Introduction
Struma ovarii is a rare form of ovarian germ cell
tumor and was first described by Gottschalk in 1899 (1). Although ~5–15% of teratomas contain
thyroid tissue, thyroid tissue must be predominant in the teratoma
for the cancer to be classified as a struma ovarii (2). Struma ovarii comprise only 1.4–2.7% of
teratomas and only 1% of ovarian tumors, worldwide (1,3,4).
Malignant struma ovarii is even less common and its associated
metastasis is documented in <5–6% of malignant struma ovarii
cases (2,5,6). The
natural history and optimal treatment strategy for malignant struma
ovarii remains controversial due to its rarity. Previous reports
have proposed that the patient should undergo complete surgical
staging for ovarian cancer, with other reports indicating that a
total thyroidectomy and adjuvant 131I radioablation
therapy may aid in the elimination of residual thyroid tissue
following surgical removal of the primary tumor (2). The current report presents a case of
long-term survival of malignant struma ovarii with multiple lung
and bone metastases by treatment with chemotherapy. Written
informed consent was obtained from the patient.
Case report
In October1989, a 45-year-old premenopausal female
(gravida 2; para 2) was referred to the Department of Obstetrics
and Gynecology, Kinki University Hospital (Osaka, Japan) for
further examination of a metastatic bone tumor. In September 1989,
the patient had consulted the local doctor due to back pain and a
tumor was identified in the right ninth rib. The patient was
admitted to the Department of Orthopedics (Kinki University
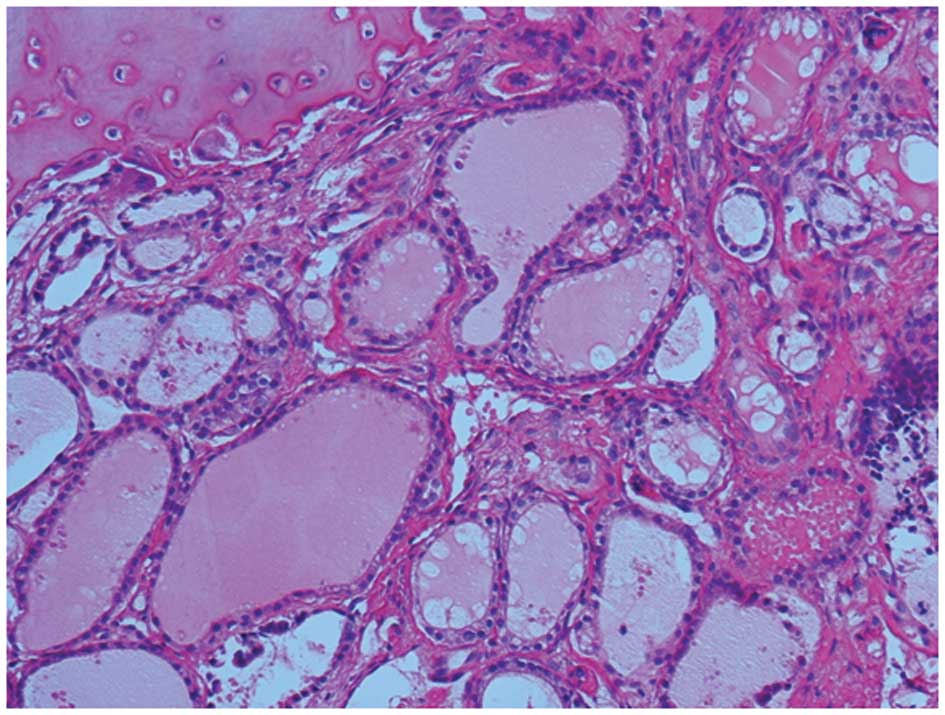
Hospital) and underwent tumor resection. Pathological diagnosis of
the surgical specimen revealed folicular structures which contain
the colloid had proliferated invasively via the destruction of the
bone trabeculae and metastasis was suspected, which resulted in a
diagnosis of a metastasizing thyroid carcinoma (Fig. 1). An ultrasound examination of the
thyroid gland and an evaluation of the thyroid hormonal function
did not present any unusual findings. Computed tomography (CT) was
then performed, which identified an ovarian tumor. The patient was
subsequently referred to the Department of Obstetrics and
Gynecology (Kinki University Hospital). Abdominal ultrasonography
revealed a hypoechoic mass in the left ovary and magnetic resonance
imaging revealed a 12×9.5×11-cm solid cystic mass with fatty tissue
elements and calcification. In January 1990, a left
salpingo-oophorectomy and a wedge resection of the right ovary were
performed. Intraoperative examination revealed that the left ovary
(diameter, 12 cm) was elastic and comprised of partially
multilocular lesions and the right ovary (diameter, 4 cm) contained
ovarian cysts. Gross examination of the uterus revealed that it was
normal, however, a small volume of ascites was detected.
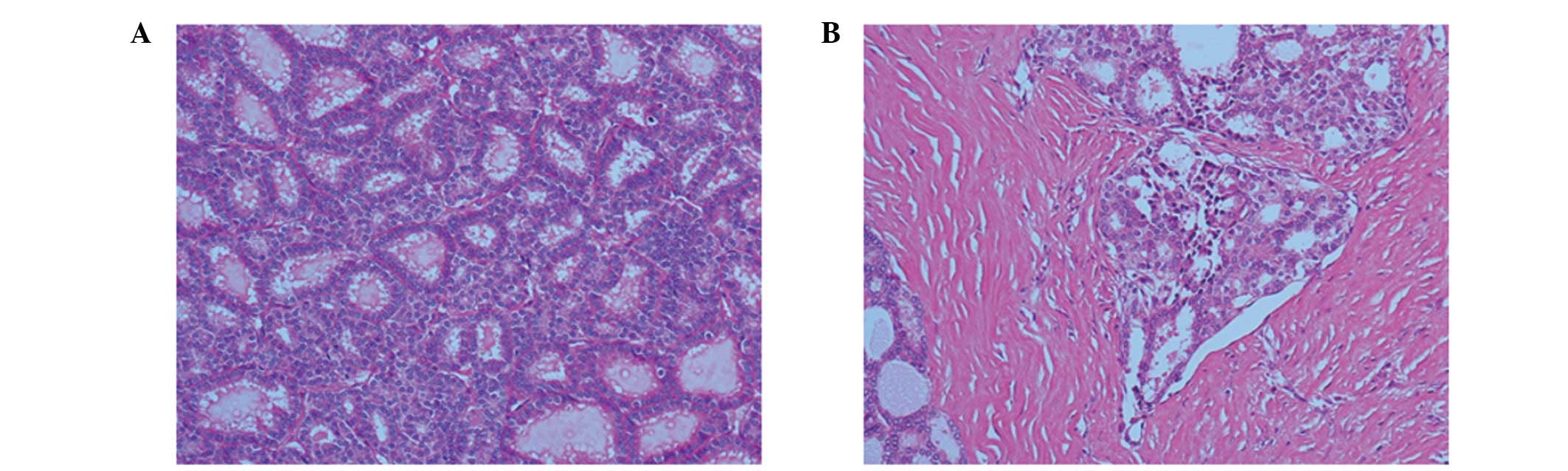
The postoperative diagnosis was determined as a
mature cystic teratoma with malignant struma ovarii (thyroid type,
follicular carcinoma) of the left ovary and mature cystic teratoma
of the right ovary. Microscopic analysis aided in the
classification of the tumor as a follicular adenocarcinoma. The
tumor cells formed tightly packed small and large follicles filled
with pink colloid-like material, and exhibited round nuclei,
increased chromatin (Fig. 2A), as
well as mitoses and vascular invasion (Fig. 2B). Furthermore, 123I
scintigraphy revealed no abnormal uptake in the thyroid or other
organs. The patient was finally diagnosed with stage IV malignant
struma ovarii with rib metastasis, according to the International
Federation of Gynecology and Obstetrics ovarian cancer staging
system, 1988 (7).
Tegafur-uracil (UFT) was administered (600 mg/day)
as an adjuvant chemotherapy for two years. Follow-up included
measurements of thyroid function (via thyroglobulin levels) and
regular chest X-rays every two months. In November 1993, three
years after commencing UFT therapy, a chest X-ray revealed multiple
lung metastases. Although systemic chemotherapy was recommended,
the patient refused and recommenced treatment with UFT at an
increased dosage of 3,000 mg/day. Following two further years of
treatment, the patient refused to continue UFT administration,
preferring to undergo observation. During this period, the lung
nodules progressed slowly, however, no thyroid dysfunction or other
symptoms were observed. In 1999, the patient identified a painless
mass under the right scapula but did not recieve medical treatment.
In 2001, the patient returned to Kinki University Hospital
presenting with pain in the pubic region and a CT scan revealed
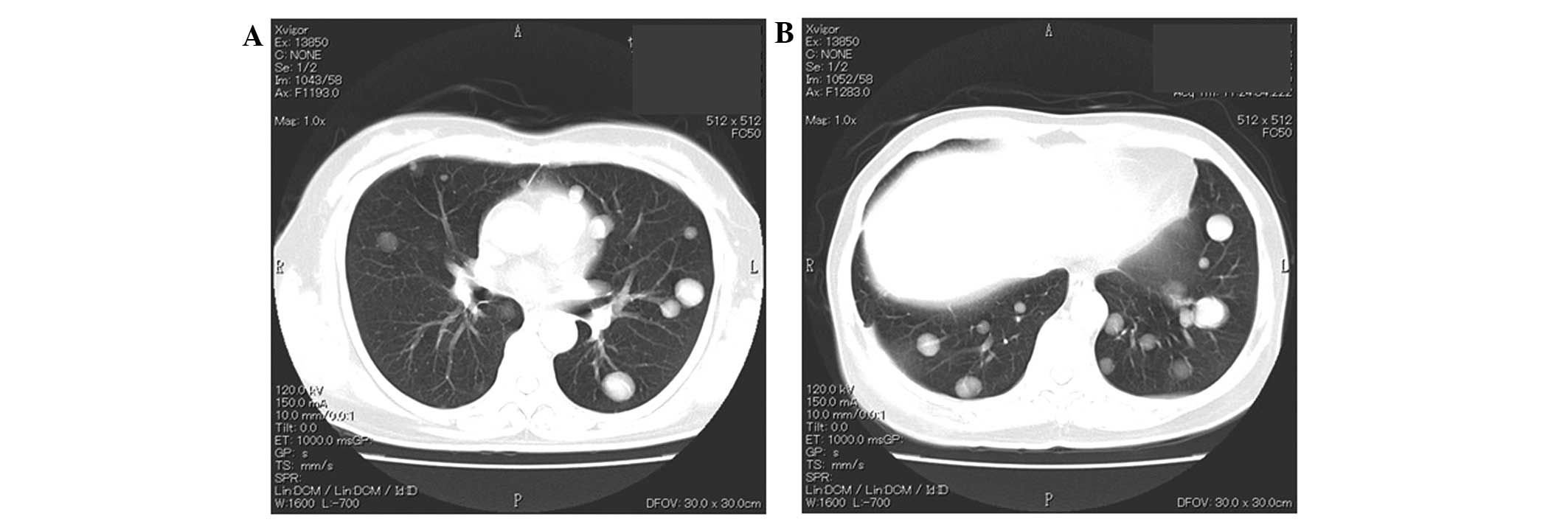
metastasis to the left acetabulum. Paclitaxel (60 mg/m2)
and carboplatin (area under the curve, 2) were administered weekly
for six months resulting in stabilization of the lung nodules and
the mass under the right scapula, however, the bone metastases in
the acetabulum continued to progress. Following six months of
paclitaxel/carboplatin therapy (Fig.
3), the patient refused any aggressive intervention and oral
etoposide (25 mg/day) was administered intermittently for eleven
years. After eleven years, due to the risk of secondary leukemia,
oral etoposide was replaced with cyclophosphamide hydrate and
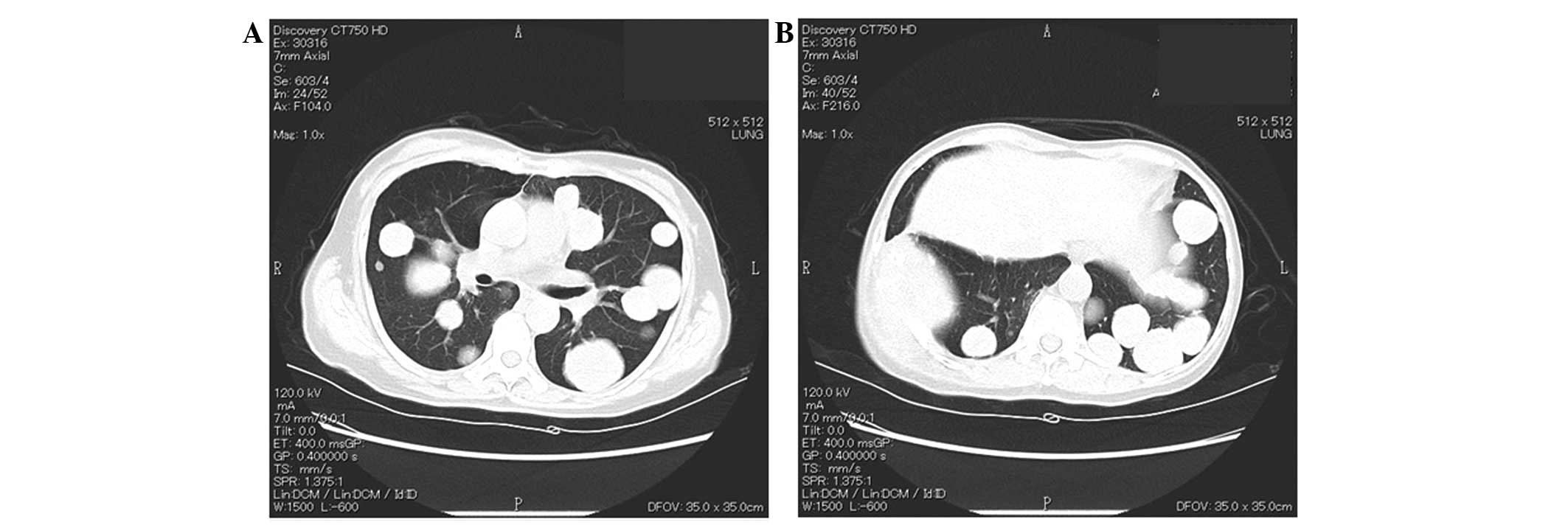
continued until the present. During this period, the metastatic
lesions progressed slowly (Fig. 4).
Leg pain that is associated with the bone metastases is currently
controlled by a non-steroidal anti-inflammatory medicine.
Consequently, the patient has survived with the disease for 24
years since the initial diagnosis of stage IV malignant struma
ovarii and for 20 years since the cancer recurred.
Discussion
Malignant struma ovarii is a particularly rare type
of tumor, which arises within mature teratomas in 0.1–0.3% of cases
(2,6,8).
According to the literature, metastasis from malignant struma
ovarii has only been reported in 5–6% of cases (7). Robboy et al (9) estimated the annual incidence as <1
in 10,000,000 females per year in the USA. Continuous follow-up
over a long period is considered to be necessary as in certain
cases, particularly in the follicular type, the cancer may recur in
subsequent years. In the present case, although the tumor was stage
IV advanced, the primary tumor and the bone metastases were
surgically resected and the patient remained tumor-free for four
years until multiple lung metastases developed.
The physical presentation of malignant struma ovarii
is non-specific. The majority of patients present with abdominal
swelling, pain or a mass (2,9,10)
and hyperthyroidism occurs in 8% of patients (8). Furthermore, the diagnosis of malignant
struma ovarii is predominantly determined subsequent to surgical
resection of the primary ovarian tumor (10,11).
Thus, the present case is rare in its process of diagnosis, which
was as follows: Initially the patient suffered from back pain and a
rib tumor was identified; pathological examination of the resected
tumor indicated a metastatic carcinoma originating from the
thyroid, however, a thorough examination of the thyroid gland
revealed no primary tumor; finally a subsequent CT scan identified
an ovarian mass. The pathological findings of the ovarian tumor
were consistent with a metastatic tumor, specifically a malignant
struma ovarii. To the best of our knowledge, only one such similar
case exists in the literature, in which metastasis to the femur
preceded the diagnosis of a primary ovarian tumor. However, the
clinical course of the case was not reported in detail (9). Although metastasis of malignant struma
ovarii is rare, an ovarian origin should be considered when a
metastatic thyroid tumor with no apparent thyroid origin is
identified.
The diagnosis of malignant struma ovarii remains
controversial. The pathological diagnosis is primarily established
according to the criteria for tumors of the thyroid gland, which
includes the presence of papillary formations lined with
overlapping ground glass nuclei and vascular invasion. Papillary
carcinoma is the most common type of malignant struma ovarii
(10,11), however, histological malignancy in
struma does not necessarily indicate a biological malignancy.
Robboy et al (9) analyzed 88
cases of malignant struma ovarii (the largest series reported thus
far) and classified the tumors into biological and histologic
malignancies. A biological malignancy was defined as: i) The
ovarian tumor having metastasized beyond the ovary, or having
penetrated the ovarian surface; or ii) the ovarian tumor having
recurred, regardless of earlier observations in the ovary. Of the
88 cases, 27 cases were classified as biologically malignant,
however, of these only 12 cases were also classified as
histologically malignant. The tissue from the remaining 15 cases
was identified as histologically benign and unremarkable thyroid
tissue. Histologically benign struma ovarii is also termed
proliferative struma ovarii and is characterized by
hyperplastic-type papillary formations and densely packed small
follicles, however, it lacks the cytologic features required for a
histologic malignancy. By contrast, among the 28 cases that were
classified as histologically malignant, only 10 cases were
identified as biologically malignant. Therefore, histological
malignancy could not effectively be used to predict the subsequent
clinical course. The 28 histologically malignant cases in the study
by Robboy et al (9)
consisted of 20 cases of papillary carcinoma, four cases of
follicular carcinoma and four cases of a follicular variant of
papillary carcinoma. Among these, 4 (20%), 4 (100%) and 2 (50%)
cases, respectively, demonstrated biological malignancy.
Furthermore, all four cases of follicular carcinoma exhibited
extra-ovarian metastasis at the time of primary surgery. The
present report, which demonstrated bone metastases at initial
diagnosis, was classified as a follicular carcinoma, indicating
that this histological subtype is more susceptible to early
metastasis. Furthermore, in primary thyroid cancer, follicular
carcinoma has a higher frequency of hematogenous metastasis when
compared with papillary carcinoma (12).
The treatment of malignant struma ovarii remains
controversial due to its rarity, as well as the difficulty in
distinguishing between biologically benign and malignant cases
(8). Surgical procedures range from
conservative surgery, in which fertility is preserved, to staging
surgery. The metastatic pattern of malignant struma ovarii
resembles that of ovarian cancer. Metastasis can occur via the
regional lymphatic system to the pelvic and paraaortic lymph nodes,
directly to the omentum, the peritoneal cavity or the contralateral
ovary, or hematogenously to the bone, lungs, liver and brain
(2,5,6,8).
Therefore, for advanced or disseminated disease, certain reports
propose performing complete staging surgery for ovarian cancer,
including pelvic and paraaortic lymph node sampling, peritoneal
washing cytology, and omentectomy (2,10,11,13).
Adjuvant therapy is not yet standardized, however, in previous
reports 131I therapy, radiation and chemotherapy have
been utilized (9). Vadmal et
al (12) and Brenner et
al (14) stated that in
patients with residual abdominal disease, or with recurrent or
metastatic tumors, a total cervical thyroidectomy followed by the
administration of 131I therapy may be effective.
DeSimone et al (2) reviewed
24 cases in the literature, in which no recurrence occurred in the
four patients treated with adjuvant 131I therapy and
seven of eight patients that were treated with 131I
therapy following recurrence initially achieved a complete
response. Thus, it was proposed that thyroidectomy and
131I therapy should be considered as the first line of
management for malignant struma ovarii. One case report described
chemotherapy as a treatment modality. Pardo-Mindan and Vazquez
(15) administered L-phenylalanine
mustard, Adriamycin and vincristine to a patient exhibiting
extended dissemination, however, the tumor continued to grow.
Chemotherapy was considered in the present case as malignant struma
ovarii is classified as a malignant germ cell tumor. The
administration of bleomycin was avoided due to the presence of
multiple lung metastases and instead paclitaxel/carboplatin was
administered, however, this treatment regimen was not effective.
The patient declined aggressive intervention, therefore, oral
anticancer agents were administered for long-term treatment. During
this period, the tumor progressed slowly. Although it is not
apparent in the present case, oral anticancer agents may be
effective in maintaining tumor dormancy in slow-growing tumors.
In conclusion, the present report describes the case
of a long-term survivor of metastatic malignant struma ovarii.
Despite extra-ovarian metastasis at the initial diagnosis, the
patient has survived with the disease for >20 years. Certain
malignant struma ovarii are slow-growing and may have a long
clinical course, therefore, aggressive cytotoxic chemotherapy may
not be effective, although long-term use of an oral chemo-reagent
may have certain benefits. These characteristics, as well as the
possible efficacy of 131I therapy, indicate that
malignant struma ovarii tumors resemble a primary thyroid carcinoma
more than an ovarian germ cell tumor. Recent advances in molecular
research of thyroid cancer have indicated the importance of
activated MAPK and PI3K/AKT signaling pathways (14). Additionally, clinical trials with an
anti-angiogenic reagent are ongoing (16). Future applications of these reagents
may benefit patients presenting with malignant struma ovarii. Due
to the rarity of malignant struma ovarii, it would be difficult to
conduct a prospective trial to establish the ideal treatment;
therefore, the development of biomarkers that distinguish between
biologically malignant and benign tumors is required.
References
|
1
|
Salvatori M, Dambra DP, D’Angelo G, Conte
LL, Locantore P, Zannoni G, Campo V and Campo S: A case of
metastatic struma ovarii treated with 131I therapy:
focus on preservation of fertility and selected review of the
literature. Gynecol Endocrinol. 24:312–319. 2008.
|
|
2
|
DeSimone CP, Lele SM and Modesitt SC:
Malignant struma ovarii: a case report and analysis of cases
reported in the literature with focus on survival and
I131 therapy. Gynecol Oncol. 89:543–548. 2003.
|
|
3
|
Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS
and Kim HS: Clinical characteristics of struma ovarii. J Gynecol
Oncol. 19:135–138. 2008.
|
|
4
|
Devaney K, Snyder R, Norris HJ and
Tavassoli FA: Proliferative and histologically malignant struma
ovarii: a clinicopathologic study of 54 cases. Int J Gynecol
Pathol. 12:333–343. 1993.
|
|
5
|
Steinman RA, De Castro IO, Shrayyef M,
Chengazi V, Giampoli E, Van Der Sloot P, Calvi LM, et al: Two cases
of malignant struma ovarii with metastasis to pelvic bone. Gynecol
Obstet Invest. 75:139–144. 2013.
|
|
6
|
Dardik RB, Dardik M, Westra W and Montz
FJ: Malignant struma ovarii: two case reports and a review of the
literature. Gynecol Oncol. 73:447–451. 1999.
|
|
7
|
Heintz AP, Odicino F, Maisonneuve P, et
al: Carcinoma of the ovary. FIGO 26th Annual Report on the Results
of Treatment in Gynecological cancer. Int J Gynaecol Obstet.
95(Suppl 1): S161–S192. 2006.
|
|
8
|
Zakhem A, Aftimos G, Kreidy R and Salem P:
Malignant struma ovarii: report of two cases and selected review of
the literature. J Surg Oncol. 43:61–65. 1990.
|
|
9
|
Robboy SJ, Shaco-Levy R, Peng RY, Snyder
MJ, Donahue J, Bentley RC, Bean S, et al: Malignant struma ovarii:
an analysis of 88 cases, including 27 with extraovarian spread. Int
J Gynecol Pathol. 28:405–422. 2009.
|
|
10
|
Makani S, Kim W and Gaba AR: Struma Ovarii
with a focus of papillary thyroid cancer: a case report and review
of the literature. Gynecol Oncol. 94:835–839. 2004.
|
|
11
|
Hatami M, Breining D, Owers RL, Del Priore
G and Goldberg GL: Malignant struma ovarii - a case report and
review of the literature. Gynecol Obstet Invest. 65:104–107.
2008.
|
|
12
|
Passler C, Scheuba C, Prager G, Kaczirek
K, Kaserer K, Zetting G and Niederle B: Prognostic factors of
papillary and follicular thyroid cancer: differences in an
iodine-replete endemic goiter region. Endocr Relat Cancer.
11:131–139. 2004.
|
|
13
|
Vadmal MS, Smilari TF, Lovecchio JL, Klein
IL and Hajdu SI: Diagnosis and treatment of disseminated struma
ovarii with malignant transformation. Gynecol Oncol. 64:541–546.
1997.
|
|
14
|
Brenner W, Bohuslavizki KH, Wolf H, Sippel
C, Clausen M and Henze E: Radiotherapy with iodine-131 in recurrent
malignant struma ovarii. Eur J Nucl Med. 23:91–94. 1996.
|
|
15
|
Pardo-Mindan FJ and Vazquez JJ: Malignant
struma ovarii. Light and electron microscopic study. Cancer.
51:337–343. 1983.
|
|
16
|
Sherman SI: Targeted therapy of thyroid
cancer. Biochem Pharmacol. 80:592–601. 2010.
|