Introduction
Renal cell carcinoma (RCC) is the third most common
urological cancer (1) and exhibits
the highest mortality rate. Clear cell renal cell carcinoma (CCRCC)
is the largest subtype of RCC and accounts for ~85% of RCC cases
(2). Half of patients suffer from
metastatic disease; the five-year survival rate for these patients
is <10% and long-term remission is infrequent (3,4).
Distant metastases and local recurrence are the main causes of
fatalities following curative resection. Therefore, the
identification of novel predictive and prognostic markers may
result in targeted adjuvant therapies, and thus improve the
prognosis in patients with early postoperative recurrence in
CCRCC.
The vascular endothelial growth factor (VEGF)
signaling pathway is important in tumor angiogenesis (5); inhibition of the pathway is currently
a clinically approved and widely used therapy for cancer. Anti-VEGF
therapy with bevacizumab has been shown to increase overall
survival (OS) and/or progression-free survival (PFS) times in
colorectal, breast and lung cancer patients (6). However, inherent or acquired
resistance to anti-VEGF therapy is frequently observed in tumors
(6), thus demonstrating the
requirement for targeting additional angiogenesis pathways to fully
exploit the strategies of anti-angiogenic cancer therapy. Notably,
the Notch signaling pathway affects numerous biological processes,
as well as cell fate determination, which has recently emerged as a
critical regulator of developmental and tumor angiogenesis
(7). In mammalian cells, Notch
signaling mediators include five transmembrane Notch ligands
[Jagged 1, Jagged 2, delta-like ligand 1 (Dll1), Dll3 and Dll4] and
four Notch receptors (Notch 1–4) (8). Recently, Dll4 signaling through the
corresponding Notch1 receptor has been identified as a critical
component of physiological and pathological neovascularization
(9). Dll4 is specifically induced
by VEGF and functions as a negative angiogenesis regulator
downstream of VEGF (10,11). Consistent with these findings,
suppression of Dll4 inhibits tumor growth by promoting excessive
and non-productive angiogenesis, even in tumors resistant to
anti-VEGF therapy (9,12). In humans, Dll4 expression has been
identified in tumors from the kidney, bladder, colon, brain and
breast (13–16). In CCRCC, Dll4 expression was
confined to the vasculature and was detected at levels nine-fold
higher than those in the normal kidney (13,17).
Previous studies have confirmed that Dll4 expression is an
independent indicator of poor prognosis in several types of human
malignancy, including lung, breast, pancreatic and bladder cancer
(14,15,18,19).
However, to the best of our knowledge, no study has been conducted
to evaluate the prognostic value of Dll4 expression levels in
patients with CCRCC. Therefore, in the present study, the
expression levels of Dll4 in CCRCC were investigated, and an
initial evaluation was conducted to analyze whether the presence of
high Dll4 expression levels was correlated with poor prognosis
following curative resection. In addition, the associations among
Dll4 expression levels, VEGF receptor-2 (VEGFR-2) expression levels
and tumor progression in patients with CCRCC were assessed.
Materials and methods
Patients and specimens
The study procedure was approved by the ethics
committee of The Second Hospital of Lanzhou University (Lanzhou,
China). For western blotting, fresh tumor tissues (later verified
as CCRCC) and adjacent normal renal tissues were obtained
intra-operatively from four patients who underwent radical
nephrectomy at the Department of Urology in The Second Hospital of
Lanzhou University. The tissue samples were then snap-frozen in
liquid nitrogen and stored at −80°C until analysis. In addition,
121 paraffin-embedded CCRCC specimens (57 male and 64 female) and
65 normal renal tissue specimens were obtained from patients with
resectable CCRCC. All patients underwent nephrectomy (partial or
radical) during hospitalization at the Department of Urology, The
Second Hospital of Lanzhou University between January 1, 2001 and
December 31, 2010. Any cases of other types of renal carcinoma were
excluded from this study. Additional exclusion criteria were a
history of another type of cancer, and preoperative radiation or
chemotherapy. All potentially eligible patients were interviewed to
obtain written informed consent prior to surgery. The tumor stage
was determined using the 2009 TNM staging classification system
(20). The tumor grade was
determined using the Fuhrman classification system
(well-differentiated, grades 1 and 2; moderately differentiated,
grade 3; and poorly differentiated, grade 4) (21). Patient profiles are summarized in
Table I.
 | Table ICorrelation between Dll4 expression
levels and clinicopathological factors. |
Table I
Correlation between Dll4 expression
levels and clinicopathological factors.
| | Dll4 expression
levels | |
|---|
| |
| |
|---|
| Parameters | N | High (53) | Low (68) | P-value |
|---|
| Age |
| ≤Mean | 70 | 29 | 41 | 0.538 |
| >Mean | 51 | 24 | 27 | |
| Gender |
| Male | 57 | 22 | 35 | 0.276 |
| Female | 64 | 31 | 33 | |
| Size, mm |
| ≤70 | 77 | 33 | 44 | 0.782 |
| >70 | 44 | 20 | 24 | |
| Metastasis |
| Metastasis | 29 | 18 | 11 | 0.012 |
| No metastasis | 92 | 35 | 57 | |
| T-stagea |
| T1+T2 | 82 | 31 | 51 | 0.023 |
| T3+T4 | 39 | 22 | 17 | |
| Gradeb |
| 1+2 | 68 | 24 | 44 | 0.033 |
| 3+4 | 53 | 29 | 24 | |
| VEGFR-2 |
| High | 75 | 42 | 33 | 0.001 |
| Low | 46 | 11 | 35 | |
Western blot analysis
The four paired samples of CCRCC tissues and
adjacent normal renal tissues were solubilized in lysis buffer on
ice. All lysates were centrifuged at 4°C for 10 min and total
proteins were extracted from the tissues by a Keygen total protein
extraction kit (SJ-200501; Nanjing Keygen Biotech. Co., Ltd,
Nanjing, China) according to the manufacturer’s instructions and
stored in a −80°C freezer until further use. For western blotting,
80 μg protein was separated by SDS-PAGE and transferred to
polyvinylidene difluoride (PVDF) membranes, which were then blocked
in 5% fat-free milk at room temperature for 2 h. Following
incubation with polyclonal rabbit anti-human Dll4 antibody (Ab7280;
dilution 1:500; Abcam, Cambridge, UK) at 4°C overnight and three
washes for 15 min in 1X Tris-buffered saline, the PVDF membrane was
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit polyclonal secondary antibodies (Ab137913; dilution
1:5,000; Abcam) at room temperature for 1 h. Subsequently, the
membranes were washed three times, for 5 min each, with 1X
Tris-buffered saline and the chemiluminescent HRP substrate was
added to the PVDF membrane. The membranes were then exposed to
X-ray medical films (Carestream Health, Inc., Rochester, NY, USA)
in the dark. Immunoreactive proteins were visualized using an
enhanced chemiluminescence HRP substrate (Millipore, Billerica, MA,
USA) and polyclonal rabbit anti-human β-actin antibody (Ab75186;
dilution 1:1,000; Abcam) served as a control for protein
loading.
Immunohistochemistry
Immunohistochemistry was performed using standard
techniques. All tissues were cut into 0.5×0.5×0.5 cm blocks, fixed
in 10% formalin solution and dehydrated through graded alcohol
(70–100%), for 1 h respectively. The tissues were then cleared in
xylene for 1 h. Parrafin wax immersion and embedding were conducted
at 54°C for 1 h, then the blocks were cooled to room temperature
and subjected to serial sections. For microscopic observation, the
paraffin-embedded tissue sections, 4 mm in size, were dewaxed in
xylene and rehydrated in graded alcohol and stained using
hematoxylin and eosin. Endogenous peroxidase was inhibited using 3%
hydrogen peroxide. Antigen retrieval was performed by boiling the
tissue sections in citrate buffer (pH 6.0) for 10 min. Non-specific
protein binding was conducted by incubating the sections with goat
serum (Hyclone, Logan, UT, USA) for 30 min incubations. These
treatments were alternated with rinses in phosphate-buffered saline
(PBS). The sections were then incubated with primary antibodies
against Dll4 (rabbit polyclonal; Ab7280; dilution 1:100; Abcam) and
VEGFR-2 (rabbit monoclonal; 55B11; dilution 1:100; Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C, respectively,
washed three times in PBS, and incubated with secondary goat
anti-rabbit polyclonal antibody (Ab137913; dilution 1:5,000; Abcam)
conjugated to horseradish peroxidase (pv6001) for 30 min at room
temperature. The membranes were washed again with PBS and then
incubated with 3,3′-diaminobenzidine (dilution 1:20) stain,
followed by counterstaining with hematoxylin blue. Non-neoplastic
renal tissues from the same sample served as a control, aiming to
omit the primary antibody in all cases.
Immunostaining evaluation
The slides were independently evaluated under a
Leica DMLP light microscope equipped with a Leica DFC camera (Leica
Mikrosysteme Vertrieb GmbH, Wetzlar, Germany) by two pathologists
(Professors Li and Shi) with no prior knowledge of the clinical
data. Dll4 expression was localized to the tumor vasculature. For
tumor vasculature, the brown particles were considered to signify
positive cells. A semiquantitative scoring system was developed to
evaluate staining intensity (0, negative; 1, weak; 2, moderate and
3, strong) and the percentage of positive cells (0, 0% cells; 1,
≤25% positive cells; 2, 26–50% positive cells and 3, >50%
positive cells). In each slide, three fields were evaluated and the
two scores were added: Low expression was designated as a total
score of 0–3 and high expression as a total score of 4–6. The
tumors were thus subdivided according to the protein expression
levels in the different groups.
Statistical analysis
The associations between Dll4 expression levels and
CCRCC clinicopathological features were assessed by the
χ2 test. The OS time period was measured as the time
period between the date of surgery and the date the patient
succumbed to disease or the date of the final follow-up. The PFS
time period was measured as the time period between the date of
surgery and either the date of disease relapse, the date the
patient succumbed to disease or the date of the final follow-up. OS
and PFS curves were calculated using the Kaplan-Meier method and
compared by the log-rank test. The Cox regression model was used
for multivariate analysis. All statistical analyses were performed
using the SPSS version 18.0 statistical software package (SPSS,
Inc., Chicago, IL, USA) and the quantification of the western blot
bands was performed using Image J software (NIH, Bethesda, MD,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 121 CCRCC patients (males, 57; females,
64) aged between 38 and 84 years (mean, 62 years) were included in
the present study. According to the pathological tumor grading
classification, 22 patients were considered to have poorly
differentiated tumors, 31 to have moderately differentiated tumors
and 68 to have well-differentiated tumors. With regard to the
T-staging of the tumors, 54 tumors were considered as T1, 28 as T2,
29 as T3 and 10 as T4. The mean follow-up duration at the time of
analysis was 48.6 months (range, 6–96 months). A total of 29
metastatic CCRCC cases and 92 non-metastatic cases were identified.
Demographic and clinicopathological variables within the cohort are
summarized in Table I.
Dll4 expression levels in CCRCC specimens
and non-cancerous tissues
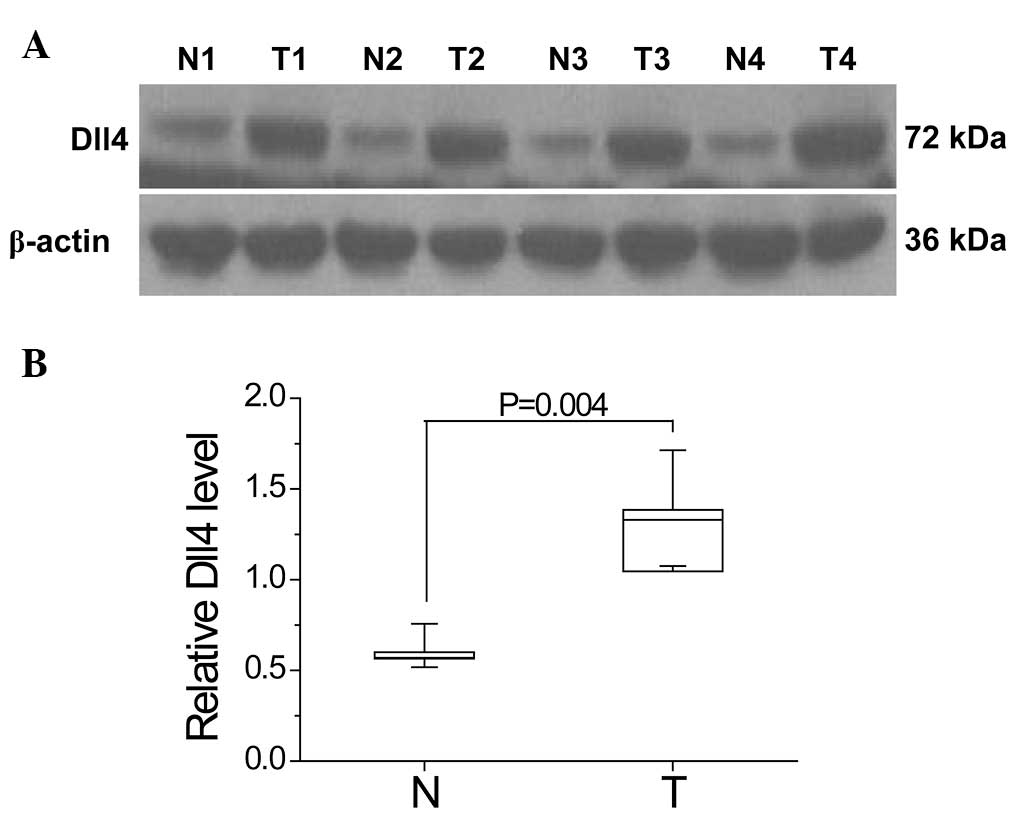
Western blot analysis was performed to analyze the
differences in Dll4 expression levels between CCRCC and
non-cancerous tissues in four CCRCC patients who had undergone
radical nephrectomy. The results show that Dll4 expression levels
were significantly increased in the CCRCC tissues compared with the
non-cancerous tissues (P=0.004; Fig.
1).
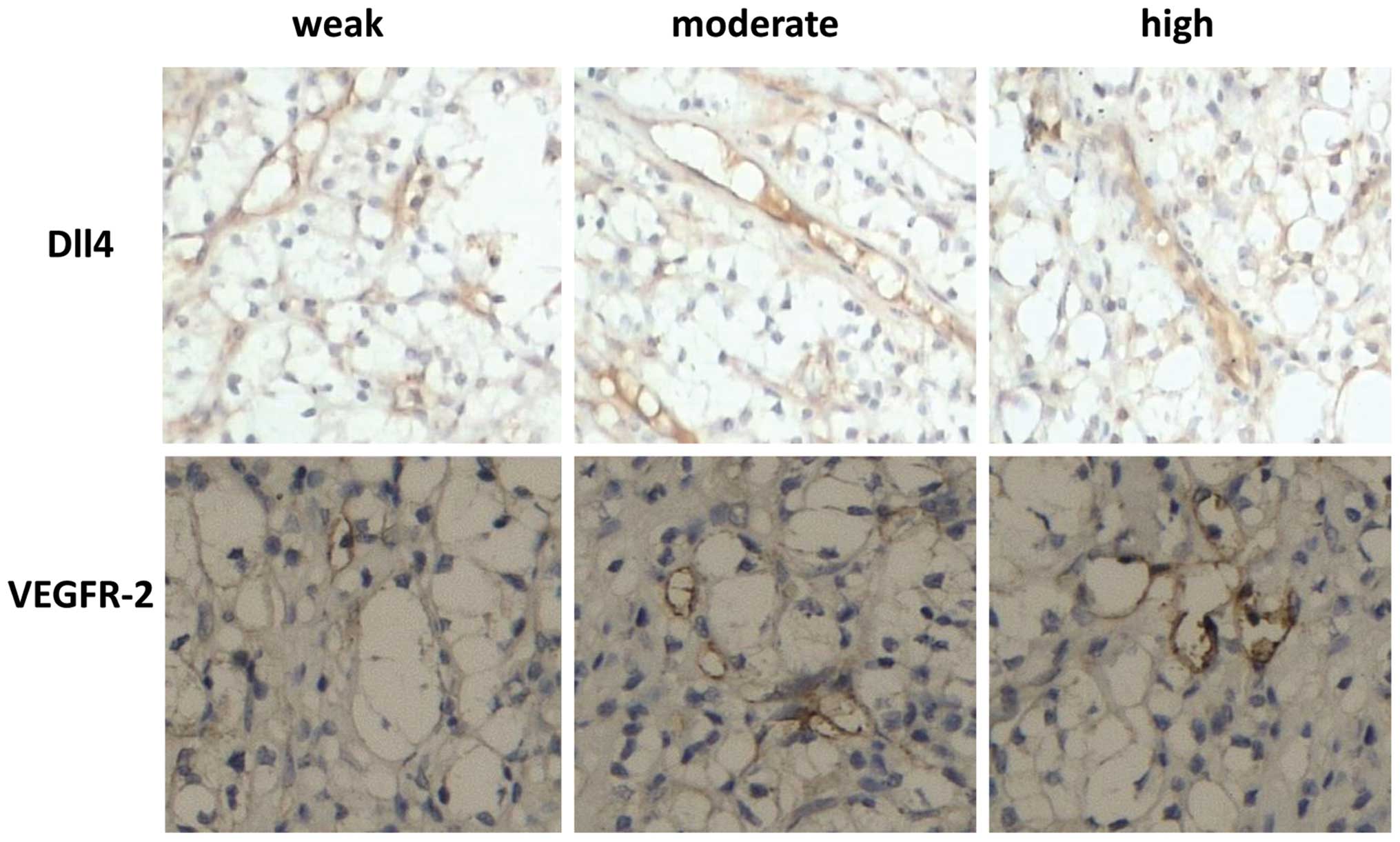
Immunohistochemical analysis was performed to
demonstrate the presence and location of Dll4 in 121 CCRCC and 65
non-cancerous tissue specimens from CCRCC patients. As shown in
Fig. 2, Dll4 and VEGFR-2 were
localized in the blood vessels in CCRCC. Significant increases in
Dll4 expression levels were observed in CCRCC tissues compared with
those of non-cancerous tissues (P<0.001, Table II).
 | Table IIDll4 expression levels in normal renal
tissues and CCRCC. |
Table II
Dll4 expression levels in normal renal
tissues and CCRCC.
| | Dll-4 expression
levels | |
|---|
| |
| |
|---|
| Variable | n | High | Low | P-value |
|---|
| CCRCC | 121 | 53 | 68 | <0.001 |
| NRT | 65 | 9 | 56 | |
Dll4 expression levels and CCRCC
features
The associations between Dll4 expression levels and
CCRCC clinicolpathological variables are summarized in Table I. No significant association between
Dll4 expression levels and patient age (P=0.538), gender (P=0.276)
or tumor size (P=0.782) was detected. However, Dll4 was found to be
significantly correlated with tumor metastasis (P=0.012), tumor
T-stage (P=0.023), tumor grade (P=0.033) and VEGFR-2 expression
levels (P=0.001). High Dll4 and VEGFR-2 expression levels were
observed in 53 (43.8%) and 75 (62.0%) patients, respectively. These
findings imply that high Dll4 expression levels are associated with
tumor differentiation, invasion, metastasis and angiogenesis, which
in turn are associated with tumorigenesis and tumor
progression.
Association between Dll4 expression
levels and CCRCC prognosis
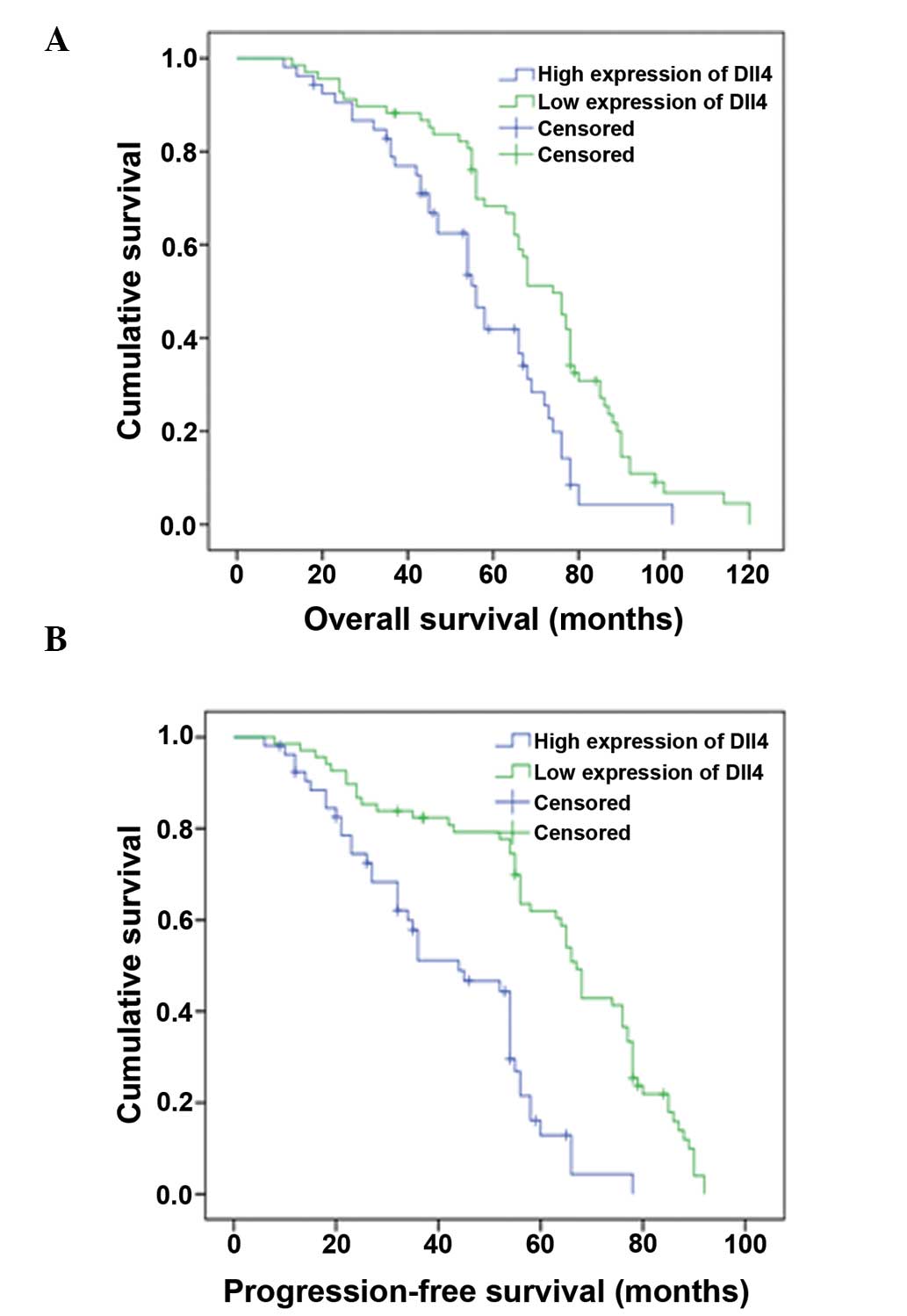
The correlations between Dll4 expression levels and
OS and PFS parameters were evaluated by Kaplan-Meier survival
analysis with log-rank statistic for determining significance. As
shown in Fig. 3, the patients with
high Dll4 expression levels had significantly worse OS (P<0.001)
and PFS (P<0.001) times than those with low Dll4 expression
levels (mean OS times, 38.9 and 52.9 months, respectively; mean PFS
times, 21.5 and 33.7 months, respectively). In a multivariate OS
and PFS analysis determined by the Cox proportional-hazards
regression model, the independent predictive value for Dll4
expression levels, as well as relevant clinical and pathological
parameters, including age, gender, tumor size, the presence of
metastases, T-stage, T-grade and VEGFR-2 expression levels, were
detected. As shown in Tables III
and IV, an increased level of Dll4
expression was shown to be an independent prognostic predictor of
OS (P=0.021) and PFS (P=0.034) times, in addition to the presence
of tumor metastasis (OS, P=0.001), tumor stage, tumor grade (OS,
P=0.045) and high expression levels of VEGFR-2. (OS, P=0.018).
 | Table IIICox regression model analysis of
overall survival times. |
Table III
Cox regression model analysis of
overall survival times.
| Variable | β | Standard error | Wald | df | P-value | Exp (β) |
|---|
| Age | | | | 1 | 0.874 | |
| Gender | | | | 1 | 0.231 | |
| Tumor size | | | | 1 | 0.064 | |
| Tumor stage | 0.874 | 0.452 | 4.487 | 1 | 0.006 | 2.457 |
| Tumor grade | 1.023 | 0.478 | 5.121 | 1 | 0.045 | 3.124 |
| Metastasis | 0.748 | 0.364 | 4.213 | 1 | 0.001 | 4.215 |
| High Dll4 | 0.645 | 0.412 | 5.456 | 1 | 0.021 | 2.741 |
| High VEGFR-2 | 0.946 | 0.547 | 4.365 | 1 | 0.018 | 3.102 |
 | Table IVCox regression model analysis of
progression-free survival times. |
Table IV
Cox regression model analysis of
progression-free survival times.
| Variable | β | Standard error | Wald | df | P-value | Exp (β) |
|---|
| Age | | | | 1 | 0.874 | |
| Gender | | | | 1 | 0.231 | |
| Tumor size | | | | 1 | 0.064 | |
| Tumor stage | 0.912 | 0.675 | 3.892 | 1 | 0.021 | 2.872 |
| Tumor grade | 1.235 | 0.364 | 4.768 | 1 | 0.025 | 3.432 |
| Metastasis | 0.872 | 0.392 | 7.113 | 1 | 0.008 | 4.214 |
| High Dll4 | 0.587 | 0.454 | 5.821 | 1 | 0.034 | 3.231 |
| High VEGFR-2 | 0.798 | 0.442 | 4.365 | 1 | 0.018 | 3.569 |
Discussion
RCC is an intractable disease due to inherent tumor
resistance to chemotherapy and radiotherapy (22). A previous study demonstrated that
the VEGF signaling pathway is important in angiogenesis in renal
cancer (5). Experimental systems
have shown that VEGF signaling induces Dll4 expression, which acts
as a negative feedback regulator to restrain vascular sprouting and
branching (10,11,23).
Functional tumor angiogenesis requires a balance of VEGF signaling
and Dll4 expression (9,12,24).
D114 expression has been detected at nine-fold higher levels in
CCRCC than in the normal kidney, with expression confined to the
vasculature (13,17). In the present study, Dll4 protein
was detected in fresh paired human CCRCC and non-cancerous tissues
through western blotting, and the expression levels of Dll4 protein
in 121 paraffin-embedded CCRCC specimens and 65 normal renal
tissues were also examined using immunohistochemistry. The results
revealed that Dll4 expression levels were higher in CCRCC than
those in the corresponding non-cancerous tissues. Furthermore, Dll4
expression levels were found to be associated with tumor
differentiation, invasion and metastasis. Compared with the primary
Dll4 tissues, metastatic CCRCC tissues exhibited more frequent Dll4
overexpression, which confirms that the presence of metastasis
predicts an inferior clinical outcome. Increased Dll4 expression
levels were clearly correlated with increased VEGFR-2 expression
levels, which was consistent with results from previous studies
(9–11,24).
All findings suggest that Dll4 expression is key in tumor
angiogenesis, which indicates that D114 overexpression may be
associated with poor prognosis in human cancer.
In the examination of prognostic factors, the
results from the Kaplan-Meier survival analysis with log-rank
statistic suggest that the presence of high Dll4 expression levels
was positively correlated with reduced OS and PFS times. In
multivariate analysis, TNM stage, grade, tumor size, age and gender
were analyzed, as well as expression levels of Dll4, using the Cox
regression model. The results revealed that the level of Dll4
expression was an independent prognostic factor for OS and PFS
times. Previous studies observed that Dll4 expression was an
independent indicator of poor prognosis in several types of human
cancer, including lung, breast, bladder and nasopharyngeal cancer
(14,15,18,25).
The present study extended this finding to CCRCC. A markedly worse
prognosis was observed in patients with high Dll4 expression levels
than in those with low Dll4 expression levels. Furthermore,
inhibition of Dll4-Notch has been shown to result in tumor growth
inhibition associated with the formation of a non-functional,
hypersprouting tumor vasculature (9,26,27).
Thus, the Dll4/Notch signaling pathway activation loop appears to
promote tumor formation and progression.
In recent years, anti-angiogenesis-targeted
therapies have been identified as promising therapeutic strategies.
Certain newly developed agents that target the VEGF signaling
pathway, such as the small-molecular VEGF receptor inhibitors,
sorafenib and sunitinib, and the anti-VEGF monoclonal antibody
bevacizumab, have shown encouraging treatment benefits in advanced
RCC patients in randomized controlled trials (28–30),
but the effects are variable and incremental, and acquired or
innate resistance is frequently encountered (31,32).
Anti-VEGF therapy acts to prune vascular sprouts and reduce new
vessel growth (27,33), which is in contrast to the
abovementioned cellular effects of inhibiting the Dll4-Notch
signaling pathway. Notably, preclinical studies have demonstrated
that Dll4 suppression exerts strong growth inhibitory effects on
tumors that are resistant to anti-VEGF therapies (9,26). In
addition, simultaneously targeting Dll4 and VEGF has been shown to
generate additive antitumor effects compared with single agents in
numerous tumor models (34).
Compared with Dll4 expression levels in lung, breast, pancreatic
and bladder cancer, Dll4 expression in CCRCC has been detected at
levels nine-fold higher than those in the normal kidney (13,17).
Therefore, CCRCC patients may benefit most from the therapeutic
targeting of angiogenesis by simultaneous inhibition of the
Dll4-Notch and VEGF signaling pathways.
Thus far, few CCRCC-specific biomarkers have been
used in the clinical setting for diagnosis and prognosis. Although
Dll4 appears not to be a specific marker of CCRCC, patients may
benefit due to the significance of D114 in determining patient
prognosis. From a clinical viewpoint, the presence of high Dll4
expression levels may be considered a risk factor for tumor
progression; therefore, implementing a strict systemic therapeutic
plan, such as immunotherapy, angiogenesis inhibitor drugs or
chemotherapy, following surgery, along with regular investigation
may improve prognosis.
In conclusion, high Dll4 expression levels were
demonstrated to be clearly associated with high VEGFR-2 expression
levels, indicators of pathological aggressiveness (such as
metastasis, pathological grade and tumor stage) and poor prognosis
in CCRCC patients. Therefore, inhibition of Dll4 may exert potent
growth inhibitory effects on CCRCC tumors that are resistant to
anti-VEGF therapies.
Acknowledgements
The authors would like to thank Dr Wentao Hu for
aiding analysis.
References
|
1
|
van Spronsen DJ, Mulders PF and De Mulder
PH: Novel treatments for metastatic renal cell carcinoma. Crit Rev
Oncol Hematol. 55:177–191. 2005.
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010.
|
|
3
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005.
|
|
4
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996.
|
|
5
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008.
|
|
6
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Pract Oncol. 3:24–40. 2006.
|
|
7
|
Lai EC: Notch signaling: control of cell
communication and cell fate. Development. 131:965–973. 2004.
|
|
8
|
Kopan R and Ilagan MX: The canonical notch
signaling pathway: unfolding the activation mechanism. Cell.
137:216–233. 2009.
|
|
9
|
Noguera-Troise I, Daly C, Papadopoulos NJ,
et al: Blockade of Dll4 inhibits tumour growth by promoting
non-productive angiogenesis. Nature. 444:1032–1037. 2006.
|
|
10
|
Hellström M, Phng LK, Hofmann JJ, et al:
Dll4 signalling through Notch1 regulates formation of tip cells
during angiogenesis. Nature. 445:776–780. 2007.
|
|
11
|
Lobov IB, Renard RA, Papadopoulos N, et
al: Delta-like ligand 4 (Dll4) is induced by VEGF as a negative
regulator of angiogenic sprouting. Proc Natl Acad Sci USA.
104:3219–3224. 2007.
|
|
12
|
Hoey T, Yen WC, Axelrod F, et al: DLL4
blockade inhibits tumor growth and reduces tumor-initiating cell
frequency. Cell Stem Cell. 5:168–177. 2009.
|
|
13
|
Mailhos C, Modlich U, Lewis J, Harris A,
Bicknell R and Ish-Horowicz D: Delta4, an endothelial specific
notch ligand expressed at sites of physiological and tumor
angiogenesis. Differentiation. 69:135–144. 2001.
|
|
14
|
Patel NS, Dobbie MS, Rochester M, et al:
Up-regulation of endothelial delta-like 4 expression correlates
with vessel maturation in bladder cancer. Clin Cancer Res.
12:4836–4844. 2006.
|
|
15
|
Jubb AM, Soilleux EJ, Turley H, et al:
Expression of vascular notch ligand delta-like 4 and inflammatory
markers in breast cancer. Am J Pathol. 176:2019–2028. 2010.
|
|
16
|
Jubb AM, Turley H, Moeller HC, et al:
Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in
colon cancer. Br J Cancer. 101:1749–1757. 2009.
|
|
17
|
Patel NS, Li JL, Generali D, Poulsom R,
Cranston DW and Harris AL: Up-regulation of delta-like 4 ligand in
human tumor vasculature and the role of basal expression in
endothelial cell function. Cancer Res. 65:8690–8697. 2005.
|
|
18
|
Donnem T, Andersen S, Al-Shibli K, Al-Saad
S, Busund LT and Bremnes RM: Prognostic impact of Notch ligands and
receptors in nonsmall cell lung cancer: coexpression of Notch-1 and
vascular endothelial growth factor-A predicts poor survival.
Cancer. 116:5676–5685. 2010.
|
|
19
|
Chen HT, Cai QC, Zheng JM, et al: High
Expression of Delta-Like Ligand 4 Predicts Poor Prognosis After
Curative Resection for Pancreatic Cancer. Ann Surg Oncol. 19(Suppl
3): S464–S474. 2012.
|
|
20
|
Martínez-Salamanca JI, Huang WC, Millán I,
et al: International Renal Cell Carcinoma-Venous Thrombus
Consortium: Prognostic impact of the 2009 UICC/AJCC TNM staging
system for renal cell carcinoma with venous extension. Eur Urol.
59:120–127. 2011.
|
|
21
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982.
|
|
22
|
Linehan WM and Zbar B: Focus on kidney
cancer. Cancer Cell. 6:223–228. 2004.
|
|
23
|
Suchting S, Freitas C, le Noble F, et al:
The Notch ligand Delta-like 4 negatively regulates endothelial tip
cell formation and vessel branching. Proc Natl Acad Sci USA.
104:3225–3230. 2007.
|
|
24
|
Scehnet JS, Jiang W, Kumar SR, et al:
Inhibition of Dll4-mediated signaling induces proliferation of
immature vessels and results in poor tissue perfusion. Blood.
109:4753–4760. 2007.
|
|
25
|
Zhang JX, Cai MB, Wang XP, et al: Elevated
DLL4 expression is correlated with VEGF and predicts poor prognosis
of nasopharyngeal carcinoma. Med Oncol. 30:3902013.
|
|
26
|
Ridgway J, Zhang G, Wu Y, et al:
Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006.
|
|
27
|
Thurston G, Noguera-Troise I and
Yancopoulos GD: The Delta paradox: DLL4 blockade leads to more
tumour vessels but less tumour growth. Nat Rev Cancer. 7:327–331.
2007.
|
|
28
|
Escudier B, Eisen T, Stadler WM, et al:
TARGET study group: Sorafenib in advanced clear-cell renal-cell
carcinoma. N Engl J Med. 356:125–134. 2007.
|
|
29
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. N Engl J Med. 356:115–124. 2007.
|
|
30
|
Yang JC, Haworth L, Sherry RM, et al: A
randomized trial of bevacizumab, an anti-vascular endothelial
growth factor antibody, for metastatic renal cancer. N Engl J Med.
349:427–434. 2003.
|
|
31
|
Ferrara N, Hillan KJ, Gerber H-P and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400.
2004.
|
|
32
|
Ebos JM, Lee CR and Kerbel RS: Tumor and
host-mediated pathways of resistance and disease progression in
response to antiangiogenic therapy. Clin Cancer Res. 15:5020–5025.
2009.
|
|
33
|
Duda DG, Batchelor TT, Willett CG and Jain
RK: VEGF-targeted cancer therapy strategies: current progress,
hurdles and future prospects. Trends Mol Med. 13:223–230. 2007.
|
|
34
|
Kuhnert F, Kirshner JR and Thurston G:
Dll4-Notch signaling as a therapeutic target in tumor angiogenesis.
Vasc Cell. 3:202011.
|