Introduction
Prostate cancer is the most common malignant tumor
in males (1). Conventional
treatment, including surgery and radiotherapy, has potential
secondary effects, such as impotence or incontinence, that can
greatly impair quality of life. By contrast, specific immunotherapy
has no severe side-effects, as it affects only the malignant cells
and spares the healthy tissue. Dendritic cell (DC) vaccination is
an important immunotherapeutic strategy. Oncolytic virotherapy and
hyperthermia can have synergistic functions with immunotherapy
(2).
The approval of the first therapeutic anticancer
vaccine, sipuleucel-T, by the Food and Drug Administration for the
treatment of metastatic hormone-refractory prostate cancer in April
2010 has enforced a new era of immunotherapy (3). In a phase III trial, following
vaccination with activated autologous DC, a prolonged overall
survival time was demonstrated in patients suffering from
castration-resistant prostate cancer (4). In addition, a number of other clinical
trials have reported the clinical benefit of DC vaccination
(5).
Another promising approach is the use of oncolytic
viruses that preferentially infect tumor cells. Newcastle disease
virus (NDV) is an avian RNA paramyxovirus with a high safety
profile in cancer patients. The three properties that make NDV
suited for fighting human cancer are its tumor-selective
replication, antitumor cytotoxicity and immunostimulation (6).
Hyperthermia has been used for the treatment of a
diverse range of solid tumors. Various techniques for the
application of heat have been developed. The cellular effects that
have been described include the induction of apoptosis and the
expression of heat shock proteins (HSPs) (7). Also, synergistic effects of
hyperthermia in combination with chemotherapy and irradiation have
been observed (8). The present
study reports the effects of combining hyperthermia with oncolytic
virotherapy and DC-based immunotherapy.
Case report
Case history
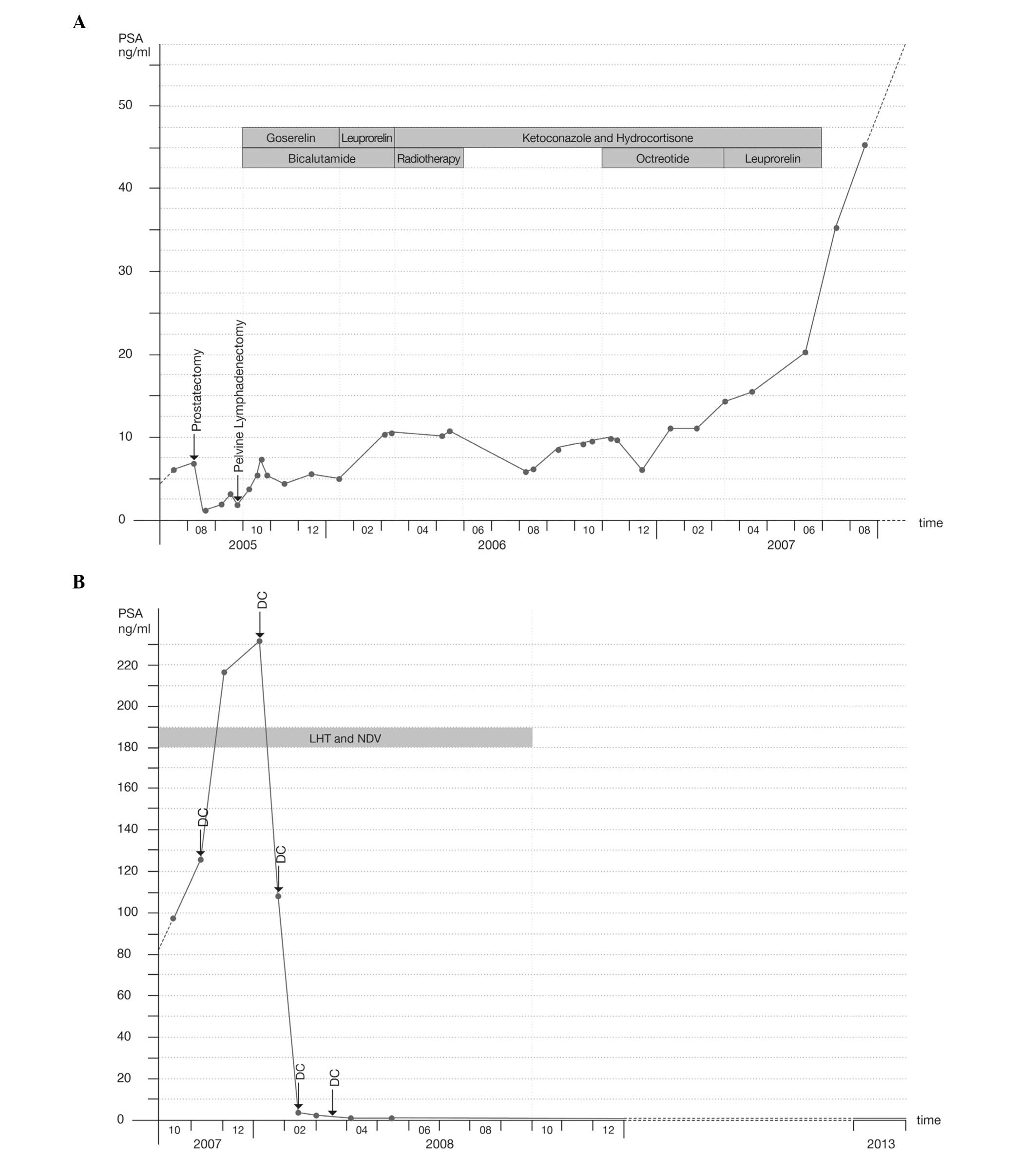
In October 2007, a 75-year-old patient presented at
the Immunological and Oncological Center (Cologne, Germany) with
progressive, hormone-refractory prostate cancer, with a
prostate-specific antigen (PSA) doubling time of 65 days and the
presence of bone metastases. The patient had previously undergone a
radical prostatectomy in August 2005, at the time of the initial
diagnosis. The post-surgical staging was pT3b pNX L1 V1 R0, with a
Gleason Score of 9 (5+4). In September 2005, a bilateral pelvic
lymphadenectomy was performed, which demonstrated no evidence of
metastases (0/32). In October 2005, the patient started androgen
suppression with goserelin (3.6 mg, once a month) and bicalutamide
(50 mg, once a day), and in January 2006, the goserelin was
switched to leuprorelin (10.72 mg, once every three months).
Despite treatment, the patient’s PSA levels rose and a subcranial
bone metastasis developed in March 2006. The tumor was classified
as hormone-refractory and the androgen suppression was
discontinued. Between March and May 2006, the patient underwent
palliative radiotherapy with 45 Gy (30×1, 5 Gy), leading to a
decrease in PSA levels from 11.6 to 6.5 ng/ml (following
prostatectomy PSA is normally undetectable). Between March 2006 and
June 2007, the patient was treated with ketoconazole (3×400 mg,
once a day) and hydrocortisone (morning dose, 20 mg and evening
dose, 10 mg, daily) in an attempt to block adrenal and testicular
androgen synthesis. Within the scope of a clinical trial,
octreotide was administered experimentally between November 2006
and March 2007. Due to rising PSA levels (from 40.8 ng/ml to 60.5
ng/ml), octreotide was discontinued in favor of another attempt
with leuprorelin (10.72 mg, once every three months) between March
and June 2007. Upon termination of androgen deprivation, rising
testosterone levels (from 0.18 ng/ml in October 2007 to 6.25 ng/ml
in May 2013; normal range, 2.14–8.27 ng/ml) were documented. In
April 2007, a scintigram revealed bone metastases in two ribs and
the left sacrum. From July 2007, the PSA levels rose further, most
likely due to the progress of osseous metastases. In September
2007, positron emission tomography/computed tomography (PET/CT)
revealed extensive, disseminated bone metastases of the entire
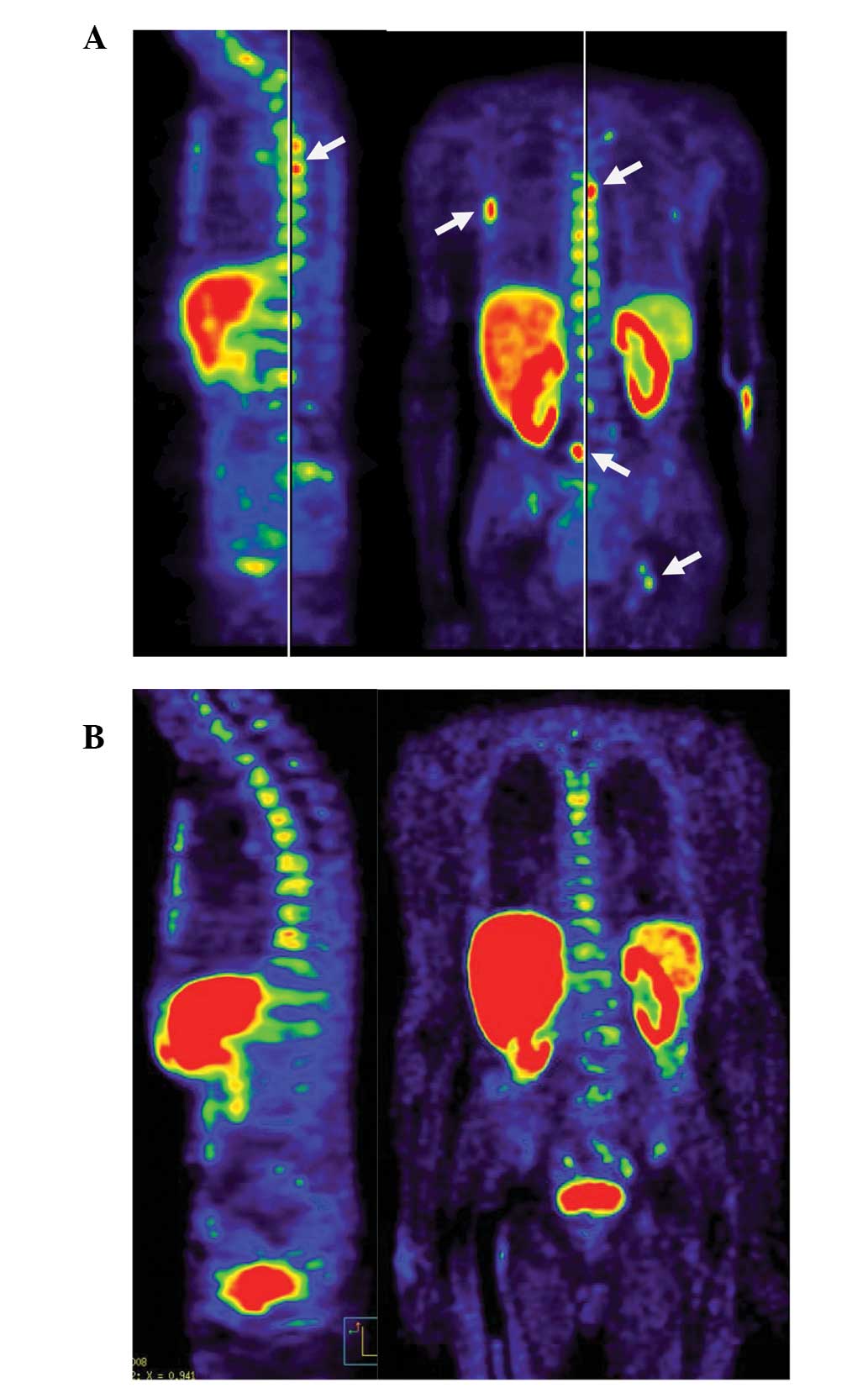
spine, pelvis, right humerus and ribcage [ECAT EXACT 47, (Siemens
Medical Systems, Erlangen, Germany); visualised using MPI-Tool,
(Advanced Tomo Vision GmbH, Kerpen, Germany)] (Fig. 1A). The university hospital at which
the patient was being treated advised the commencement of
chemotherapy, but the patient decided to begin immunotherapy. By
October 2007, the patient’s PSA level had risen to 98.1 ng/ml
(Diamond Select GEMINI GXL 16 PET/CT System, Philips, Eindhoven,
Netherlands; visualised using Syntegra Imaging software version
2.1, Philips)(Fig. 2A).
Immunotherapy
Between October 2007 and June 2008, the patient was
treated at the Immunological and Oncological Center (Cologne,
Germany) with locoregional hyperthermia of the pelvis and thorax,
and systemic oncolytic NDV virotherapy approximately twice a week.
In addition, in November 2007, the patient received two sessions of
local hyperthermia (LHT) of the occiput.
Between November 2007 and March 2008, the patient
received five vaccinations with autologous antigen-pulsed DCs
combined with moderate whole-body hyperthermia. Between July and
September 2008, the patient was treated with LHT of the pelvis and
thorax, and oncolytic NDV virotherapy once a month to sustain the
immune response. After September 2008, no further immunological
treatment was considered necessary.
Hyperthermia was administered with the Oncothermia
EHY-2000 device (Oncotherm GmbH, Troisdorf, Germany) with a
radiofrequency of 13 MHz. In total, the patient received 46
hyperthermia treatments to the thorax, 54 to the pelvis and two to
the occiput. The duration of the sessions was 50 min each, starting
at 60 W and increasing to 130 W. Intravenous administration of 109
plaque-forming units of NDV (strain MTH-68) were provided per
session.
DCs were differentiated from autologous monocytes
with granulocyte-macrophage colony-stimulating factor and
interleukin-4. Immature DCs were pulsed with a lysate from
NDV-infected DU145 prostate carcinoma cells, termed the oncolysate.
Subsequent to maturation, the cells were administered intradermally
simultaneously with interferon-γ (IFN-γ; 0.1 mg), followed by
moderate whole-body hyperthermia (temperature, 38.5–39.0°C;
infrared device, Heckel-HT2000; Heckel Medizintechnik GmbH,
Esslingen, Germany).
Outcome and follow-up
By early January 2008, the PSA levels had reached a
maximum of 233.8 ng/ml. In late January and throughout February,
the PSA levels decreased to 0.8 ng/ml. In March 2008, a reduction
in the bone metastases was detected by PET/CT (Fig. 1B). The PSA level has remained low up
to the present time and was <0.03 ng/ml in December 2013
(Fig. 2B).
Since there was sustained cancer remission, the
patient was tested for the development of an immunological
antitumor memory T-cell response. An Enzyme-Linked ImmunoSpot assay
(Autoimmun Diagnostika GmbH, Strassberg, Germany) was performed in
July 2011 to quantify the numbers of T cells that were secreting
IFN-γ upon short-term (48-h) contact with oncolysate-pulsed
autologous DCs. Overall, 150±10 patient blood-derived circulatory T
cells per 100,000 T cells were found to respond, but without
oncolysate pulsing there was only a background response of T cells
and DCs of 2±1 cells.
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Discussion
The first conclusion that can be made from this case
study is that immunotherapy can have an impact on metastatic
prostate cancer. The described procedures provoked no relevant
side-effects. The successful experimental approach involved a
combination of LHT, oncolytic virotherapy and DC vaccination. It is
likely that the observed immunological memory T-cell response
contributed to the long-term effects of treatment, as has been
described recently for colon carcinoma (9).
The second conclusion that can be made is that this
case is of importance and relevance, as it demonstrates that
immunotherapy is not restricted to early-stage cancer, as has been
previously assumed (10). The
results were achieved by a novel and scientifically well-founded
combination of biological (NDV and DC) and physical (LHT) treatment
procedures that produce synergistic effects. The strategy is not
restricted to a particular type of cancer and may thus have broad
implications for clinical oncology in general.
Lately, the traditional therapy for prostate cancer,
in particular radical prostatectomy, has been questioned (11). Generally, the presence of metastases
should be determined prior to prostatectomy. The remaining and
quickly rising post-surgical PSA-levels in the present patient
suggested that there had been metastases at the time of the
surgical intervention. Therefore, in this particular case, the
prostatectomy had not only been in vain, but had also led to
permanent incontinence. All further conventional therapies had
failed. Apart from the immunotherapy described, the patient was not
treated with any other conventional or alternative therapy and did
not undergo any significant change in lifestyle.
In the following analysis, an explanation for the
success of this novel combined treatment is outlined. To avoid
possible immune escape mechanisms, including antigen shift and the
induction of tolerance by the tumor, a DC vaccine with multiple
prostate carcinoma antigens was used and two strategies for the
introduction of danger signals into the tumor cells were utilized,
consisting of oncolytic viruses and hyperthermia.
NDV infection introduces foreign viral RNA,
activating the endosomal Toll-like receptor-3, cytoplasmic retinoic
acid-inducible gene 1 and the plasma membrane-expressed viral
hemagglutinin-neuraminidase proteins, leading to the induction of
IFN-α and -β, and to the potentiation of T-cell-mediated antitumor
immunity (10). The rationale for
the combination of virus infection with hyperthermia was an
expected synergy, as LHT has been reported to enhance virus tumor
targeting and replication (2,12). In
addition, viral infection and hyperthermia each cause an
endoplasmic reticulum stress response (5,13),
change the expression of HSP 70/90 and the surface properties
(calreticulin) of tumor cells, and induce immunogenic tumor cell
death mechanisms (14). This leads
to antigen uptake by host DCs, cross-presentation of autologous
tumor antigens and priming of specific T cells. Subsequent
vaccination with oncolysate-pulsed DCs results in further priming
and activation of T-cell antitumor immunity. Ideally, this elicits
an effective antitumor cytotoxic reaction and leads to long-lasting
antitumor T-cell memory. DC vaccination was combined with moderate
whole-body hyperthermia, as DC function can be enhanced at elevated
temperatures (15). More than three
years after the DC vaccination, a good antitumor memory T-cell
response was detected in the present patient.
Taken together, the combination of different
immunotherapeutic strategies led to a surprising therapeutic
success in the patient. In a different patient with inoperable
metastasized prostate carcinoma, treated at the Immunological and
Oncological Center, a similar strategy has led to sustained disease
stabilization over a time-period of more than four years. The
combination of DC vaccination, oncolytic NDV application and
hyperthermia appears to be effective and deserves further
investigation.
Single case studies, such as the present study, can
provide important innovation and additions to our medical
knowledge. Single case use, as approved by the German
Pharmaceuticals Act (16), is
important for patients and for medical progress.
Abbreviations:
|
DC
|
dendritic cell
|
|
HSP
|
heat shock protein
|
|
IFN
|
interferon
|
|
LHT
|
local hyperthermia
|
|
NDV
|
Newcastle disease virus
|
References
|
1
|
Robert-Koch Institut. The Federal Health
Monitoring-Prostate Diseases. Robert Kock-Institut; Berlin: pp.
362007
|
|
2
|
Eisenberg DP, Carpenter SG, Adusumilli PS,
Chan MK, Hendershott KJ, Yu Z and Fong Y: Hyperthermia potentiates
oncolytic herpes viral killing of pancreatic cancer through a heat
shock protein pathway. Surgery. 148:325–334. 2010.
|
|
3
|
Dendreon Press Release: FDA Approves
PROVENGE(R) for the Treatment of Men with Advanced Prostate Cancer.
http://http://investor.dendreon.com/releasedetail.cfm?ReleaseID=533434.
Accessed August 7, 2013
|
|
4
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, Xu Y, Frohlich MW and Schellhammer PF; IMPACT study
investigators. Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010.
|
|
5
|
Draube A, Klein-González N, Mattheus S,
Brillant C, Hellmich M, Engert A and von Bergwelt-Baildon M:
Dendritic cell based tumor vaccination in prostate and renal cell
cancer: a systematic review and meta-analysis. PLoS One.
6:e188012011.
|
|
6
|
Fournier P, Bian H, Szeberényi J and
Schirrmacher V: Analysis of three properties of Newcastle disease
virus for fighting cancer: tumor-selective replication, antitumor
cytotoxicity, and immunostimulation. Methods Mol Biol. 797:177–204.
2012.
|
|
7
|
Hildebrandt B, Wust P, Ahlers O, Dieing A,
Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and
molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56.
2002.
|
|
8
|
Rao W, Deng ZS and Liu J: A review of
hyperthermia combined with radiotherapy/chemotherapy on malignant
tumors. Crit Rev Biomed Eng. 38:101–116. 2010.
|
|
9
|
Schirrmacher V, Fournier P and Schlag P:
Autologous tumor cell vaccines for post-operative active-specific
immunotherapy of colorectal carcinoma: long-term patient survival
and mechanism of function. Expert Rev Vaccines. 13:117–130.
2014.
|
|
10
|
Gulley JL, Madan RA and Schlom J: Impact
of tumour volume on the potential efficacy of therapeutic vaccines.
Curr Oncol. 18:e150–e157. 2011.
|
|
11
|
Wilt TJ, Brawer MK, Jones KM, et al:
Prostate Cancer Intervention versus Observation Trial (PIVOT) Study
Group: Radical prostatectomy versus observation for localized
prostate cancer. N Engl J Med. 367:203–213. 2012.
|
|
12
|
Chang E, Chalikonda S, Friedl J, Xu H,
Phan GQ, Marincola FM, Alexander HR and Bartlett DL: Targeting
vaccinia to solid tumors with local hyperthermia. Hum Gene Ther.
16:435–444. 2005.
|
|
13
|
Workenhe ST and Mossman KL: Oncolytic
virotherapy and immunogenic cancer cell death: sharpening the sword
for improved cancer treatment strategies. Mol Ther. 22:251–256.
2014.
|
|
14
|
Hou W, Zhang Q, Yan Z, Chen R, Zeh HJ III,
Kang R, Lotze MT and Tang D: Strange attractors: DAMPs and
autophagy link tumor cell death and immunity. Cell Death Dis.
4:e9662013.
|
|
15
|
Knippertz I, Stein MF, Dörrie J, Schaft N,
Müller I, Deinzer A, Steinkasserer A and Nettelbeck DM: Mild
hyperthermia enhances human monocyte-derived dendritic cell
functions and offers potential for applications in vaccination
strategies. Int J Hyperthermia. 27:591–603. 2011.
|
|
16
|
Gagnier JJ, Kienle G, Altman DG, Moher D,
Sox H and Riley D; CARE Group. The CARE guidelines: consensus-based
clinical case report guideline development. J Clin Epidemiol.
67:46–51. 2014.
|