying molecular
mechanism of this supra-additive effect.
The present study had certain shortcomings, namely
the retrospective nature, the relatively small number of patients
and possible patient selection bias. Despite these limitations,
this remains, to the best of our knowledge, the first study
describing the effect and toxicity of pemetrexed plus cisplatin in
refractory/metastatic osteosarcoma patients, and comparing this
treatment regimen with gemcitabine plus docetaxel therapy. Although
the two drug combinations were well-tolerated and easily
administered, the pemetrexed plus cisplatin treatment appears to
have fewer incidences of severe toxicity. To determine whether
treatment with pemetrexed plus cisplatin results in longer survival
times than those that achieved with pemetrexed alone, prospective
studies are required.
Acknowledgements
Editorial assistance in the preparation of this
manuscript was provided by Dr Daliu Min from the Department of
Oncology, Affiliated Sixth People’s Hospital, Shanghai Jiaotong
University, Shanghai, China.
References
|
1
|
Arndt CA and Crist WM: Common
musculoskeletal tumors of childhood and adolescence. N Engl J Med.
341:342–352. 1999.
|
|
2
|
Friedman MA and Carter SK: The therapy of
osteogenic sarcoma: current status and thoughts for the future. J
Surg Oncol. 4:482–510. 1972.
|
|
3
|
Ozaki T, Flege S, Kevric M, et al:
Osteosarcoma of the pelvis: experience of the Cooperative
Osteosarcoma Study Group. J Clin Oncol. 21:334–341. 2003.
|
|
4
|
Tabone MD, Kalifa C, Rodary C, et al:
Osteosarcoma recurrences in pediatric patients previously treated
with intensive chemotherapy. J Clin Oncol. 12:2614–2620. 1994.
|
|
5
|
Kempf-Bielack B, Bielack SS, Jürgens H, et
al: Osteosarcoma relapse after combined modality therapy: an
analysis of unselected patients in the Cooperative Osteosarcoma
Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
|
|
6
|
Biccoli A, Rocca M, Salone M, et al:
Resection of recurrent pulmonary metastases in patients with
osteosarcoma. Cancer. 104:1721–1725. 2005.
|
|
7
|
Bielack SS, Kempf-Bielack B, Branscheid D,
et al: Second and subsequent recurrences of osteosarcoma:
presentation, treatment, and outcomes of 249 consecutive
cooperative osteosarcoma study group patients. J Clin Oncol.
27:557–565. 2009.
|
|
8
|
Briccoli A, Rocca M, Salone M, et al: High
grade osteosarcoma of the extremities metastatic to the lung:
long-term results in 323 patients treated combining surgery and
chemotherapy, 1985–2005. Surg Oncol. 19:193–199. 2010.
|
|
9
|
Zhang Y, Xiao JP, Zhang HZ, et al:
Stereotactic body radiation therapy favors long-term overall
survival in patients with lung metastases: five-year experience of
a single-institution. Chin Med J (Engl). 124:4132–4137. 2011.
|
|
10
|
Hogendoorn PC, Athanasou N and Bielack S;
ESMO/EUROBONET Working Group. Bone sarcomas: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
21(Suppl 5): 204–213. 2010.
|
|
11
|
Qi WX, He AN, Tang LN, Shen Z and Yao Y:
Evaluation of pirarubicin-cisplatin chemotherapy in the treatment
for refractory and recurrent high-grade osteosarcoma: experience of
a single institute. Med Oncol. 29:2229–2233. 2012.
|
|
12
|
Seibel NL, Krailo M, Chen Z, et al:
Upfront window trial of topotecan in previously untreated children
and adolescents with poor prognosis metastatic osteosarcoma:
children’s Cancer Group (CCG) 7943. Cancer. 109:1646–1653.
2007.
|
|
13
|
Crews KR, Stewart CF, Liu T, Rodriguez
Galindo C, Santana VM and Daw NC: Effect of fractionated ifosfamide
on the pharmacokinetics of irinotecan in pediatric patients with
osteosarcoma. J Pediatr Hematol Oncol. 26:764–767. 2004.
|
|
14
|
Bond M, Bernstein ML, Pappo A, Schultz KR,
Krailo M, et al: A phase II study of imatinib mesylate in children
with refractory or relapsed solid tumors: A Children’s Oncology
Group study. Pediatr Blood Cancer. 50:254–258. 2008.
|
|
15
|
De Sio L, Milano GM, Castellano A, Jenkner
A, Fidani P, et al: Temozolamide in resistant or relapsed pediatric
solid tumors. Pediatr Blood Cancer. 47:30–36. 2006.
|
|
16
|
Carmichael J: The role of gemcitabine in
the treatment of other tumours. Br J Cancer. 78(Suppl 3): 21–25.
1998.
|
|
17
|
Trudeau ME: Docetaxel: a review of its
pharmacology and clinical activity. Can J Oncol. 6:443–457.
1996.
|
|
18
|
Merimsky O, Meller I, Kollender Y and
Inbar M: Palliative effect of gemcitabine in osteosarcoma resistant
to standard chemotherapy. Eur J Cancer. 34:1296–1297. 1998.
|
|
19
|
Okuno S, Edmonson J, Mahoney M, Buckner
JC, Frytak S and Galanis E: Phase II trial of gemcitabine in
advanced sarcomas. Cancer. 94:3225–3229. 2002.
|
|
20
|
Leu KM, Ostruszka LJ, Shewach D, et al:
Laboratory and clinical evidence of synergistic cytotoxicity of
sequential treatment with gemcitabine followed by docetaxel in the
treatment of sarcoma. J Clin Oncol. 22:1706–1712. 2004.
|
|
21
|
Navid F, Willert JR, McCarville MB, et al:
Combination of gemcitabine and docetaxel in the treatment of
children and young adults with refractory bone sarcoma. Cancer.
113:419–425. 2008.
|
|
22
|
Mora J, Cruz CO, Parareda A and de Torres
C: Treatment of relapsed/refractory pediatric sarcomas with
gemcitabine and docetaxel. J Pediatr Hematol Oncol. 31:723–729.
2009.
|
|
23
|
Geoerger B, Chisholm J, Le Deley MC, et
al: European Consortium Innovative Therapies for Children with
Cancer: Phase II study of gemcitabine combined with oxaliplatin in
relapsed or refractory paediatric solid malignancies: An innovative
therapy for children with Cancer European Consortium Study. Eur J
Cancer. 47:230–238. 2011.
|
|
24
|
Qi WX, He AN, Tang LN, et al: Efficacy and
safety of gemcitabine-docetaxel combination therapy for recurrent
or refractory high-grade osteosarcoma in China: a retrospective
study of 18 patients. Jpn J Clin Oncol. 42:427–431. 2012.
|
|
25
|
He A, Qi W, Huang Y, et al: Comparison of
pirarubicin-based versus gemcitabine-docetaxel chemotherapy for
relapsed and refractory osteosarcoma: a single institution
experience. Int J Clin Oncol. 18:498–505. 2013.
|
|
26
|
Shih C, Habeck LL, Mendelsohn LG, et al:
Multiple folate enzyme inhibition: mechanism of a novel
pyrrolopyrimidine-based antifolate LY231514 (MTA). Adv Enzyme
Regul. 38:135–152. 1998.
|
|
27
|
Hartmann JT, Bauer S, Egerer G, et al:
Pemetrexed in patients with refractory soft tissue sarcoma: a
non-comparative multicenter phase II study of the German Sarcoma
Group AIO-STS 005. Invest New Drugs. 31:167–174. 2013.
|
|
28
|
Argiris A, Pennella E, Koustenis A, et al:
Pemetrexed in head and neck cancer: a systematic review. Oral
Oncol. 49:492–501. 2013.
|
|
29
|
Tomasini P, Greillier L, Khobta N and
Barlesi F: The place of pemetrexed in the management of
non-small-cell lung cancer patients. Expert Rev Anticancer Ther.
13:257–266. 2013.
|
|
30
|
Boons CC, VAN Tulder MW, Burgers JA, et
al: The value of pemetrexed for the treatment of malignant pleural
mesothelioma: a comprehensive review. Anticancer Res. 33:3553–3561.
2013.
|
|
31
|
Walling J: From methotrexate to pemetrexed
and beyond. A review of the pharmacodynamic and clinical properties
of antifolates. Invest New Drugs. 24:37–77. 2006.
|
|
32
|
Duffaud F, Egerer G, Ferrari S, et al: A
phase II trial of second-line pemetrexed in adults with
advanced/metastatic osteosarcoma. Eur J Cancer. 48:564–570.
2012.
|
|
33
|
Urba S, van Herpen CM, Sahoo TP, et al:
Pemetrexed in combination with cisplatin versus cisplatin
monotherapy in patients with recurrent or metastatic head and neck
cancer: final results of a randomized, double-blind,
placebo-controlled, phase 3 study. Cancer. 118:4694–4705. 2012.
|
|
34
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003.
|
|
35
|
Jänne PA, Wozniak AJ, Belani CP, et al:
Pemetrexed expanded access program investigators: Pemetrexed alone
or in combination with cisplatin in previously treated malignant
pleural mesothelioma: outcomes from a phase IIIB expanded access
program. J Thorac Oncol. 1:506–512. 2006.
|
|
36
|
Trotti A, Byhardt R, Stetz J, et al:
Common toxicity criteria: version 2.0. an improved reference for
grading the acute effects of cancer treatment: impact on
radiotherapy. Int J Radiat Oncol Biol Phys. 47:13–47. 2000.
|
|
37
|
Cirillo M, Venturini M, Ciccarelli L, et
al: Clinician versus nurse symptom reporting using the National
Cancer Institute-Common Terminology Criteria for Adverse Events
during chemotherapy: results of a comparison based on patient’s
self-reported questionnaire. Ann Oncol. 20:1929–1935. 2009.
|
|
38
|
Bacci G, Briccoli A, Ferrari S, et al:
Neoadjuvant chemotherapy for osteosarcoma of the extremity:
long-term results of the Rizzoli’s 4th protocol. Eur J Cancer.
37:2030–2039. 2001.
|
|
39
|
Bacci G, Ferrari S, Longhi A, et al:
Italian Sarcoma Group/Scandinavian Sarcoma Group: High dose
ifosfamide in combination with high dose methotrexate, adriamycin
and cisplatin in the neoadjuvant treatment of extremity
osteosarcoma: preliminary results of an Italian Sarcoma
Group/Scandinavian Sarcoma Group pilot study. J Chemother.
14:198–206. 2002.
|