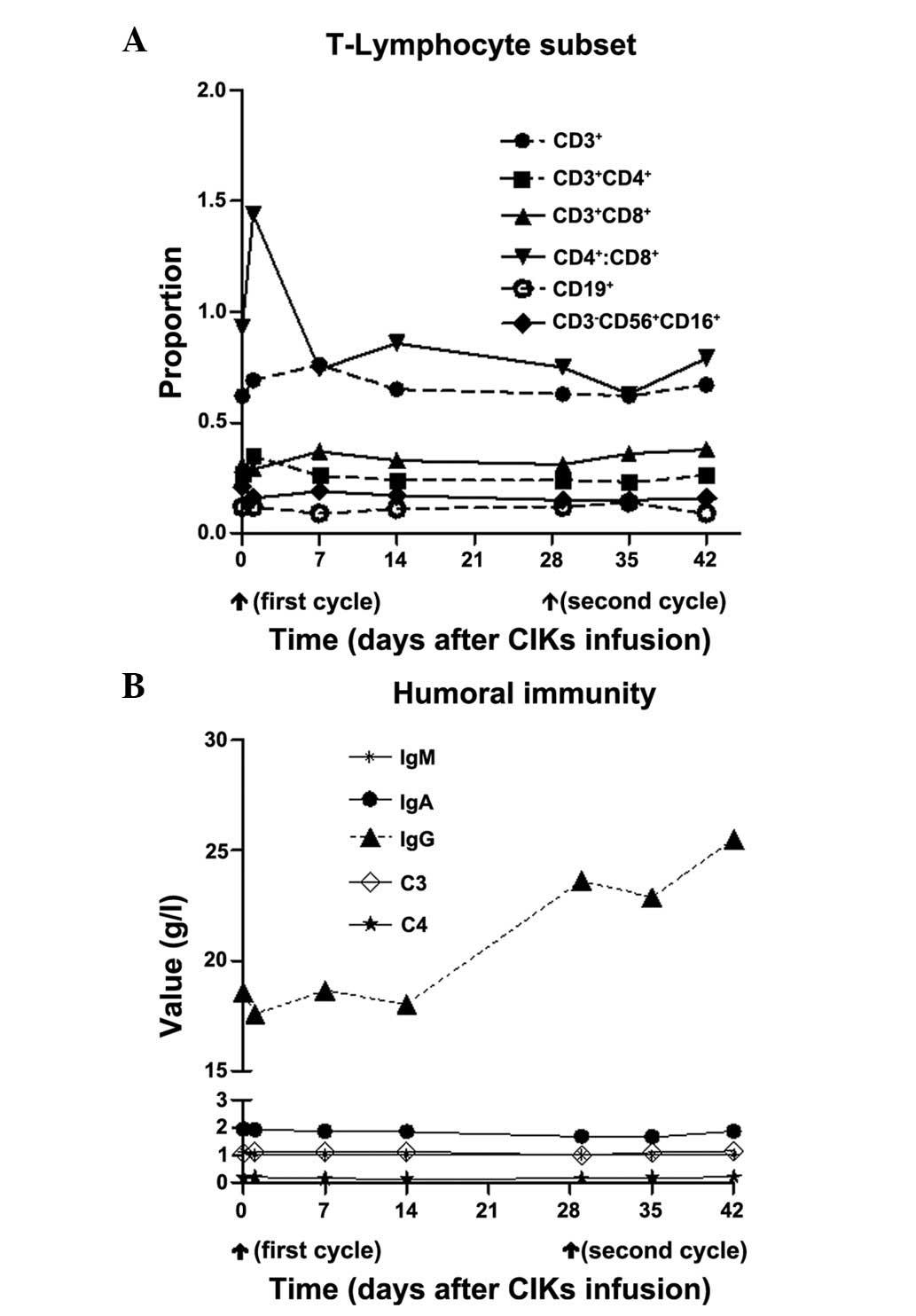
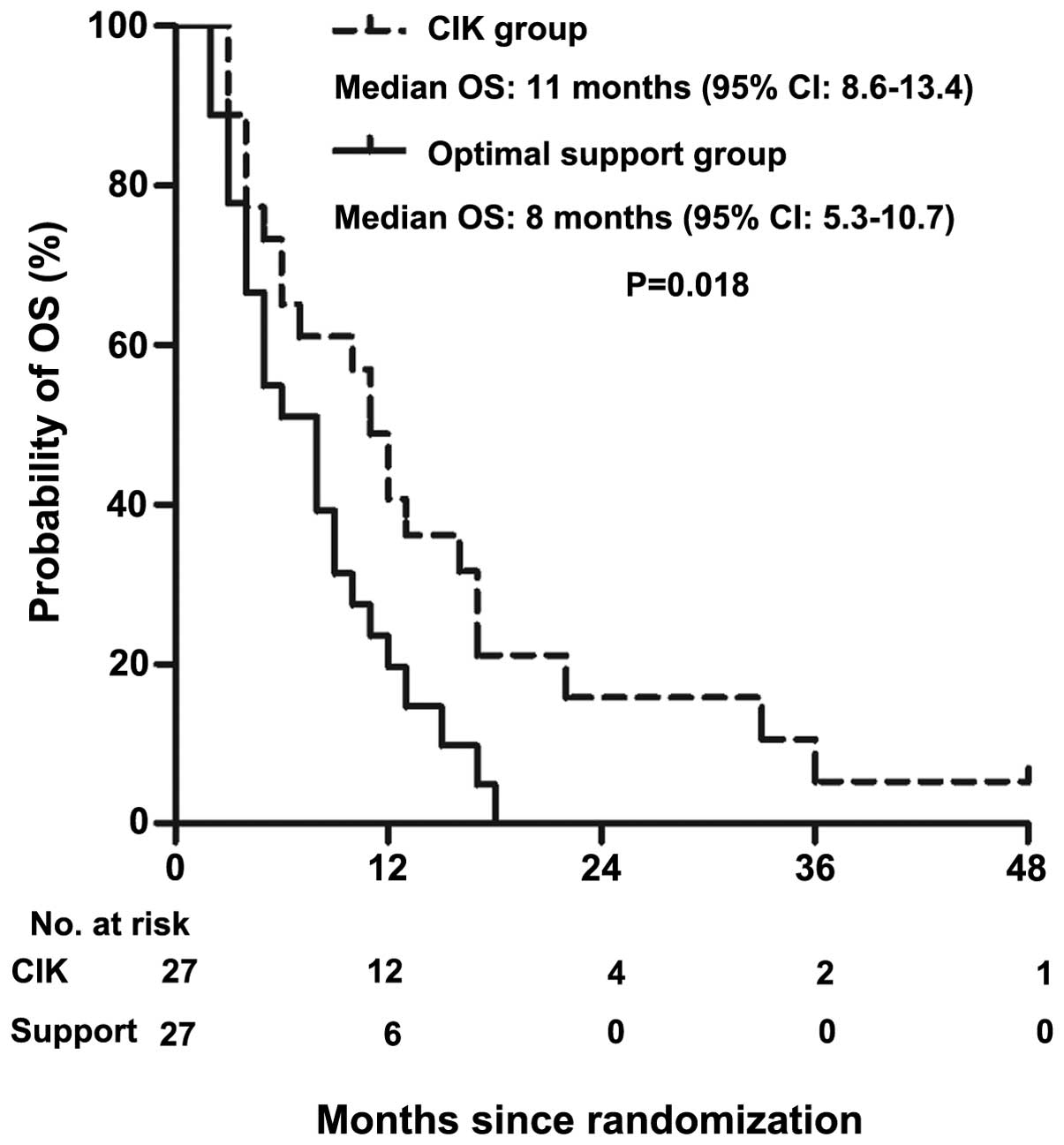
|
1
|
Li XD, Xu B, Wu J, Ji M, Xu BH, Jiang JT
and Wu CP: Review of Chinese clinical trials on CIK cell treatment
for malignancies. Clin Transl Oncol. 14:102–108. 2012.
|
|
2
|
Mesiano G, Todorovic M, Gammaitoni L, et
al: Cytokine-induced killer (CIK) cells as feasible and effective
adoptive immunotherapy for the treatment of solid tumors. Expert
Opin Biol Ther. 12:673–684. 2012.
|
|
3
|
Ma Y, Zhang Z, Tang L, Xu YC, Xie ZM, Gu
XF and Wang HX: Cytokine-induced killer cells in the treatment of
patients with solid carcinomas: a systematic review and pooled
analysis. Cytotherapy. 14:483–493. 2012.
|
|
4
|
Olioso P, Giancola R, Di Riti M, Contento
A, Accorsi P and Iacone A: Immunotherapy with cytokine induced
killer cells in solid and hematopoietic tumours: a pilot clinical
trial. Hematol Oncol. 27:130–139. 2009.
|
|
5
|
Li R, Wang C, Liu L, et al: Autologous
cytokine-induced killer cell immunotherapy in lung cancer: a phase
II clinical study. Cancer Immunol Immunother. 61:2125–2133.
2012.
|
|
6
|
Liu L, Zhang W, Qi X, et al: Randomized
study of autologous cytokine-induced killer cell immunotherapy in
metastatic renal carcinoma. Clin Cancer Res. 18:1751–1759.
2012.
|
|
7
|
Wu C, Jiang J, Shi L and Xu N: Prospective
study of chemotherapy in combination with cytokine-induced killer
cells in patients suffering from advanced non-small cell lung
cancer. Anticancer Res. 28:3997–4002. 2008.
|
|
8
|
Zhong R, Teng J, Han B and Zhong H:
Dendritic cells combining with cytokine-induced killer cells
synergize chemotherapy in patients with late-stage non-small cell
lung cancer. Cancer Immunol Immunother. 60:1497–1502. 2011.
|
|
9
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: first report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011.
|
|
10
|
Li JJ, Gu MF, Pan K, Liu LZ, Zhang H, Shen
WX and Xia JC: Autologous cytokine-induced killer cell transfusion
in combination with gemcitabine plus cisplatin regimen chemotherapy
for metastatic nasopharyngeal carcinoma. J Immunother. 35:189–195.
2012.
|
|
11
|
Hui D, Qiang L, Jian W, Ti Z and Da-Lu K:
A randomized, controlled trial of postoperative adjuvant
cytokine-induced killer cells immunotherapy after radical resection
of hepatocellular carcinoma. Dig Liver Dis. 41:36–41. 2009.
|
|
12
|
Sangiolo D: Cytokine induced killer cells
as promising immunotherapy for solid tumors. J Cancer. 2:363–368.
2011.
|
|
13
|
Wang S, Du W, Zhang H, et al: Biological
characteristics and antitumor activity of CIK cells activated by
recombinant human fibronectin for human lung cancer cell lines
in vitro. Zhongguo Fei Ai Za Zhi. 13:277–281. 2010.(In
Chinese).
|
|
14
|
Beasley MB, Brambilla E and Travis WD: The
2004 World Health Organization classification of lung tumors. Semin
Roentgenol. 40:90–97. 2005.
|
|
15
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
|
|
16
|
Niu Q, Wang W, Li Y, et al: Cord
blood-derived cytokine-induced killer cells biotherapy combined
with second-line chemotherapy in the treatment of advanced solid
malignancies. Int Immunopharmacol. 11:449–456. 2011.
|
|
17
|
Li Y, Schmidt-Wolf IG, Wu YF, et al:
Optimized protocols for generation of cord blood-derived
cytokine-induced killer/natural killer cells. Anticancer Res.
30:3493–3499. 2010.
|
|
18
|
Laport GG, Sheehan K, Baker J, et al:
Adoptive immunotherapy with cytokine-induced killer cells for
patients with relapsed hematologic malignancies after allogeneic
hematopoietic cell transplantation. Biol Blood Marrow Transplant.
17:1679–1687. 2011.
|
|
19
|
Zhong ZD, Luo Y, Zou P, et al: Infusions
of recipient-derived cytokine-induced killer cells of donor origin
eradicated residual disease in a relapsed leukemia patient after
allo-hematopoietic stem cell transplantation. Chin Med J (Engl).
125:1669–1671. 2012.
|
|
20
|
Sangiolo D, Mesiano G, Carnevale-Schianca
F, Piacibello W, Aglietta M and Cignetti A: Cytokine induced killer
cells as adoptive immunotherapy strategy to augment graft versus
tumor after hematopoietic cell transplantation. Expert Opin Biol
Ther. 9:831–840. 2009.
|
|
21
|
Sangiolo D, Martinuzzi E, Todorovic M, et
al: Alloreactivity and anti-tumor activity segregate within two
distinct subsets of cytokine-induced killer (CIK) cells:
implications for their infusion across major HLA barriers. Int
Immunol. 20:841–848. 2008.
|
|
22
|
Liu C, Wang S, Jiao X, Liu D, Huang W, Ma
K and Wang J: Antitumor effect of antigen-pulsed DC-CIK cells
against prostate cancer DU145 cells. Progress of Anatomical
Sciences. 18:222–226. 2012.
|
|
23
|
Wang QJ, Wang H, Pan K, et al: Comparative
study on anti-tumor immune response of autologous cytokine-induced
killer (CIK) cells, dendritic cells-CIK (DC-CIK), and
semi-allogeneic DC-CIK. Chin J Cancer. 29:641–648. 2010.
|
|
24
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013.
|
|
25
|
Tan G, Zhang X, Feng H, Luo H and Wang Z:
The therapeutic effect of cytokine-induced killer cells on
pancreatic cancer enhanced by dendritic cells pulsed with K-ras
mutant peptide. Clin Dev Immunol. 2011:649359–649367. 2011.
|
|
26
|
Wu X, Wang S, Jiao X, et al: The dynamic
changes of hTERT expression in long-term cultured CIK cells. J
Modern Oncology. 20:667–671. 2012.
|
|
27
|
Wolchok JD, Hoos A, O’Day S, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009.
|
|
28
|
Yang B, Lu XC, Yu RL, et al: Repeated
transfusions of autologous cytokine-induced killer cells for
treatment of haematological malignancies in elderly patients: a
pilot clinical trial. Hematol Oncol. 30:115–122. 2012.
|