Introduction
Cancer often leads to hypercoagulability, which may
result in a variety of clinical manifestations, including migratory
superficial thrombophlebitis, venous thrombosis, non-bacterial
thrombotic endocarditis and disseminated intravascular coagulation.
The release or expression of procoagulants by tumor cells and the
demonstration of procoagulant activity by monocytes, platelets and
endothelial cells are hypothesized to cause vascular complications
in patients exhibiting malignancies. The common treatments for
cancer include surgery, high-dose chemotherapy, bone marrow
transplantation and numerous chemotherapeutic regimens. In
addition, an indwelling central venous catheter may be used, which
may significantly increase the risk of thrombotic events in
patients with malignancy (1).
Angiogenesis inhibitors are increasingly
administered for the treatment of patients with malignancies.
Bevacizumab is a humanized monoclonal antibody that inhibits
angiogenesis by inhibiting vascular endothelial growth factor
(VEGF)-A. Bevacizumab was initially approved in 2004 for
combinational use with standard chemotherapy for metastatic colon
cancer treatment. However, the use of bevacizumab has been
associated with an increased risk of venous thromboembolic events
and bleeding in cancer patients (2,3).
Currently, there is insufficient data regarding the safety and
activity of thrombolytic agents in the treatment of massive
pulmonary thromboembolisms that have developed in cancer patients
undergoing bevacizumab-based therapy.
In the present study, the case of a patient with an
acute massive pulmonary thromboembolism is presented, who received
fibrinolytic treatment, whilst receiving a bevacizumab-based
combination regimen for metastatic colon cancer. Written informed
consent was obtained from the patient.
Case report
In September 2013, a 66-year-old female with a
metastatic colon carcinoma was admitted to the emergency department
of Hitit University, Corum Educational and Research Hospital
(Corum, Turkey) with acute dyspnea, palpitations and dizziness. The
patient exhibited hypertension, however, the patient’s medical
history did not include smoking, diabetes mellitus, ischemic heart
disease or any thrombotic disease. The patient underwent nine
cycles of the FOLFIRI (90 min intravenous infusion of 180
mg/m2 irinotecan, 400 mg/m2 fluorouracil and
400 mg/m2 leucovorin, followed by a 46 h intravenous
infusion of 2,400 mg/m2, entire regimen delivered twice
a week, for 18 weeks) plus bevacizumab combination therapy. The
patient’s symptoms developed 10 days following the last cycle of
chemotherapy. On physical examination the patient’s blood pressure
was 70/50 mmHg and heart rate was 120 bpm. The patient exhibited
tachypnea, tachycardia, jugular venous distention and a systolic
2/6 murmur was identified on all cardiac points. An emergency
two-dimensional ultrasonographic echocardiography revealed right
heart dilatation, moderate tricuspid regurgitation and pulmonary
hypertension. Thus, as the patient was considered to have a high
risk of pulmonary embolism [PE; Wells score (4), 7 points], a PE was suspected. Thoracic
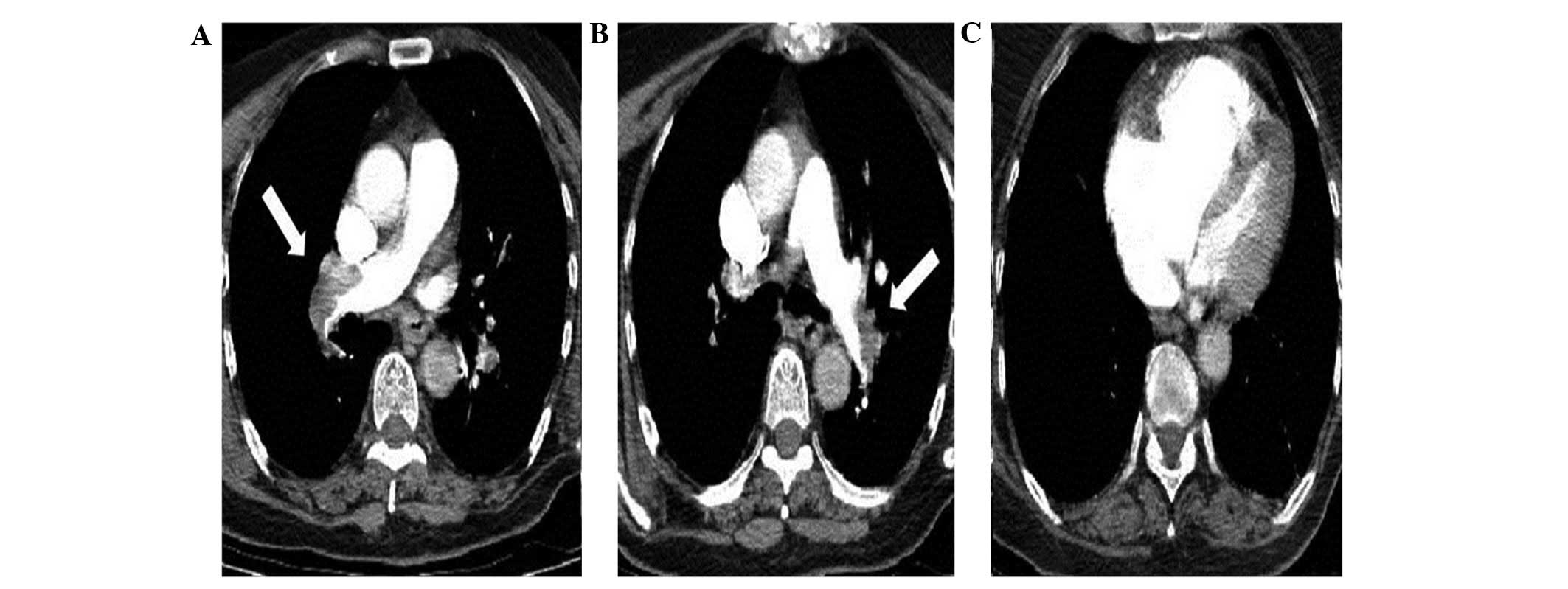
computed tomography (CT) angiography demonstrated a bilateral
pulmonary arterial embolism (Fig.
1). The patient was diagnosed with a massive PE and hemodynamic
instability. Due to the patient’s malignancy and risk of bleeding,
prolonged low-dose thrombolytic therapy [25 mg tissue plasminogen
activator (tPA) infusion for 6 h] was administered to the
peripheral vein, according to a previous study by Aykan et
al (5), rather than a standard
thrombolytic regime. Following treatment, there was an increase in
blood pressure (100/70 mmHg) and improvement in the patient’s
clinical condition. No bleeding complications were observed.
Control echocardiography revealed an improvement in the right heart
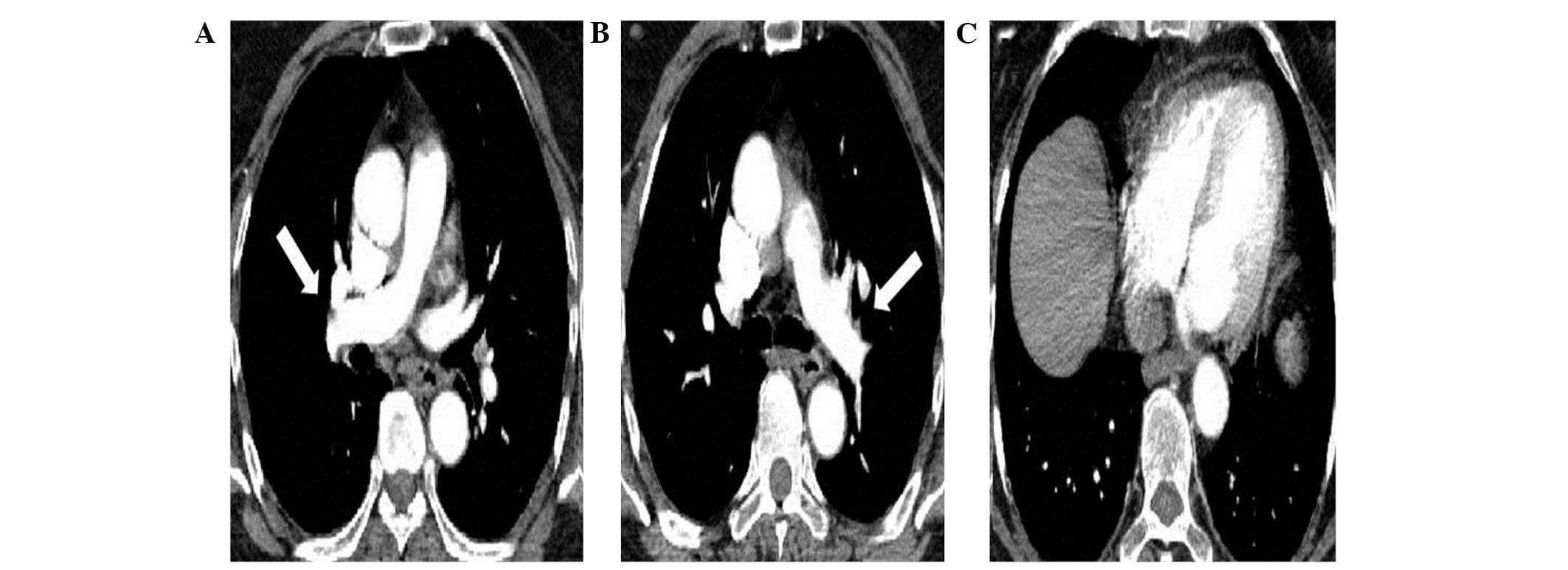
chambers and a decrease in pulmonary pressure. The CT angiography
revealed that the peripheral vascular bed was reperfused (Fig. 2). Additionally, Doppler ultrasound
revealed acute deep vein thrombosis (DVT) in the right lower
extremities, which was considered to be the source of the pulmonary
embolism.
Discussion
Numerous studies have indicated that
progression-free survival and overall survival are improved by the
administration of bevacizumab, in combination with various
chemotherapy regimens, for patients with metastatic colon cancer
(5,6). The class-effects of VEGF axis
inhibition include cardiovascular effects, for example hypertension
and left ventricular dysfunction, and non-cardiovascular effects,
including proteinuria, delayed wound healing, gastrointestinal
perforation, fatigue and dysphonia. In addition, the administration
of bevacizumab in conjunction with chemotherapy is associated with
an increased risk of thromboembolic and bleeding events (2,3). PE
occurs in 2–5% of cases where bevacizumab and chemotherapy are used
in combination (6,7). In the present study, the patient was
diagnosed with a massive PE and DVT following bevacizumab
combination therapy.
It is recommended that thrombolytic therapy is
followed by anticoagulation therapy, rather than anticoagulation
alone, for patients exhibiting acute PE who are persistently
hypotensive as a result of the PE (systolic blood pressure <90
mmHg or a decrease in systolic blood pressure of ≥40 mmHg from
baseline) and who do not exhibit an increased risk of bleeding. The
results of previous studies indicate that thrombolytic therapy
leads to early hemodynamic improvement, however, is associated with
an increased risk of major bleeding (8,9).
Results regarding the effect of thrombolytic therapy
on the improvement of mortality are controversial. Despite the
inconsistent results of controlled clinical trials, one
observational study of 72,230 unstable patients with acute PE
revealed that thrombolytic therapy was associated with lower
all-cause mortality when compared with no treatment (15 vs. 47%,
respectively) and lower mortality attributable to PE when compared
with no treatment (8.4 vs. 42%) (10). However, observational results
obtained from the same population revealed that thrombolytic
therapy was underutilized and less likely to be administered in
older patients (aged >60 years) and in patients with comorbid
conditions, highlighting a possible lack of confidence exhibited by
clinicians regarding the use of thrombolytic therapy (11). While the reported effect size is
large in the two above-mentioned studies, the observational design
and the potential influence of bias demonstrates that the efficacy
of thrombolytic therapy in this clinical setting remains unclear.
Although bevacizumab may increase the risk of bleeding, due to the
patient’s hemodynamic instability in the present case, thrombolytic
therapy was initiated immediately.
In patients exhibiting massive pulmonary embolisms,
the guidelines for conducting thrombolytic therapy recommend
peripheral venous administration of 100 mg tPA for 2 h (12). The risk of bleeding, a significant
complication of thrombolytic therapy, has been reported to be as
high as 20% in older patients with a large body mass index and a
history of previous catheterization (13–15).
However, to the best of our knowledge, no sufficient data exists
regarding a prolonged low-dose tPA regime. In previous studies, it
has been demonstrated that prolonged low-dose tPA may be
effectively and reliably administered to elderly patients in whom
the risk of bleeding is high (16),
in patients with a prosthetic valve (17) or in patients who exhibit hemoptysis
secondary to an embolism (18)
without an increased risk of bleeding.
In conclusion, thrombolytic therapy in patients with
malignancies is associated with an increased risk of bleeding,
regardless of the presence of metastasis. Certain chemotherapeutic
agents, including bevacizumab, may elevate the risk of bleeding.
However, in the present study, despite the increased risk of
bleeding, low-dose and prolonged tPA infusion was effectively and
reliably administered in a patient with a massive PE, although it
did result in hemodynamic instability.
References
|
1
|
Schiffer CA, Mangu PB, Wade JC, et al:
Central venous catheter care for the patient with cancer: American
Society of Clinical Oncology clinical practice guideline. J Clin
Oncol. 31:1357–1370. 2013.
|
|
2
|
Scappaticci FA, Skillings JR, Holden SN,
et al: Arterial thromboembolic events in patients with metastatic
carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer
Inst. 99:1232–1239. 2007.
|
|
3
|
Nalluri SR, Chu D, Keresztes R, et al:
Risk of venous thromboembolism with the angiogenesis inhibitor
bevacizumab in cancer patients: a meta-analysis. JAMA.
300:2277–2285. 2008.
|
|
4
|
Wells PS, Anderson DR, Rodger M, et al:
Derivation of a simple clinical model to categorize patients
probability of pulmonary embolism: increasing the models utility
with the SimpliRED D-dimer. Thromb Haemost. 83:416–420. 2000.
|
|
5
|
Aykan AC, Boyaci F and Hatem E: Successful
treatment of a pulmonary embolism with low dose prolonged infusion
of tissue typed plasminogen activator in a 37 year old female in
early postoperative period. Anadolu Kardiyol Derg. 14:400–402.
2014.
|
|
6
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342.
2004.
|
|
7
|
Kabbinavar FF, Schulz J, McCleod M, et al:
Addition of bevacizumab to bolus fluorouracil and leucovorin in
first-line metastatic colorectal cancer: results of a randomized
phase II trial. J Clin Oncol. 23:3697–3705. 2005.
|
|
8
|
Goldhaber SZ, Haire WD, Feldstein ML, et
al: Alteplase versus heparin in acute pulmonary embolism:
randomised trial assessing right-ventricular function and pulmonary
perfusion. Lancet. 341:507–511. 1993.
|
|
9
|
Chatterjee S, Chakraborty A, Weinberg I,
et al: Thrombolysis for pulmonary embolism and risk of all-cause
mortality, major bleeding, and intracranial hemorrhage: a
meta-analysis. JAMA. 311:2414–2421. 2014.
|
|
10
|
Stein PD and Matta F: Thrombolytic therapy
in unstable patients with acute pulmonary embolism: saves lives but
underused. Am J Med. 125:465–470. 2012.
|
|
11
|
Stein PD and Matta F: Treatment of
unstable pulmonary embolism in the elderly and those with comorbid
conditions. Am J Med. 126:304–310. 2013.
|
|
12
|
Kearon C, Kahn SR, Agnelli G, et al:
American College of Chest Physicians: Antithrombotic therapy for
venous thromboembolic disease: American College of Chest Physicians
Evidence-Based Clinical Practice Guidelines (8th Edition). Chest.
133(Suppl 6): S454–S545. 2008.
|
|
13
|
Fiumara K, Kucher N, Fanikos J and
Goldhaber SZ: Predictors of major hemorrhage following fibrinolysis
for acute pulmonary embolism. Am J Cardiol. 97:127–129. 2006.
|
|
14
|
Mikkola KM, Patel SR, Parker JA, et al:
Increasing age is a major risk factor for hemorrhagic complications
after pulmonary embolism thrombolysis. Am Heart J. 134:69–72.
1997.
|
|
15
|
Thabut G, Thabut D, Myers RP, et al:
Thrombolytic therapy of pulmonary embolism: a meta-analysis. J Am
Coll Cardiol. 40:1660–1667. 2002.
|
|
16
|
Biteker M, Duran NE, Gündüz S and Ozkan M:
Treatment of pulmonary embolism with low-dose prolonged infusion of
tissue-type plasminogen activator in an 85-year-old woman. J Am
Geriatr Soc. 57:745–746. 2009.
|
|
17
|
Özkan M, Çakal B, Karakoyun S, et al:
Thrombolytic therapy for the treatment of prosthetic heart valve
thrombosis in pregnancy with low-dose, slow infusion of tissue-type
plasminogen activator. Circulation. 128:532–540. 2013.
|
|
18
|
Karavelioğlu Y, Karapınar H, Kucukdurmaz
Z, et al: Worm-like thrombus in the right heart treated with low
dose fibrinolytic therapy in a patient with pulmonary embolism.
Koşuyolu Heart Journal. DOI: 10.5578/kkd.5793. (Epub ahead of
print).
|