Introduction
Lung cancer remains the leading cause of
cancer-related mortality worldwide, and is particularly prevalent
in males (1). In China, the
prevalence of lung cancer has grown rapidly over the past five
years (2). The main types of lung
caner are small-cell lung carcinoma (SCLC) and non-small-cell lung
carcinoma (NSCLC). Among all lung cancer cases, almost 85% are
NSCLC (3), which is further
categorized into two predominant types: Non-squamous carcinoma
(including adenocarcinoma, large-cell carcinoma and other cell
types) and squamous cell carcinoma. Despite recent advances in the
diagnosis and treatment of lung cancer, the improvement in survival
rate has only been modest, with an overall five-year survival rate
of <15% (4). Cancer cell
invasion, identified in 30–40% of NSCLC patients with poor
prognosis, is a critical determinant of survival. Therefore,
elucidating the potential biological markers of metastasis is
urgently required for guidance on postoperative surveillance and
therapeutic decisions.
The migration and invasion inhibitor protein (MIIP),
also known as invasion inhibitory protein 45 (IIp45), is a recently
characterized putative tumor suppressor gene in glioma (5). MIIP was initially identified in a
yeast two-hybrid screen for proteins that interact with protein
insulin-like growth factor binding protein 2 (IGFBP2) (6). MIIP is located on chromosome 1p36.22
and recent genome analysis has determined that MIIP contains 10
exons that span 12.6 kb genomic DNA. The full-length transcript
contains 1,588 bp. The MIIP protein is comprised of 388 amino acids
and has a predicted molecular weight of 43 kDa. MIIP is a
hydrophilic protein and contains three segments of low
compositional complexity domains and an arginine-glycine-aspartate
motif (7). The 1p36 region is
deleted in a number of types of cancer, including neuroblastoma,
breast cancer, colon and rectum cancer, and prostate cancer, as
well as lung cancer (8–12). Recently, MIIP has emerged as a key
protein in regulating cell migration, cell invasion and the mitosis
checkpoint and, thus, may exert a critical role in cancer
physiology (6,13). However, MIIP expression profiles
have, to the best of our knowledge, not been described in NSCLC.
The present study aimed to detect MIIP expression using real-time
polymerase chain reaction (PCR) and immunohistochemistry (IHC)
methods in resected tissue samples and formalin-fixed paraffin
sections from patients with NSCLC. Whether MIIP expression
correlated with pathological and clinical features was also
evaluated. In addition, the association between MIIP expression and
the five-year overall survival rate was analyzed.
Materials and methods
Study population and samples
Fresh tumor tissues and matched normal tissues from
37 NSCLC patients (diagnosed in 2011 and 2012) were immediately
transferred to liquid nitrogen and stored at −80°C for subsequent
real-time PCR analysis, and 94 formalin-fixed paraffin-embedded
samples of NSCLC tissues (patients diagnosed in 2007 and 2008) were
obtained for immunohistochemical analysis. All patients were
diagnosed in the First Affiliated Hospital of Liaoning Medical
University (Jinzhou, China) and samples from the patients were
stored in the pathology archive. Permission from patients was
obtained prior to specimen collection. None of the patients had
received chemotherapy, radiotherapy or immunotherapy prior to
surgery. The study was approved by the Ethics Committee of the
First Affiliated Hospital of Liaoning Medical University.
Histopathological evaluation was conducted independently by two
pathologists. All cases were classified according to the World
Health Organization revised proposal for histological types of lung
and pleural tumors (14). TNM
patient evaluation was performed according to the criteria
indicated in the staging procedures of the International
Association for the Study of Lung Cancer (15). The patients enrolled in the IHC
analysis were followed-up for five years to determine survival
time, which was defined as the time period between the date of
surgery on the primary tumor and when the patient succumbed to
disease or the date of the final follow-up. Patient survival times
were individually provided by family members by telephone. The
clinicopathological data are summarized in Table I.
 | Table IClinicopathological features of the
NSCLC patients. |
Table I
Clinicopathological features of the
NSCLC patients.
| Total no. of patients
(n) |
|---|
|
|
|---|
| Clinicopathological
feature | Real-time PCR
analysis (n=37) | Immunohistochemical
analysis (n=94) |
|---|
| Gender |
| Male | 19 | 54 |
| Female | 18 | 40 |
| Age (years) |
| <60 | 17 | 43 |
| ≥60 | 20 | 51 |
| Pathology |
| Adenocarcinoma | 19 | 45 |
| Squamous cell
carcinoma | 18 | 49 |
| Differentiation
status |
| Well | 14 | 29 |
| Moderate | 11 | 43 |
| Poor | 12 | 22 |
| Tumor staging |
| IA–IB | 14 | 30 |
| IIA–IIB | 17 | 45 |
| IIIA | 6 | 19 |
Real-time PCR
Total RNA was isolated from the frozen NSCLC and
matched normal tissues using the TRIzol RNA kit (Invitrogen,
Carlsbad, CA, USA) and quantified using an Eppendorf Biophotometer
(Eppendorf, Hamburg, Germany). Reverse transcription was conducted
using a PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio,
Inc., Shiga, Japan). Under the thermal conditions recommended by
the manufacturer, 2 μg total RNA was transcribed to cDNA in a 20 μl
reaction using random hexamers. The following primers were used for
quantification of MIIP mRNA expression levels: Forward, 5′-GGT CCA
TCC TGG CTC AAC AGA-3′ and reverse, 5′-GCA ATC CAG TCA TAG CCC AGG
TA-3′. The length of the PCR product was 118 bp. Real-time PCR was
performed using Mastercycler® ep realplex (Eppendorf,
Hamburg, Germany) and the SYBR® Premix Ex
Taq™ kit (Takara Bio., Inc.). GAPDH served as a control
with the following primers used: Forward, 5′-GCA CCG TCA AGG CTG
AGA AC-3′ and reverse, 5′-TGG TGA AGA CGC CAG TGG A-3′, with the
length of PCR product at 138 bp. PCR was run for 40 cycles with 5
sec per 95°C denaturation, 30 sec/55°C annealing and 30 sec/72°C
elongation. To verify the accuracy of the amplification, a melting
curve analysis was conducted subsequent to amplification. In
addition, the PCR products were verified by electrophoretic
analysis on a 3% agarose gel. To determine the relative expression
levels of MIIP, the comparative Ct method was used. The Ct value of
the target gene was normalized to that of the endogenous reference
[ΔCT = CT(target) − CT(GAPDH)] and compared with a calibrator [ΔΔCT
= ΔCT(target) − ΔCT (calibrator)]. The relative expression levels
of the target gene were calculated via the 2−ΔΔCT
method.
Immunohistochemical staining for
MIIP
MIIP protein expression on the formalin-fixed
paraffin sections was determined by IHC. Briefly, 5-μm tissue
sections were dewaxed in xylene, rehydrated and incubated in 0.3%
(v/v) hydrogen peroxide in 0.01 M phosphate-buffered saline (PBS;
pH 7.6) for 20 min to inactivate endogenous peroxidase. Antigen
retrieval was performed by heating the sections with 10 mm citrate
buffer (pH 6.0) for 15 min in a microwave. The sections were
incubated with rabbit anti-human polyclonal primary anti-MIIP
antibody (1:300; Sigma-Aldrich, St. Louis, MO, USA) at 4°C
overnight, washed in PBS three times for 15 min and incubated with
horseradish peroxidase/Fab Polymer-conjugated mouse anti-rabbit
monoclonal secondary antibody (Zhongshan Biotechnology Inc., China)
for 30 min at room temperature. Thereafter, the antibody was
revealed by incubation with diaminobenzidine at room temperature
for 1 min. The sections were counterstained with hematoxylin,
dehydrated and mounted. The MIIP immunoreactivity level was
classified using the proportion of positive cells: 0, <5%
positive cells; 1+, 5–30% positive cells; 2+, 31–50% positive
cells; and 3+, >50% positive cells. The intensity of MIIP
expression was scored as follows: 0, negative to weak; 1, moderate;
and 2, strong. The final staining score was the sum of the
intensity and the percentage of positive cells scores. A score of
≤1 was applied as a cut-off point for loss of MIIP expression.
Statistical analysis
The real-time PCR values are presented as mean ±
standard error of the mean, and MIIP protein expression was
dichotomized as either ‘negative’ or ‘positive’, according to the
criteria described above. A two-sample t-test for independent
samples was used for continuous variables. The correlation between
MIIP expression and clinicopathological characteristics was then
analyzed using the χ2-test. One-way analysis of variance
was used to compare the means of two or more independent groups.
The survival curves for patient with MIIP-positive and -negative
tumors were plotted using the Kaplan-Meier method and the log-rank
test was used to assess the statistical difference between the
groups. Univariate and multivariate analyses were conducted using
the Cox proportional-hazards regression model, and the results are
expressed as hazard ratios with 95% confidence intervals.
Two-tailed P<0.05 was considered to indicate a statistically
significant difference. All analyses were performed using SPSS for
Windows, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Expression levels of MIIP mRNA in NSCLC
and adjacent normal lung samples
The MIIP mRNA expression levels in cancer tissues
and matched normal tissues from 37 NSCLC patients were examined
using the ΔΔCT method. Melting curve analysis confirmed the
specific amplification of the target and reference genes.
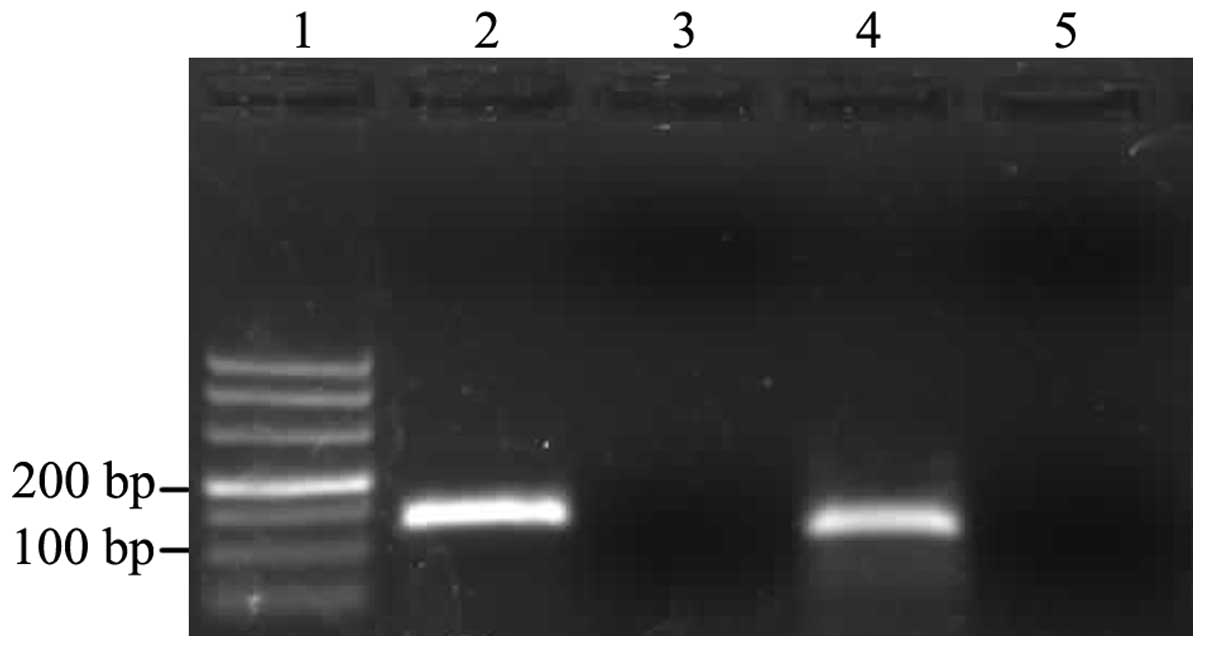
Furthermore, gel electrophoresis analysis of the amplification
products revealed single bands of the expected sizes for MIIP (118
bp) and GAPDH (138 bp) (Fig. 1).
The results demonstrated that MIIP expression was downregulated in
the cancer tissues. The average MIIP mRNA expression level in the
37 cancer tissue samples was 0.1867±0.0217.
Correlation between MIIP mRNA expression
levels and various clinicopathological parameters
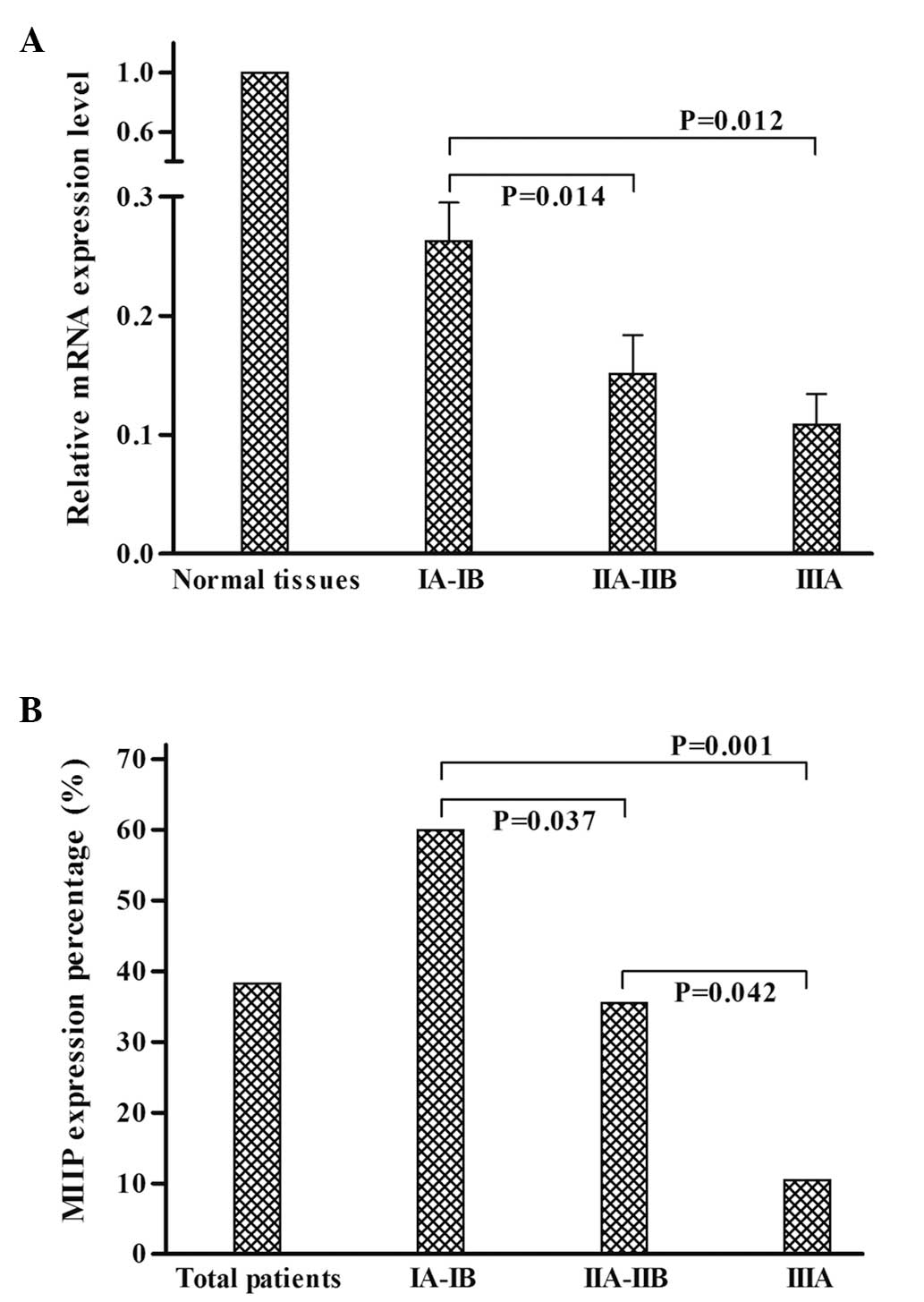
The association between MIIP mRNA expression levels
in the cancer tissues and various clinicopathological parameters
was further analyzed. The results revealed that the MIIP mRNA
expression levels in adenocarcinoma were significantly higher than
those in squamous cell carcinoma (P=0.002). A statistically
significant correlation was also observed between MIIP mRNA
expression and tumor stage (P=0.014), with reduced MIIP mRNA
expression levels in advanced tumor stage samples, as compared with
specimens from tumors at the lower stages (Fig. 2A). However, no significant
correlations were observed between MIIP expression levels and
gender, age or differentiation status (all P>0.05), as shown in
Table II.
 | Table IICorrelation between MIIP mRNA
expression and clinicopathological features. |
Table II
Correlation between MIIP mRNA
expression and clinicopathological features.
| Clinicopathological
feature | No. of patients | MIIP expression level
(mean ± SE) | P-value |
|---|
| Gender | | | 0.832 |
| Male | 19 | 0.1822±0.0306 | |
| Female | 18 | 0.1916±0.0315 | |
| Age (years) | | | 0.265 |
| <60 | 17 | 0.1642±0.0281 | |
| ≥60 | 20 | 0.2133±0.0334 | |
| Pathology | | | 0.002 |
| Adenocarcinoma | 19 | 0.2488±0.0282 | |
| Squamous cell
carcinoma | 18 | 0.1213±0.0257 | |
| Differentiation
status | | | 0.435 |
| Well | 14 | 0.2117±0.0356 | |
| Moderate | 11 | 0.1990±0.0460 | |
| Poor | 12 | 0.1464±0.0316 | |
| Tumor staging | | | 0.014 |
| IA–IB | 14 | 0.2631±0.0319 | |
| IIA–IIB | 17 | 0.1513±0.0321 | |
| IIIA | 6 | 0.1091±0.0253 | |
Correlation between MIIP protein
expression and various clinicopathological parameters
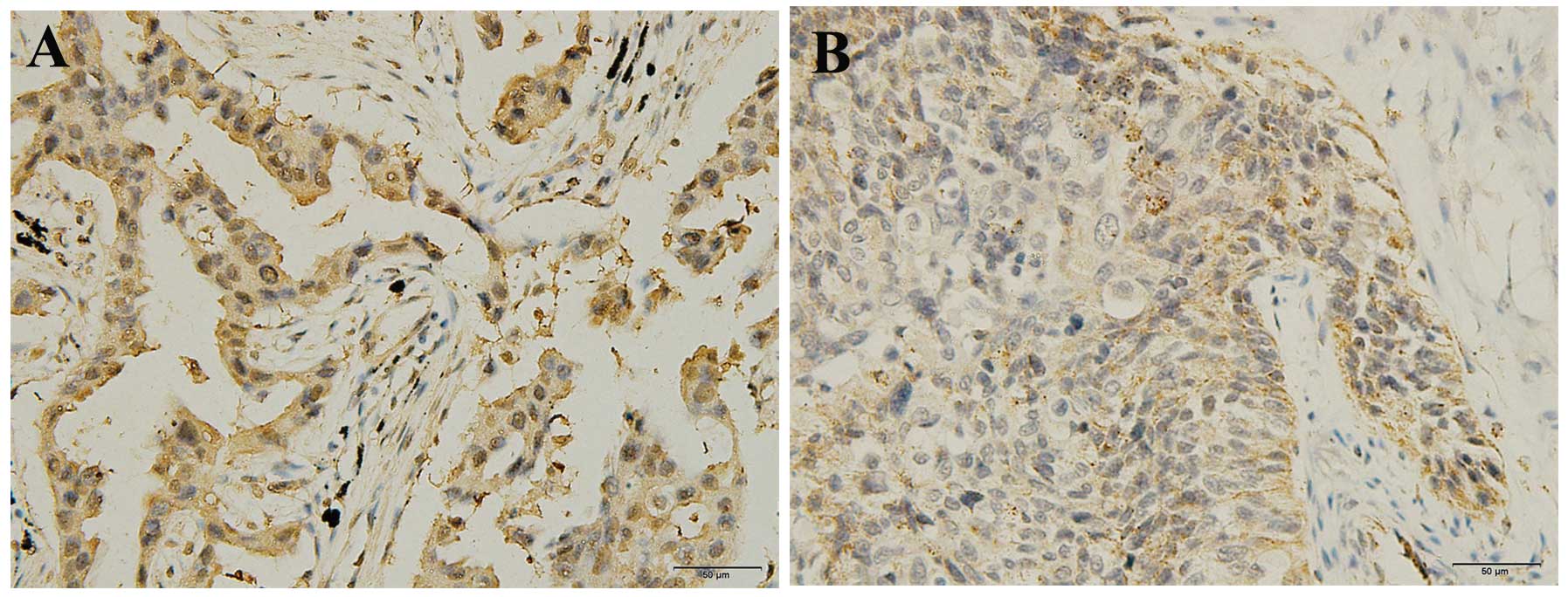
Immunohistochemical staining of MIIP in the cancer
tissue sections was conducted. Immunoreactivity for the MIIP
antibody was predominantly identified in the cytoplasm of cancer
cells with marginal immunoreactivity in the nucleus (Fig. 3). Among the 94 tissue samples, MIIP
protein expression was positive in 36 (38.3%) and negative in 58
(61.7%) cases. The results revealed that MIIP protein expression
was downregulated in cancer tissues, a finding in concordance with
the mRNA expression result. The association between MIIP expression
in NSCLC tissues and various clinicopathological parameters was
also analyzed. Analysis of MIIP expression revealed that expression
was significantly associated with pathology and tumor staging
(P=0.014 and P=0.002, respectively), but not with gender, age or
NSCLC differentiation status (all P>0.05), as shown in Table III. To determine whether the
downregulation of MIIP protein expression was correlated with
disease progression, the staining degrees within tumor staging
groups were compared. The results demonstrated that the positive
percentage of MIIP protein expression was significantly reduced in
advanced tumor stage samples, as compared with specimens from less
advanced tumors (Fig. 2B; Table III).
 | Table IIICorrelation between MIIP3 protein
expression and various clinicopathological parameters. |
Table III
Correlation between MIIP3 protein
expression and various clinicopathological parameters.
| | MIIP expression | |
|---|
| |
| |
|---|
| Clinicopathological
feature | No. of patients | Positive (n=36) | Negative (n=58) | P-value |
|---|
| Gender | | | | 0.250 |
| Male | 54 | 18 | 36 | |
| Female | 40 | 18 | 22 | |
| Age | | | | 0.133 |
| <60 | 43 | 20 | 23 | |
| ≥60 | 51 | 16 | 35 | |
| Pathology | | | | 0.014 |
| Adenocarcinoma | 45 | 23 | 22 | |
| Squamous cell
carcinoma | 49 | 13 | 36 | |
| Differentiation
status | | | | 0.680 |
| Well | 29 | 13 | 16 | |
| Moderate | 43 | 15 | 28 | |
| Poor | 22 | 8 | 14 | |
| Tumor staging | | | | 0.002 |
| IA–IB | 30 | 18 | 12 | |
| IIA–IIB | 45 | 16 | 29 | |
| IIIA | 19 | 2 | 17 | |
Survival analysis
The total follow-up time period for the patients who
were alive at the time of analysis was five years. A total of 66
(70.2%) of the 94 patients succumbed to disease during the
follow-up period. The five-year survival rate within the patient
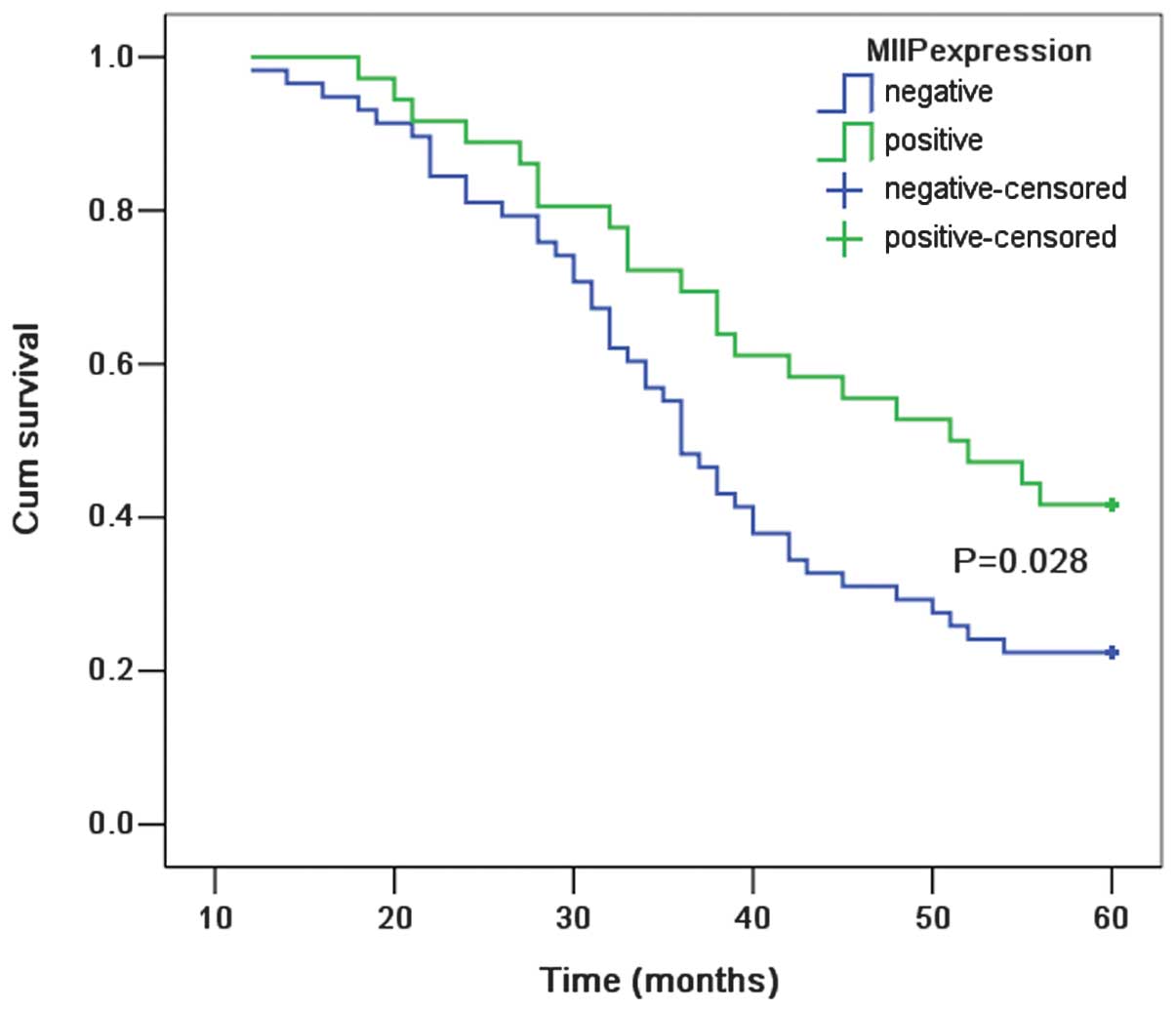
population was 29.8%. With regard to MIIP expression status, the
five-year survival rate for patients who were MIIP-positive was
41.7% as compared with 22.4% for patients who were MIIP-negative.
According to the Kaplan-Meier survival analysis, the overall
survival rate curve demonstrated statistically significant
differences between NSCLC patients with and without MIIP expression
(P=0.028; Fig. 4). Cox
proportional-hazards regression model was employed to perform
univariate and multivariate analysis of survival. The results
revealed that positive MIIP expression is an independent and
significant factor associated with improved five-year survival. The
detailed results are shown in Table
IV.
 | Table IVUnivariate and multivariate analyses
of MIIP protein expression with regard to overall survival. |
Table IV
Univariate and multivariate analyses
of MIIP protein expression with regard to overall survival.
| Overall survival |
|---|
|
|
|---|
| Variables | HRa | 95% CIb | P-value |
|---|
| Univariate
analysis | | 0.337–0.954 | 0.033 |
| Negative
expression | 1.000 | | |
| Positive
expression | 0.567 | | |
| Multivariate
analysis | | 0.205–0.963 | 0.040 |
| Negative
expression | 1.000 | | |
| Positive
expression | 0.444 | | |
Discussion
Cell motility is important for normal tissue
development and remodeling as well as for pathological conditions,
such as cancer invasion and metastasis (16). Lung cancer is the leading cause of
cancer-related mortality. Approximately 90% of all cancer mortality
is the result of metastases, rather than the primary tumor
(17). Thus, further understanding
of the underlying pathways and molecular mechanisms of lung cancer
metastasis is required to develop novel therapeutic approaches.
MIIP has emerged as a key protein in regulating cell migration and
invasion (6). However, the
importance of MIIP in NSCLC is unknown. The present study aimed to
examine the expression of MIIP in NSCLC with respect to prognosis.
The results of real-time PCR and immunohistochemical staining
clearly demonstrated that MIIP expression was downregulated in
cancer tissues, as compared with matched normal tissues, a finding
consistent with the results previously reported in a study
analyzing glioblastoma multiforme (6). In addition, in the present study, MIIP
expression levels were significantly correlated with pathology and
tumor staging, suggesting a potential role for MIIP protein in the
pathogenesis of NSCLC. Furthermore, the prognostic significance of
MIIP expression in formalin-fixed paraffin-embedded tissues was
examined. Notably, patients with positive MIIP expression had a
significantly improved overall survival rate (P=0.028). A
significant association was also observed between positive MIIP
expression and improved prognosis using univariate and multivariate
analyses. These results suggest that MIIP may be a functional
genetic marker of NSCLC development and prognosis, and that MIIP
may be an attractive therapeutic target in the treatment of lung
cancer.
The MIIP protein has been previously demonstrated to
bind to a product of an oncogene, IGFBP-2, which is commonly
upregulated in the advanced stages of cancer, and to inhibit the
migration- and invasion-enhancing functions of IGFBP-2 (6). Studies have revealed that MIIP
expression levels were reduced in glioblastoma multiforme and that
IIp45 expression levels were low in advanced glioma (6,18). In
the present study, similar results were observed in NSCLC. The data
demonstrate that MIIP expression levels were significantly
correlated with tumor staging, and reduced MIIP mRNA and protein
expression levels were detected in advanced tumor stage samples.
The MIIP protein has been previously observed to inhibit glioma
cell migration and invasion in vitro and in vivo, and
a xenograft model study revealed that tumors formed from
MIIP-expressing cells were also less invasive as compared with the
controls (6). Another study
reported that MIIP mediates histone deacetylase 6 activity, which
regulates microtubule dynamics/cytoskeletal structure, increases
cell migration and increases acetylated alpha-tubulin (18). In addition to an antimigration and
-invasion function, MIIP has also been shown to be involved in
mitosis (13), as elevated MIIP
expression levels inhibited glioma cell colony formation and cell
growth in vitro, and MIIP expression in a glial-specific
mouse model suppressed glioma development and progression (13). Epidemiology studies have provided
further evidence that MIIP is important in cancer development.
Several MIIP single nucleotide polymorphisms (SNPs) were recently
evaluated in a molecular epidemiology study involving 1,524 breast
cancer patients and 1,592 healthy females, which found that the
rs2295283 (K167E) SNP was not only associated with breast cancer
risk but also with various tumor phenotypes in cancer patients
(19). All these results from
recent studies raise the possibility that MIIP is a putative
tumor-suppressor gene, critical in cancer physiology.
In conclusion, the present study has revealed that
MIIP was downregulated in NSCLC tissues. According to the survival
analysis, MIIP expression was an independent prognostic factor
associated with improved survival. All data suggest that MIIP is a
tumor suppressor gene, with a critical role in NSCLC physiology.
Since the present study was a preliminary investigation, the number
of patients with NSCLC was relatively small (94 patients). Further
experiments are required in order to investigate the exact
mechanism of MIIP involvement in lung carcinogenesis. The
significant association with survival rate observed in the present
study requires confirmation in additional patient cohorts.
Acknowledgements
This study was supported by the Natural Science
Foundation of Liaoning Province of China (grant no. 201102122) and
the Scientific and Technological Project of Liaoning Province
(grant no. 2013225002 ).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
|
|
2
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250. 2005.
|
|
3
|
Esposito L, Conti D, Ailavajhala R, Khalil
N and Giordano A: Lung Cancer: Are we up to the Challenge? Curr
Genomics. 11:513–518. 2010.
|
|
4
|
Alberg AJ, Ford JG and Samet JM; American
College of Chest Physicians. Epidemiology of lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132(3 Suppl): 29S–55S. 2007.
|
|
5
|
Song SW, Fuller GN, Zheng H and Zhang W:
Inactivation of the invasion inhibitory gene IIp45 by alternative
splicing in gliomas. Cancer Res. 65:3562–3567. 2005.
|
|
6
|
Song SW, Fuller GN, Khan A, et al: IIp45,
an insulin-like growth factor binding protein 2 (IGFBP-2) binding
protein, antagonizes IGFBP-2 stimulation of glioma cell invasion.
Proc Natl Acad Sci USA. 100:13970–13975. 2003.
|
|
7
|
Wang Y, Wen J and Zhang W: MIIP, a
cytoskeleton regulator that blocks cell migration and invasion,
delays mitosis, and suppresses tumorogenesis. Curr Protein Pept
Sci. 12:68–73. 2011.
|
|
8
|
Munirajan AK, Ando K, Mukai A, et al:
KIF1Bbeta functions as a haploinsufficient tumor suppressor gene
mapped to chromosome 1p36.2 by inducing apoptotic cell death. J
Biol Chem. 283:24426–24434. 2008.
|
|
9
|
Fujita T, Igarashi J, Okawa ER, et al:
CHD5, a tumor suppressor gene deleted from 1p36.31 in
neuroblastomas. J Natl Cancer Inst. 100:940–949. 2008.
|
|
10
|
Ragnarsson G, Eiriksdottir G,
Johannsdottir JT, et al: Loss of heterozygosity at chromosome 1p in
different solid human tumours: association with survival. Br J
Cancer. 79:1468–1474. 1999.
|
|
11
|
Gibbs M, Stanford JL, McIndoe RA, et al:
Evidence for a rare prostate cancer-susceptibility locus at
chromosome 1p36. Am J Hum Genet. 64:776–787. 1999.
|
|
12
|
Yanada M, Yaoi T, Shimada J, et al:
Frequent hemizygous deletion at 1p36 and hypermethylation
downregulate RUNX3 expression in human lung cancer cell lines.
Oncol Rep. 14:817–822. 2005.
|
|
13
|
Ji P, Smith SM, Wang Y, et al: Inhibition
of gliomagenesis and attenuation of mitotic transition by MIIP.
Oncogene. 29:3501–3508. 2010.
|
|
14
|
Shimosato Y: Histological Typing of Lung
and Pleural Tumors (3rd edition, 1999): Malignant epithelial
tumors. Nihon Rinsho. 60(Suppl 5): 123–131. 2002.(In Japanese).
|
|
15
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
|
|
16
|
Cunha-Ferreira I, Bento I and
Bettencourt-Dias M: From zero to many: control of centriole number
in development and disease. Traffic. 10:482–498. 2009.
|
|
17
|
Mehlen P and Puisieux A: Metastasis: a
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
|
|
18
|
Wu Y, Song SW, Sun J, et al: IIp45
inhibits cell migration through inhibition of HDAC6. J Biol Chem.
285:3554–3560. 2010.
|
|
19
|
Song F, Ji P, Zheng H, et al: Definition
of a functional single nucleotide polymorphism in the cell
migration inhibitory gene MIIP that affects the risk of breast
cancer. Cancer Res. 70:1024–1032. 2010.
|