Introduction
The life span of companion animals has been
prolonged by the advent of routine vaccinations, improved nutrition
and living environments, and advances in veterinary medicine. As a
result, the incidence of aging-associated illnesses has increased
in the companion animal population. Specifically, cancer is
considered to be a significant issue. As in human medicine, there
are three major modalities for cancer treatment within veterinary
medicine; surgery, chemotherapy and radiation therapy. However, it
is difficult to treat all of the affected animals with these types
of therapy due to the cost and the limited number of facilities
available. Therefore, the development of novel treatment strategies
is required.
Conventional hyperthermia has long been established
as a treatment for cancer, particularly for superficially located
tumors (1). Conventional
hyperthermia is performed alone or as an adjunct to radio- or
chemotherapy (2–5) and has previously been adopted to treat
spontaneous tumors in veterinary medicine (6–9).
Various studies have focused on two common strategies; conventional
hyperthermia at mild temperatures (range, 42–45°C) (1,10,11)
and ablation therapy at high temperatures (>70°C) (12). Our previous study demonstrated that
high temperature hyperthermia (HTH) treatment ranging between 60
and 70°C suppressed glioma tumor growth and induced necrosis and
apoptosis in a rat model (13). In
the present study, the efficacy of HTH therapy in the treatment of
spontaneous tumors in canines is evaluated.
Case report
Case 1
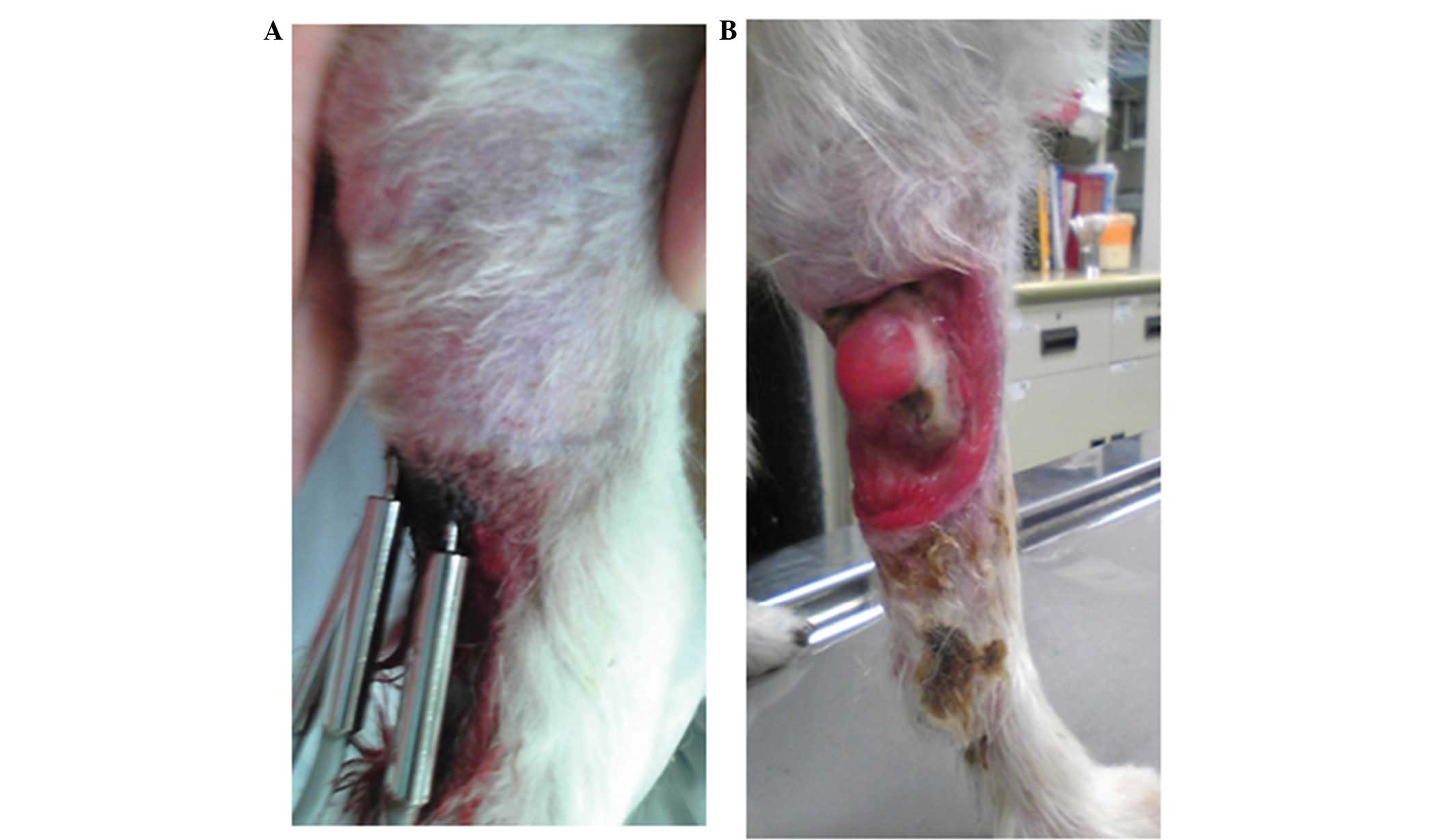
An 18-year-old female Papillon (weight, 3.2 kg) was
referred to the Yamaguchi University Veterinary Teaching Hospital
(Yamaguchi, Japan) in April, 2010 for evaluation of a right
forelimb tumor (Fig. 1A). Surgical
excision of the tumor had been performed twice previously, however,
the tumor had recurred. Histological analysis revealed that the
tumor was a rhabdomyosarcoma. On initial examination, the caudal
right forelimb was covered by the tumor and the animal was
incapacitated in the affected limb. The risk of recurrence and the
treatment options were explained to the owners, which included
surgery, radiation therapy and chemotherapy. Complete surgical
excision was considered to be too complex, as the tumor border was
unclear. HTH experimental therapy was recommended and the animal
was enrolled in the clinical trial, with the owners’ written
informed consent. A tissue ablation device for veterinary medicine
(AMTC 200; AdMeTech Co., Ltd., Ehime, Japan) was used to administer
the HTH treatment. On day 0, HTH therapy was performed with no
anesthesia or sedation. Three needles of the device were inserted
into the tumor tissue at 6-mm intervals and the HTH therapy was
performed for 10 min at 65°C. On day 21, the tumor volume had
decreased from that which was observed on day 0, and the subject
had regained improved function of the limb (Fig. 1B). Following four weeks of HTH
therapy, the tumor disappeared.
Case 2
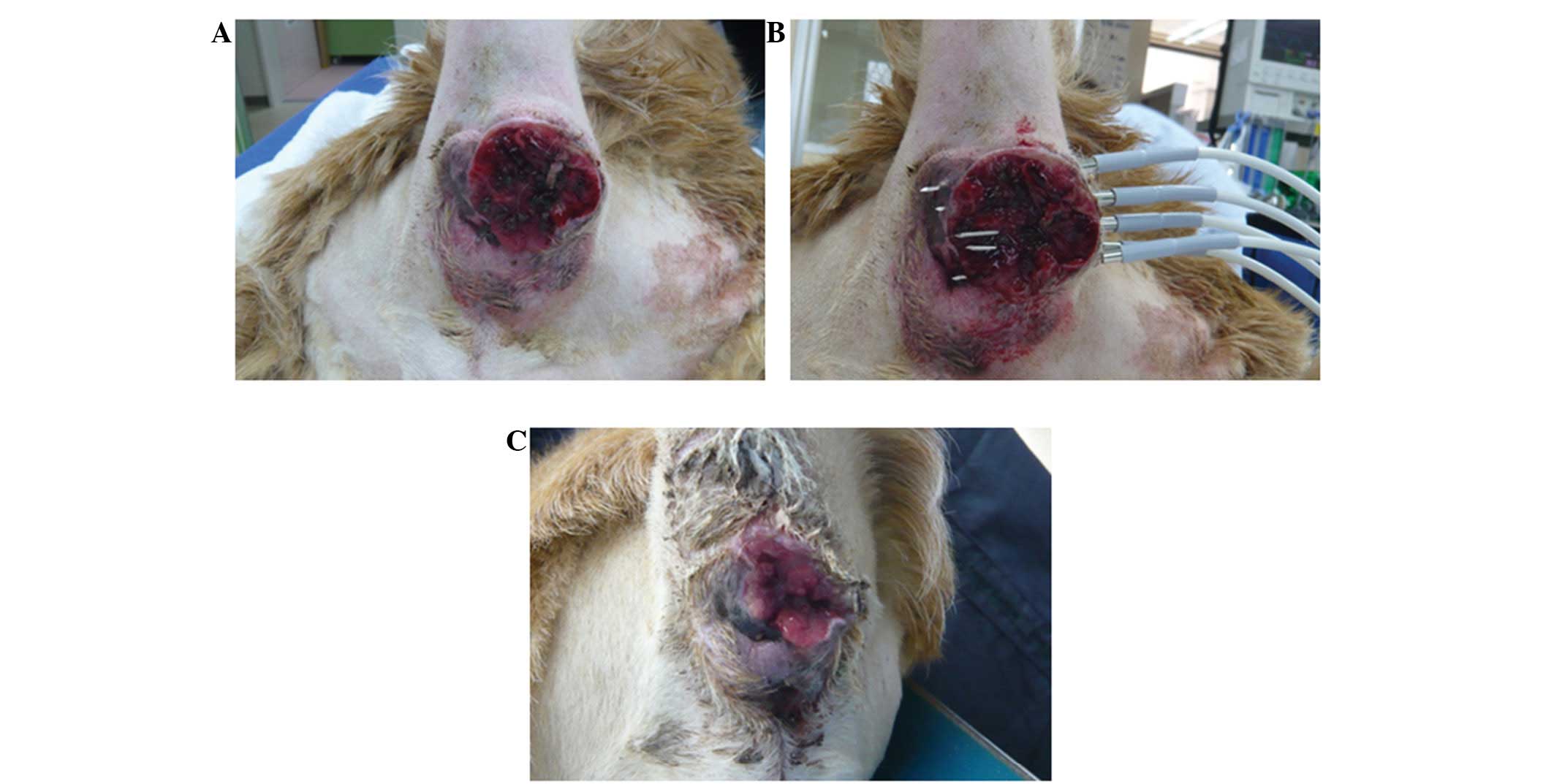
A 14-year-old male Golden Retriever (weight, 32.7
kg) was referred to the Takagi Animal Clinic (Saijo, Japan) in
February, 2011 for the evaluation of a tumor surrounding the anus
(Fig. 2A). Biopsy and
histopathological analysis identified the tumor as a perianal gland
adenocarcinoma. The risk of recurrence and the treatment options
were described to the owners, which included surgery, radiation
therapy and chemotherapy. Complete surgical excision was considered
to be too difficult, as the tumor border was unclear. HTH
experimental therapy was recommended and the animal was enrolled in
the clinical trial, with the owners’ written informed consent. On
day 0, HTH therapy was performed under general anesthesia, which
was administered by inhalation of isoflurane. Five needles of the
device were inserted into the tumor at 1-cm intervals, and HTH was
performed for 10 min at 65°C (Fig.
2B) and repeated one additional time. On day 21, the tumor
volume had decreased from that which was observed on day 0
(Fig. 2C). HTH therapy was repeated
using the same protocol, however, the dog succumbed one week later
due to old age.
Case 3
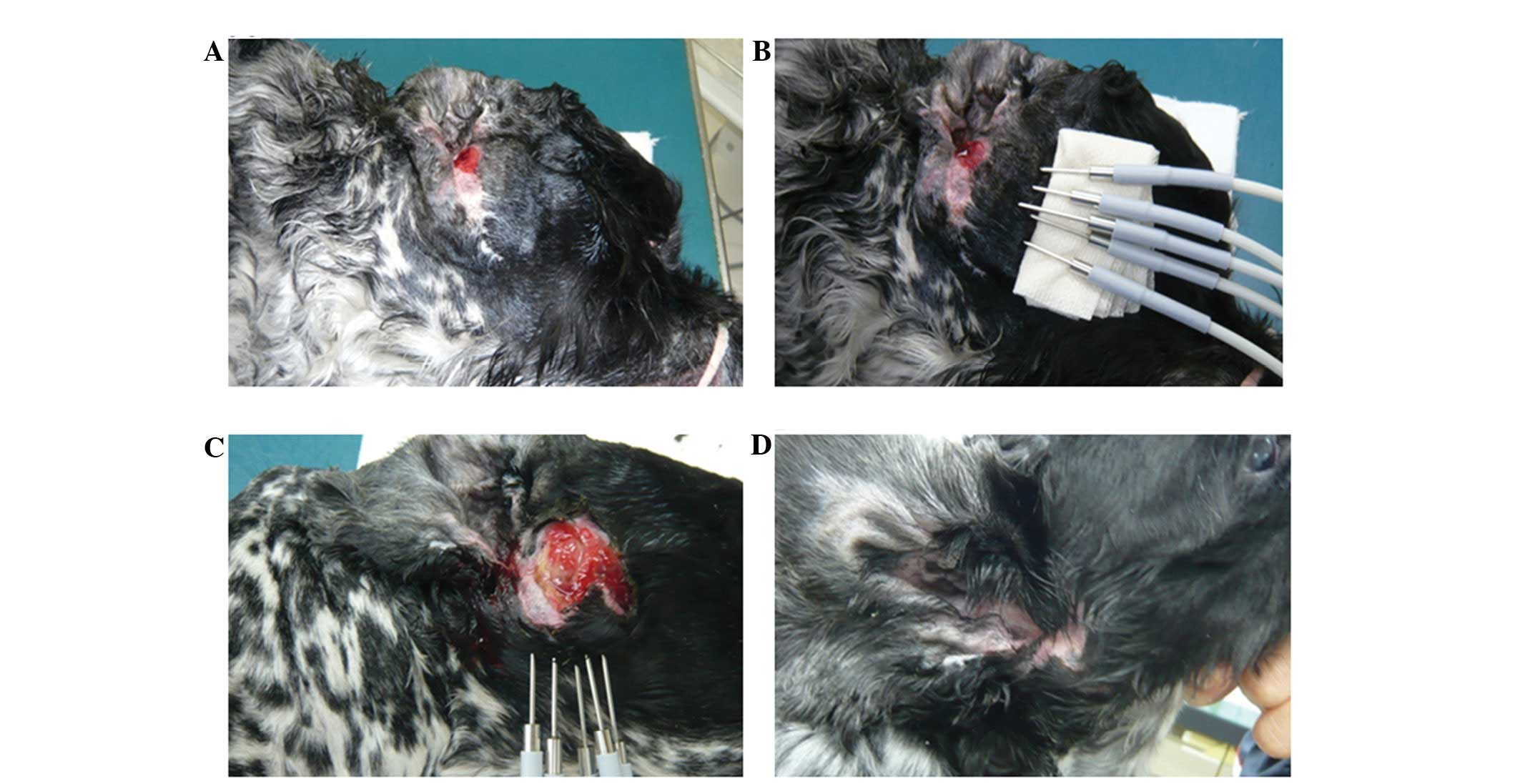
A 13-year-old male English Cocker Spaniel (weight,
12.3 kg) was referred to the Takagi Animal Clinic in February, 2011
for the evaluation of a tumor in the right external auditory canal
(Fig. 3A). A right total ear canal
ablation was performed and subsequent histopathological analysis
revealed a ceruminous adenocarcinoma. Two months after the
intervention, the tumor recurred at the surgical site. The risk of
recurrence and the treatment options were explained to the owner,
which included surgery, radiation therapy and chemotherapy.
Specifically, surgery presented the risks of vestibular disorders
and facial paralysis. HTH experimental therapy was recommended and
the animal was enrolled in a clinical trial, with the owner’s
written informed consent. On day 0, HTH therapy was performed under
general anesthesia, which was maintained using inhaled isoflurane.
Five needles of the device were inserted into the tumor and HTH
therapy was performed for 10 min at 65°C (Fig. 3B). On day 22, the tumor volume had
decreased from that which was observed on day 0. On day 28, the HTH
therapy was repeated using the same protocol. On day 78, the tumor
volume had decreased further and a third HTH procedure was
performed. On day 133, the tumor had disappeared and did not
recur.
Discussion
To the best of our knowledge, the beneficial effects
of HTH therapy for the treatment of superficial tumors have not yet
been reported in veterinary medicine. The HTH protocol used in the
current study was simple to conduct and was only performed on
spontaneous tumors that had presented in canines. In the three
cases presented, the tumor volumes decreased following HTH therapy;
furthermore, no severe side effects were observed in any of the
cases.
In recent years, various innovative and minimally
invasive cancer therapies have been developed as alternatives to
surgery. Ablation, which uses high temperatures, radio waves or
microwaves, is considered to be a potent alternative therapeutic
strategy (14).
High temperatures (>46°C) directly damage cells,
resulting in severe protein denaturation and DNA damage (15,16),
which induces irreversible changes that ultimately result in cell
death. Tumor cells express specific tumor-associated antigens and
in high temperature conditions (>46°C), the tumor cells swell
and break into fragments, which releases antigens; this large
antigen load generates antitumor immunity. The high temperatures
also lead to severe protein denaturation that appears to destroy
the immunogenicity of tumor cells (17–21).
When thermal ablation temperatures (>70°C) are achieved, there
is a high risk of shock syndrome that is induced by the sudden and
large production of necrotic tumor material (22). Therefore, the case for ablation
therapy in medicine is limited. Ablation therapy is commonly
performed on tumors measuring ≤3 cm in diameter (23). In the present cases, tumor sizes
were >3 cm in diameter, although this was not measured
precisely. In our previous study, it was reported that HTH therapy
administered at temperatures between 50 and 70°C induces necrosis
and apoptosis in a rat glioma model (13). However, HTH therapy at 50°C did not
exert adequate suppressive effects when compared with treatment at
60 and 70°C. The present results coincide with our previous data.
The optimal therapeutic protocol, including the effective
temperature, time and frequency must be established in order to
extend the application of HTH therapy for routine use in veterinary
oncology.
In conclusion, HTH treatment is a simple therapeutic
option with no severe side effects and is expected to become a
useful alternative therapy for superficial tumors in companion
animals.
References
|
1
|
Soares PI, Ferreira IM, Igreja RA, Novo CM
and Borges JP: Application of hyperthermia for cancer treatment:
recent patents review. Recent Pat Anticancer Drug Discov. 7:64–73.
2012.
|
|
2
|
Wust P, Hildebrandt B, Sreenivasa G, et
al: Hyperthermia in combined treatment of cancer. Lancet Oncol.
3:487–497. 2002.
|
|
3
|
Falk MH and Issels RD: Hyperthermia in
oncology. Int J Hyperthermia. 17:1–18. 2001.
|
|
4
|
Ross MI: Current status of hyperthermic
limb perfusion for in-transit melanoma. Int J Hyperthermia.
24:205–217. 2008.
|
|
5
|
Pennacchioli E, Fiore M and Gronchi A:
Hyperthermia as an adjunctive treatment for soft-tissue sarcoma.
Expert Rev Anticancer Ther. 9:199–210. 2009.
|
|
6
|
Brewer WG Jr and Turrel JM: Radiotherapy
and hyperthermia in the treatment of fibrosarcomas in the dog. J Am
Vet Med Assoc. 181:146–150. 1982.
|
|
7
|
Page RL and Thrall DE: Clinical
indications and applications of radiotherapy and hyperthermia in
veterinary oncology. Vet Clin North Am Small Anim Pract.
20:1075–1092. 1990.
|
|
8
|
Gillette EL: Hyperthermia effects in
animals with spontaneous tumors. Natl Cancer Inst Monogr.
61:361–364. 1982.
|
|
9
|
Grier RL, Brewer WG Jr and Theilen GH:
Hyperthermic treatment of superficial tumors in cats and dogs. J Am
Vet Med Assoc. 177:227–233. 1980.
|
|
10
|
Stojkovic R and Radacic M: Cell killing of
melanoma B16 in vivo by hyperthermia and cytotoxins. Int J
Hyperthermia. 18:62–71. 2002.
|
|
11
|
Ito A, Fujioka M, Yoshida T, et al:
4-S-Cysteaminylphenol-loaded magnetite cationic liposomes for
combination therapy of hyperthermia with chemotherapy against
malignant melanoma. Cancer Sci. 98:424–430. 2007.
|
|
12
|
Haen SP, Pereira PL, Salih HR, Rammensee
HG and Gouttefangeas C: More than just tumor destruction:
immunomodulation by thermal ablation of cancer. Clin Dev Immunol.
2011:1602502011.
|
|
13
|
Takagi H, Azuma K, Tsuka T, Imagawa T,
Osaki T and Okamoto Y: Anti-tumor effects of high-temperature
hyperthermia on a glioma rat model. Oncol Lett. 7:1007–1010.
2014.
|
|
14
|
Baisi A, De Simone M, Raveglia F and
Cioffi U: Thermal ablation in the treatment of lung cancer: present
and future. Eur J Cardiothorac Surg. 43:683–686. 2013.
|
|
15
|
Diederich CJ: Thermal ablation and
high-temperature thermal therapy: overview of technology and
clinical implementation. Int J Hyperthermia. 21:745–753. 2005.
|
|
16
|
Roti Roti JL: Cellular responses to
hyperthermia (40–46 degrees C): cell killing and molecular events.
Int J Hyperthermia. 24:3–15. 2008.
|
|
17
|
den Brok MH, Sutmuller RP, van der Voort
R, Bennink EJ, Figdor CG, Ruers TJ and Adema GJ: In situ tumor
ablation creates an antigen source for the generation of antitumor
immunity. Cancer Res. 64:4024–4029. 2004.
|
|
18
|
Baronzio G, Gramaglia A and Fiorentini G:
Hyperthermia and immunity. A brief overview. In Vivo. 20:689–695.
2006.
|
|
19
|
Zerbini A, Pilli M, Penna A, et al:
Radiofrequency thermal ablation of hepatocellular carcinoma liver
nodules can activate and enhance tumor-specific T-cell responses.
Cancer Res. 66:1139–1146. 2006.
|
|
20
|
Mukhopadhaya A, Mendecki J, Dong X, et al:
Localized hyperthermia combined with intratumoral dendritic cells
induces systemic antitumor immunity. Cancer Res. 67:7798–7806.
2007.
|
|
21
|
Zhang HG, Mehta K, Cohen P and Guha C:
Hyperthermia on immune regulation: a temperature’s story. Cancer
Lett. 271:191–204. 2008.
|
|
22
|
Moroz P, Jones SK and Gray BN:
Magnetically mediated hyperthermia: current status and future
directions. Int J Hyperthermia. 18:267–284. 2002.
|
|
23
|
Wiggermann P, Puls R, Vasilj A, Sieroń D,
Schreyer AG, Jung EM, Wawrzynek W and Stroszczynski C: Thermal
ablation of unresectable liver tumors: factors associated with
partial ablation and the impact on long-term survival. Med Sci
Monit. 18:CR88–CR92. 2012.
|