Introduction
Malignant gastrointestinal neuroectodermal tumor
(GNET), named by Stockman et al (1) in 2012, is a rare tumor of the
gastrointestinal tract. It has been previously referred to as clear
cell sarcoma-like gastrointestinal tumor (CCSLGT) (1,2) or
clear cell sarcoma-like tumor with osteoclast-like giant cells of
the gastrointestinal tract (3–5), and
is also commonly reported in the literature as clear cell sarcoma
of the gastrointestinal tract (CCS-GI) (6–15).
Clear cell sarcoma (CCS) was initially described by Enzinger
(16) in 1965, and often occurs in
the distal limb deep soft tissue, particularly in tendons and
aponeuroses; therefore, it is also known as clear cell sarcoma of
the tendons and aponeuroses (16,17).
Subsequently, researchers have demonstrated that the tumor has
obvious characteristics of melanocytic differentiation, but differs
from malignant melanoma with respect to clinical, genetic and
biological factors. Therefore, in 1983, CSS was renamed as
malignant melanoma of soft parts by Chung and Enzinger (18).
In 1993, Ekfors et al (17) reported a case of CCS in the
duodenum, which was the first visceral case reported. Following
this, in 1998, Kothaj et al (19) reported the initial case of CCS of
the stomach. Subsequently, a number of CCS cases in the
gastrointestinal tract were reported successively, the majority of
which lacked melanocytic differentiation features, and were
commonly reported as CCSLGT. Stockman et al (1) retrospectively analyzed 16 cases of
CCSLGT and observed that the tumor exhibited neural differentiation
potential; therefore, the authors suggested GNET as a more
appropriate name for this tumor type, an assessment that we agree
with. The current study reports a case of GNET in the stomach and
reviews the literature, focusing on similar cases and tumor
classification. Written informed consent was obtained from the
patient’s family.
Case report
Clinical features
A 17-year-old male was admitted to the Department of
Gastrointestinal Surgery at the First Affiliated Hospital of Bengbu
Medical College (Bengbu, China) with a two-month history of
fatigue, discontinuous low temperature and anemia. The patient
initially felt weakness in the limbs, which was particularly
apparent following physical activity and, subsequently, weakness
and fatigue affected the whole body. Concomitantly, the patient
exhibited mild symptoms of abdominal distension and melena. On
examination, a hemoglobin level of 66 g/l was recorded (normal
reference range, 110–160 g/l), therefore, blood transfusion therapy
was administered; however, no clear response was observed.
Subsequently, positron emission tomography-computed tomography
examination revealed a soft tissue mass in the gastric antrum,
which exhibited increased fluoride deoxidization glucose. The
gastroscopy results revealed irregular hyperplasia at the gastric
antrum, as well as ulcers and signs of necrosis. Due to these
observations, a radical distal gastric resection was performed.
During the surgery, several swollen lymph nodes were identified and
dissected; these were located under the pylorus and around the
common hepatic artery, left gastric artery and celiac artery.
Gross and histological features
Pathological examination of the resected stomach
specimen revealed a gray ulcerated mass, measuring 6.0×4.0×3.5
cm3. The microscopic examination demonstrated that the
tumor had invaded the serosa layer of the stomach. No tumor tissue
was apparent in the swollen lymph nodes. The medium-sized and
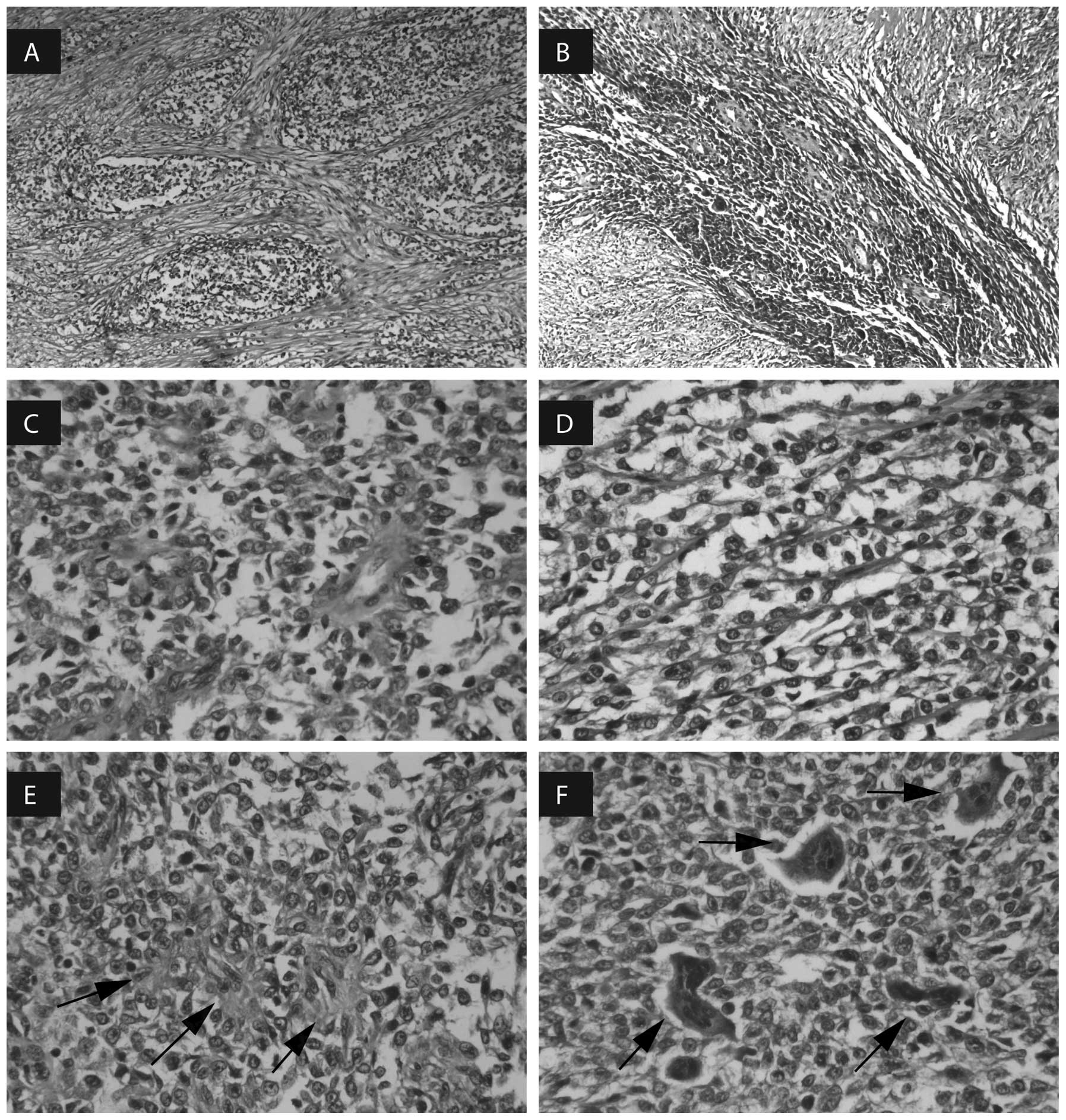
round, oval or spindle-shaped tumor cells were arranged in a nest,
and focally formed fasciculate, pseudopapillary, pseudoalveolar and
rosette-like growth patterns (Fig.
1A–E) surrounded by fibrous connective tissue. A number of
multinucleated osteoclast-like giant cells were also identified
(Fig. 1F), composed of five to 20
nuclei, which was the most prominent morphological characteristic.
The tumor cells consisted of weak eosinophilic cytoplasm,
vacuolated nuclear chromatin and basophilic nucleoli.
Immunohistochemical and molecular genetic
features
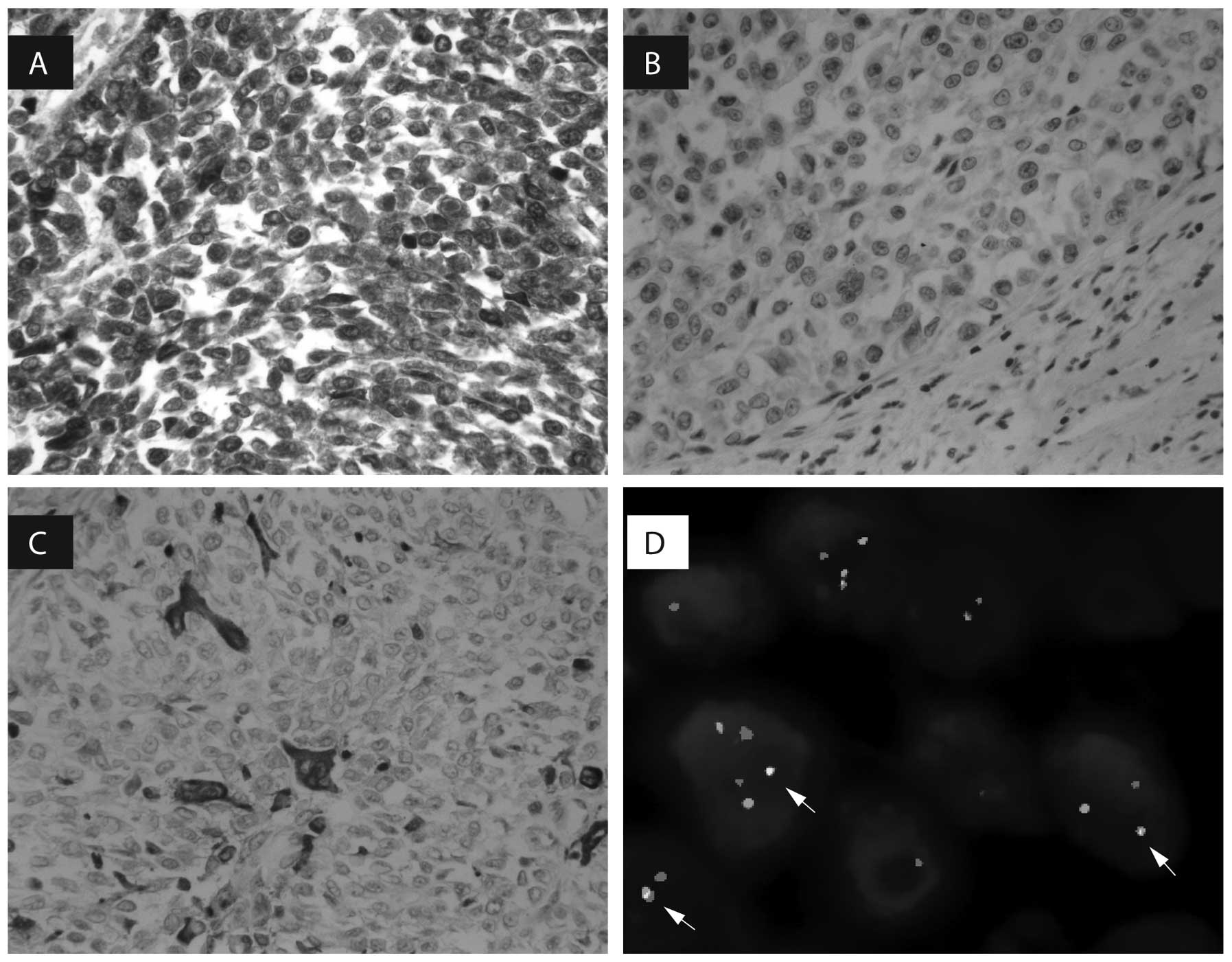
The tumor cells were diffusely positive for S-100
protein (Fig. 2A), strongly
positive for vimentin, and focally positive for BCL-2 and CD57. By
contrast, the tumor cells were negative for HMB45 and Melan-A,
which are markers of melanocytic differentiation (Fig. 2B). The osteoclast-like giant cells
were positive for CD68 (Fig. 2C).
In addition, several other indicators were negative, including
cytokeratin, smooth muscle actin, desmin, CD117, CD34, MyoD1, CD99,
calponin, WT-1, CD21, CD23, CD35, D2-40, CD1α, EMA, synaptophysin,
CD56, neuron-specific enolase, CD30 and ALK1. Furthermore, 5–10% of
tumor cells exhibited Ki-67 expression. At the genetic level,
fluorescence in situ hybridization demonstrated EWSR1
gene rearrangement. The proportion of cells exhibiting an abnormal
signal indicating the genetic disruption of EWSR1 was 71%
(Fig. 2D).
Discussion
In a review of the literature, the majority of
CCS-GI cases were found to be cases of GNET; however, we
hypothesize that GNET and CCS represent two distinct tumor types.
Where CCS tumors are arranged in nests or fascicles (16) and have multinucleated Touton-like
giant cells (2), GNETs exhibit
alternative arrangements in addition to nests and fascicles,
including a number of multinucleated osteoclast-like giant cells.
Immunohistochemically, CCS tumors have been demonstrated to express
melanocytic differentiation-related markers, including HMB45,
Melan-A and MiTF (2,20,21),
which are not a characteristic of GNET. CCS tumors have been
associated with a balanced chromosome translocation
t(12;22)(q13;q12), which results in the fusion of EWSR1
(located at 22q12) and ATF1 (located at 12q13) (2). GNETs also exhibit EWSR1 gene
rearrangements, confirming that this is not a tumor-specific
characteristic. Furthermore, such rearrangements have been detected
in Ewing’s sarcoma (22),
angiomatoid fibrous histiocytoma (23), primary pulmonary myxoid sarcoma
(23) and hyalinizing clear cell
carcinoma of the salivary gland (23).
A search of the relevant literature revealed a total
of 39 published case reports that may be considered as GNET,
occurring in the stomach (eight cases) (1,4,5,7,10),
ileum (14 cases) (1,4,11–13,15,22),
jejunum (nine cases) (1,3,4,6,8,14),
colon (three cases) (1,22) and small intestine (five cases)
(1,12). Overall, GNETs (including the present
case) span a wide age range of 10–81 years (median, 36 years; mean,
39 years) and the male to female ratio is 18:22. Patients commonly
exhibit symptoms of abdominal pain and abdominal distension or
weight loss, and a few patients present with anemia, melena, fever
or other symptoms. The mean tumor size is 5.49 cm (range, 2.4–15.0
cm). Histologically, GNET consists of epithelioid or
oval-to-spindle tumor cells arranged in sheets or nests, which
focally form pseudoalveolar, pseudopapillary, microcystic,
fascicular, cord-like or rosette-like growth patterns. A number of
multinucleated osteoclast-like giant cells have also been observed.
GNETs express a primitive neural phenotype, such as positivity for
S-100 protein, SOX10, NSE, synaptophysin, CD56 and NB84, with no
expression of melanocytic markers. Notably, BCL-2 was positively
expressed in the present case. Under the electron microscope, GNET
exhibits evidence of neural differentiation, including multiple
interdigitating cell processes containing dense core granules and
clear vesicles resembling synaptic bulbs (1). At the molecular genetic level, GNET is
associated with EWSR1 gene rearrangements, which results in
the fusion of EWSR1 and ATF1, or EWSR1 and
CREB1 (1,22,23).
The characteristics of the case reported in the current study are
consistent with those of other GNET cases.
The various diagnoses of GNET include
gastrointestinal stromal tumor (GIST), alveolar rhabdomyosarcoma,
synovial sarcoma and malignant peripheral nerve sheath tumor
(MPNST). However, characteristic properties of each diagnosis have
been observed. GIST is positive for CD34 and CD117, alveolar
rhabdomyosarcoma is positive for desmin and MyoD1, and is
associated with a t(2;13)(q35;q14) chromosome translocation and
synovial sarcoma often expresses the epithelial membrane antigens,
CK7, CK19, and CD99. Furthermore, almost all synovial sarcomas
exhibit the constant translocation t(X;18)(p11;q11). MPNST is
positive for the S-100 protein, Leu-7, PGP9.5 and myelin basic
protein. The NF1 gene inactivation may be used to confirm
the diagnosis of MPNST. The most common treatment for patients with
GNET is excision of the tumor. Following the initial surgical
resection, five of 40 cases exhibited liver metastasis (3,4,8,11,22),
whereas 19 of 40 cases showed lymph node metastasis at the time of
diagnosis (1,4, 5,12–14,22).
In total, eight of 40 cases succumbed to the disease (1,4). After
10 months of follow-up, no evidence of recurrence or metastasis was
identified in the present case.
In general, the diagnosis of GNET is based on the
histological, immunohistochemical and molecular genetic features.
The information presented in this study contributes further much
required knowledge of GNET, which may aid in the diagnosis,
treatment and prognosis of the tumor; however, clinical data from
additional patients are required due to the rarity of the
tumor.
Acknowledgements
The authors are grateful to Professor Zenong Cheng
at the Department of Pathology (Bengbu Medical College) for the
excellent technical assistance. This study was supported by the
Natural Science Foundation of Anhui Province (grant no.
1208085MH152).
Abbreviations:
|
GNET
|
malignant gastrointestinal
neuroectodermal tumor
|
|
CCSL-GI
|
clear cell sarcoma-like
gastrointestinal tumor
|
|
CCS-GI
|
clear cell sarcoma of the
gastrointestinal tract
|
|
CCS
|
clear cell sarcoma
|
|
GIST
|
gastrointestinal stromal tumor
|
|
MPNST
|
malignant peripheral nerve sheath
tumor
|
References
|
1
|
Stockman DL, Miettinen M, Suster S, et al:
Malignant gastrointestinal neuroectodermal tumor:
clinicopathologic, immunohistochemical, ultrastructural, and
molecular analysis of 16 cases with a reappraisal of clear cell
sarcoma-like tumors of the gastrointestinal tract. Am J Surg
Pathol. 36:857–868. 2012.
|
|
2
|
Kosemehmetoglu K and Folpe AL: Clear cell
sarcoma of tendons and aponeuroses, and osteoclast-rich tumour of
the gastrointestinal tract with features resembling clear cell
sarcoma of soft parts: a review and update. J Clin Pathol.
63:416–423. 2010.
|
|
3
|
Friedrichs N, Testi MA, Moiraghi L, et al:
Clear cell sarcoma-like tumor with osteoclast-like giant cells in
the small bowel: further evidence for a new tumor entity. Int J
Surg Pathol. 13:313–318. 2005.
|
|
4
|
Zambrano E, Reyes-Mugica M, Franchi A and
Rosai J: An osteoclast-rich tumor of the gastrointestinal tract
with features resembling clear cell sarcoma of soft parts: reports
of 6 cases of a GIST simulator. Int J Surg Pathol. 11:75–81.
2003.
|
|
5
|
Huang WB, Zhang XH, Li DJ, et al:
Osteoclast-rich tumor of the gastrointestinal tract with features
resembling those of clear cell sarcoma of soft parts. Virchows
Arch. 448:200–203. 2006.
|
|
6
|
Lasithiotakis K, Protonotarios A, Lazarou
V, Tzardi M and Chalkiadakis G: Clear cell sarcoma of the jejunum:
a case report. World J Surg Oncol. 11:17–21. 2013.
|
|
7
|
Pauwels P, Debiec-Rychter M, Sciot R,
Vlasveld T, den Butter B, Hagemeijer A and Hogendoorn PC: Clear
cell sarcoma of the stomach. Histopathology. 41:526–530. 2002.
|
|
8
|
D’Amico FE, Ruffolo C, Romeo S, Massani M,
Dei Tos AP and Bassi N: Clear cell sarcoma of the ileum: report of
a case and review of the literature. Int J Surg Pathol. 20:401–406.
2012.
|
|
9
|
Taminelli L, Zaman K, Gengler C, et al:
Primary clear cell sarcoma of the ileum: an uncommon and misleading
site. Virchows Arch. 447:772–777. 2005.
|
|
10
|
Lagmay JP, Ranalli M, Arcila M and Baker
P: Clear cell sarcoma of the stomach. Pediatr Blood Cancer.
53:214–216. 2009.
|
|
11
|
Yang JC, Chou AJ, Oeffinger KC, La Quaglia
MP and Wolden SL: Clear cell sarcoma of the gastrointestinal tract
after very low-dose therapeutic radiation therapy: a case report. J
Pediatr Surg. 47:1943–1945. 2012.
|
|
12
|
Shenjere P, Salman WD, Singh M, et al:
Intra-abdominal clear-cell sarcoma: a report of 3 cases, including
1 case with unusual morphological features, and review of the
literature. Int J Surg Pathol. 20:378–385. 2012.
|
|
13
|
Venkataraman G, Quinn AM, Williams J and
Hammadeh R: Clear cell sarcoma of the small bowel: a potential
pitfall. Case report. APMIS. 113:716–719. 2005.
|
|
14
|
Joo M, Chang SH, Kim H, Gardner JM and Ro
JY: Primary gastrointestinal clear cell sarcoma: report of 2 cases,
one case associated with IgG4-related sclerosing disease, and
review of literature. Ann Diagn Pathol. 13:30–35. 2009.
|
|
15
|
Comin CE, Novelli L, Tornaboni D and
Messerini L: Clear cell sarcoma of the ileum: report of a case and
review of literature. Virchows Arch. 451:839–845. 2007.
|
|
16
|
Enzinger FM: Clear-cell sarcoma of tendons
and aponeuroses an analysis of 21 cases. Cancer. 18:1163–1174.
1965.
|
|
17
|
Ekfors TO, Kujari H and Isomäki M: Clear
cell sarcoma of tendons and aponeuroses (malignant melanoma of soft
parts) in the duodenum: the first visceral case. Histopathology.
22:255–259. 1993.
|
|
18
|
Chung EB and Enzinger FM: Malignant
melanoma of soft parts. A reassessment of clear cell sarcoma. Am J
Surg Pathol. 7:405–413. 1983.
|
|
19
|
Kothaj P, Turcan I, Marko L, Cunderlík P,
Fukal J and Okapec S: Malignant melanoma of soft parts (clear cell
sarcoma) - a rare case of multiorgan localization. Rozhl Chir.
77:328–333. 1998.(In Slovak).
|
|
20
|
Hisaoka M, Ishida T, Kuo TT, et al: Clear
cell sarcoma of soft tissue: a clinicopathologic,
immunohistochemical, and molecular analysis of 33 cases. Am J Surg
Pathol. 32:452–460. 2008.
|
|
21
|
Fukuda T, Kakihara T, Baba K, Yamaki T,
Yamaguchi T and Suzuki T: Clear cell sarcoma arising in the
transverse colon. Pathol Int. 50:412–416. 2000.
|
|
22
|
Antonescu CR, Nafa K, Segal NH, Dal Cin P
and Ladanyi M: EWS-CREB1: a recurrent variant fusion in clear cell
sarcoma - association with gastrointestinal location and absence of
melanocytic differentiation. Clin Cancer Res. 12:5356–5362.
2006.
|
|
23
|
Thway K and Fisher C: Tumors with
EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg
Pathol. 36:e1–e11. 2012.
|