Introduction
Cytokeratins (CKs) are proteins of the intermediary
filaments of keratin, situated intracytoplasmically and present in
the cytoskeleton of all epithelial cells. The term CK was initially
used at the end of the 1970s (1)
when the protein subunits of the intermediary filaments from the
inside of cells were identified and characterized for the first
time. A novel nomenclature for keratins was established in 2006,
and the proteins that were previously known as CKs were termed
keratins (Ks) (2). There are two
types of Ks: Acidic (type I) and basic (type II). K5 is present at
the level of the basal layer of the keratinized and non-keratinized
stratified squamous epithelia. K5 expression is decreased at the
level of the spinosum stratum of the normal oral mucosa, and in the
dysplastic epithelium, K5 is positive in the basal, parabasal and
stratum spinosum cells. In the case of cancers, Ks serve
predominantly as tumor markers for diagnostic procedures.
Therefore, in the case of breast cancer, an association between the
young age of patients at presentation and the basal-like subtype
(characterized by the absence of the expression of estrogen,
progesterone and human epidermal growth factor receptor 2
receptors, and the presence of epidermal growth factor receptor and
K5 and K6 expression) has been recorded. Additionally, a higher
tumor grade has been associated with a prognosis of approximately
five years of disease-free-survival (3,4).
Squamocellular carcinomas, independently from their
origin, are characterized by the expression of the Ks of the
stratified epithelia (K5, K14 and K17) and overexpression of K6 and
K16 in hyperproliferating strata (5). The combined overexpression of K5 and
K14 was demonstrated in tumors of the oral cavity, in the
oropharyngeal, hypopharyngeal and laryngeal areas (6,7) and in
the basal actively mitotic cells of the squamous stratified
epithelium (8). The expression of
K5 and K14 remains high even if the malignant grading decreases
(9–11).
Using these data as a stratification method, the aim
of the present study was to identify the K5 expression features in
the squamocellular carcinoma that were located in various regions
of the oral and maxillofacial area in order to improve the
diagnostic accuracy.
Materials and methods
Patients and specimens of squamocellular
carcinoma
A total of 13 biopsy fragments were evaluated, which
were obtained from patients that had been diagnosed with
squamocellular carcinoma in the following areas; larynx (n=2),
pharynx (n=2), hard palate (n=1), tongue (n=2), submandibular
(n=1), lip (n=1), gingival sulcus (n=1), nasal pyramid (n=1),
maxilla (n=1) and zygomatic (n=1). Patients provided written
informed consent.
Immunohistochemistry analysis
The biopsy fragments were placed into buffered
formalin (10%) for 48 h and subsequently paraffin was added. All
stages of the immunohistochemical technique were facilitated by the
use of an automated immunohistochemistry instrument (Leica
Bond-III; Leica Microsystems, GmbH, Wetzlar, Germany), according to
the manufacturer’s instructions and using a primary monoclonal
mouse antibody specific for CK5 (clone XM26, ready to use;
Novocastra Laboratories Ltd., Newcastle upon Tyne, UK). Following
dehydration in pure alcohol, the sections were placed in benzene to
replace ethanol prior to embedding in paraffin and subsequently
fitted using Canada balsam (Sigma-Aldrich, St. Louis, MO, USA). The
microscopic evaluation was performed with a Nikon Eclipse E600
microscope (Nikon Corporation, Tokyo, Japan) and the images were
obtained using the Laboratory Universal Computer Image Analysis G
system (Laboratory Imaging Co., Prague, Czech Republic). The
immunohistochemistry for K5 in the tumor cells was evaluated
according to the following scores: 0 (0% CK5-positive cells), 1
(<10% CK5-positive cells), 2 (10–30% CK5-positive cells) and 3
(>30% CK5-positive cells).
Results
Score of CK5 expression in various
squamocellular carcinoma
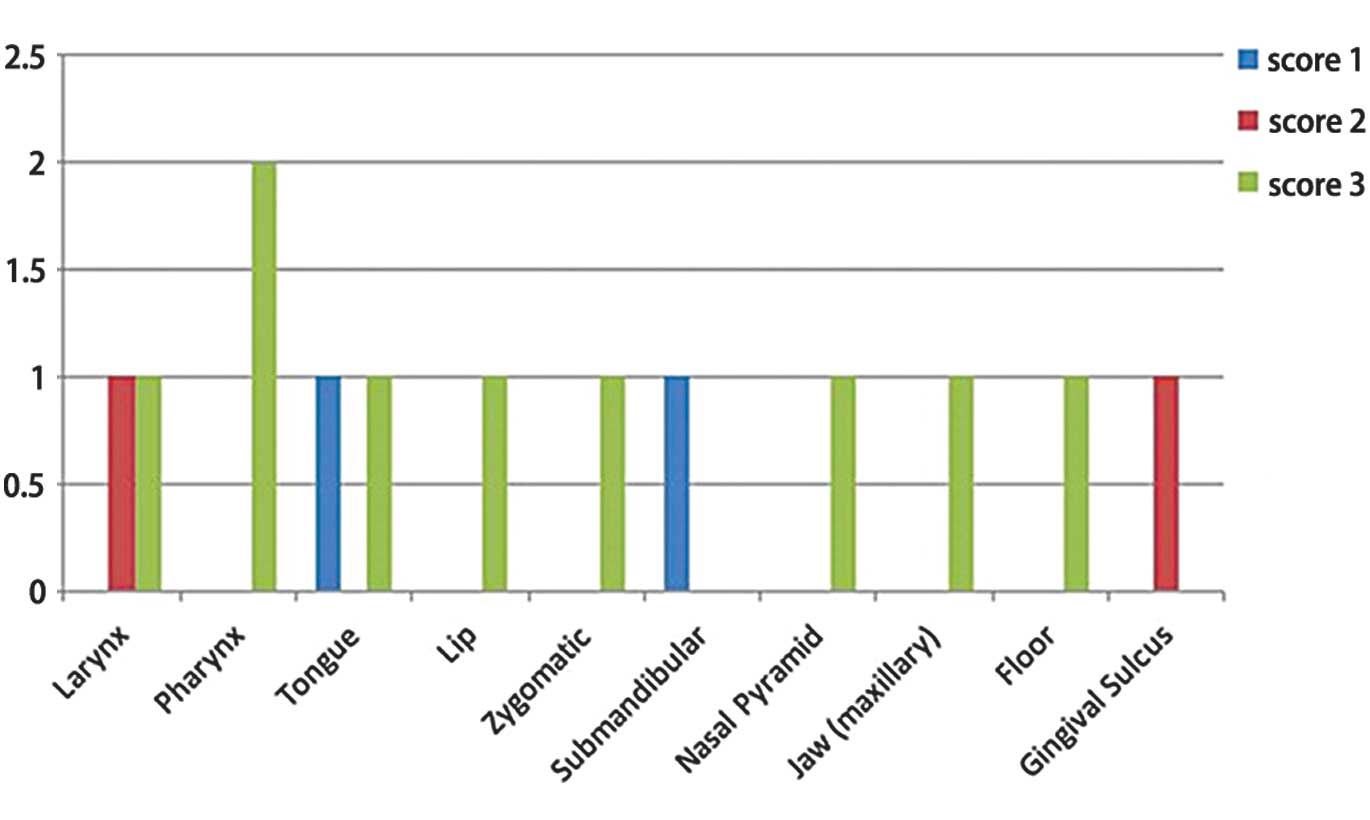
The morphological labeling indicated the presence of
seven cases of well-differentiated squamocellular carcinoma (two
larynx, two pharynx, one nasal pyramid, one lip and one zygomatic),
two were moderately differentiated (tongue and hard palate) and
four were poorly differentiated (tongue, submandibular area,
maxillary and gingival sulcus). In certain cases, at the level of
the larynx, scores of 2 were observed in addition to the presence
of two distribution models, which were as follows: i) Homogeneous
distribution, all the cells in the tumor area were positive for CK5
labeling; and ii) heterogeneous distribution, the CK5-positive
cells were prevalent at the periphery of the tumor areas. However,
the distribution of the immunohistochemical staining score was
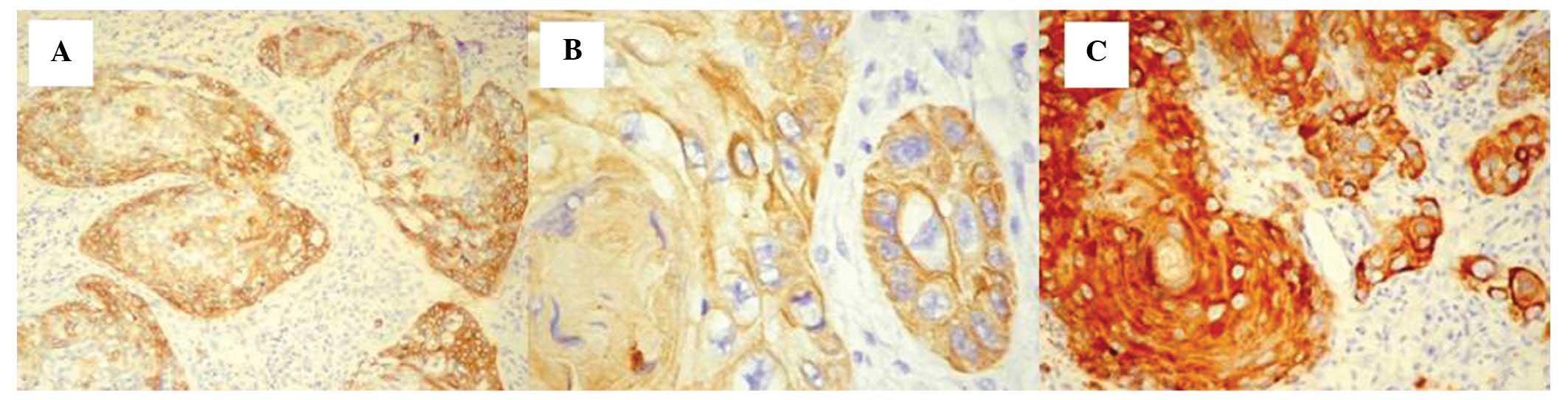
always 2 (Fig. 1A).
For the second case that originated from the larynx,
the score was 3, the distribution was homogeneous and a prevalent
cytoplasmic pattern was demonstrated (Fig. 1B).
The well-differentiated squamocellular carcinoma
cases, originating from the pharynx, exhibited a CK5 expression
score of 3 with a homogeneous distribution pattern and intense
immunohistochemical staining (Fig.
1C). All of the squamocellular carcinoma cases originating from
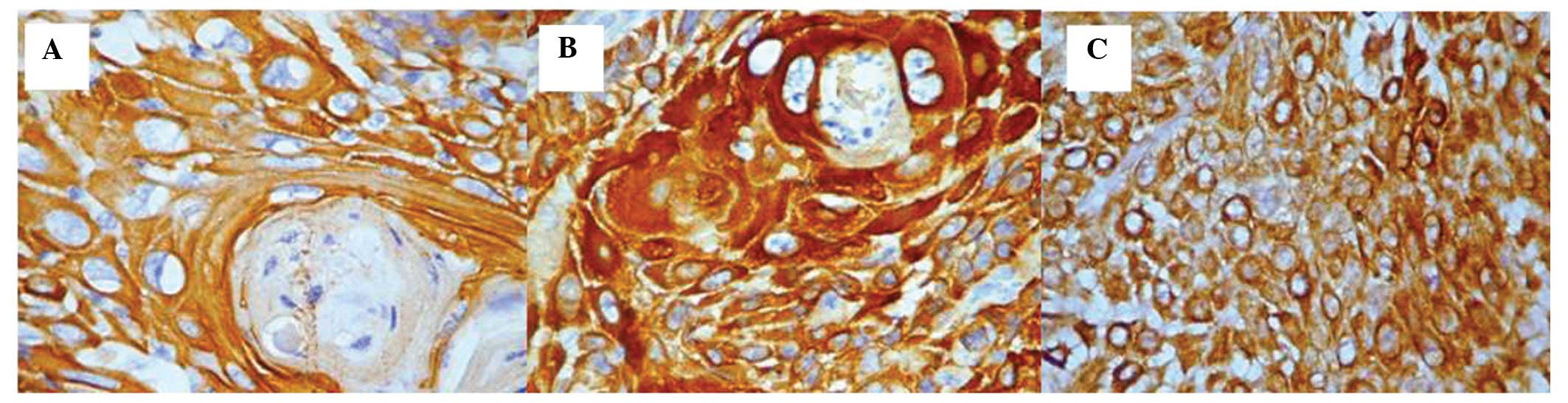
the lip, nasal pyramid and zygomatic area had a well-differentiated
grade. These cases were all highly positive for CK5 expression
(score, 3) with either a cytoplasmic or mixed (cytoplasmic and
membrane) pattern and a homogeneous distribution (Fig. 2A–C).
Moderately-differentiated squamocellular carcinoma
cases originating from the lip and hard palate demonstrated a
homogeneous distribution of CK5 in all the cells of the tumor area
with a score of 3.
Intensity and expression pattern of
CK5
With regard to the intensity of the
immunohistochemical staining and the associated expression pattern,
the following were observed: i) Maximal intensity and a cytoplasmic
pattern in the isolated cells, in addition to a mixed pattern in
the carcinomas located in the lip (Fig.
3A). ii) Heterogeneity of the intensity of the
immunohistochemical staining (moderate and intense), however, with
an expression pattern that was comparable between those that
originated from the lip and the carcinomas that originated from the
hard palate (Fig. 3B).
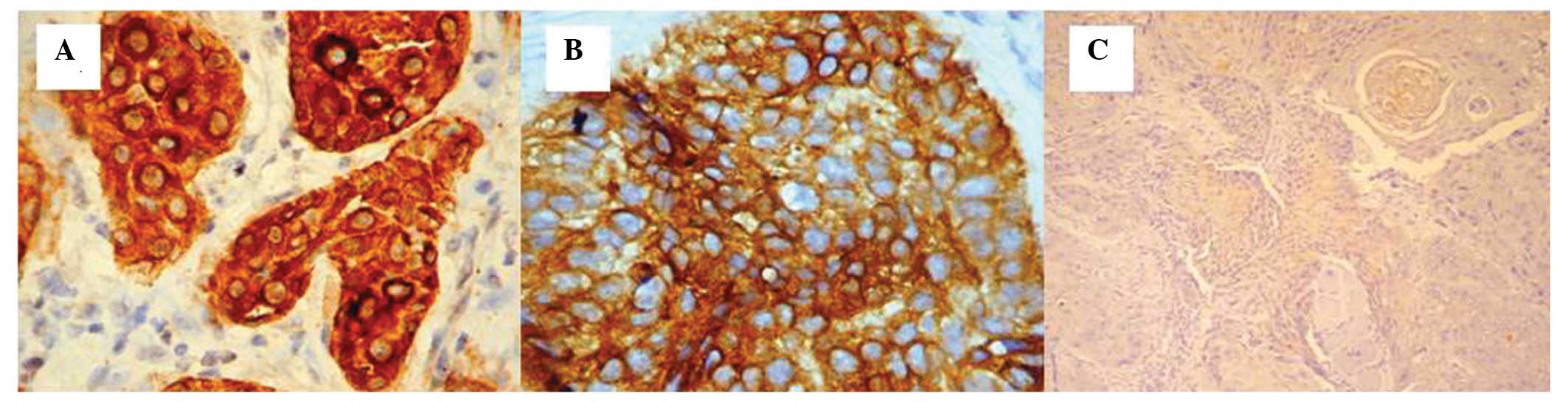
For all the four cases that were included in the
poorly-differentiated grade carcinoma category located in the
tongue, submandibular area, maxillary and gingival sulcus, a marked
reduction in the scores for the CK5 immunohistochemistry was
observed; for squamocellular carcinoma originating from the tongue,
a score of 1 was observed (Fig.
3C). The cells that were positive for CK5 were dispersed among
the cells that did not express CK5 and exhibited a decreased
reaction intensity and cytoplasmic expression pattern.
The squamocellular carcinoma case originating from
the submandibular area presented isolated regions with positive
cells in the tumor area (score, 1) as well as cytoplasmic
expression (Fig. 4A). For the case
originating from the upper jaw area, a high score of 3 was observed
(Fig. 4B).
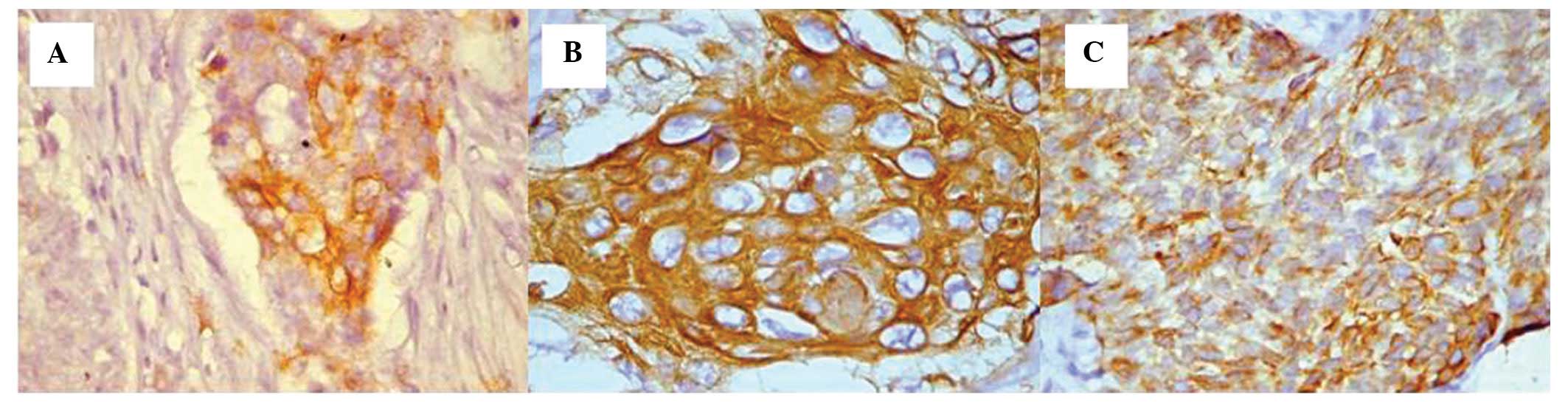
The squamocellular carcinoma that originated from
the gingival sulcus area preserved the same mixed aspect with
positive and negative cells present at the level of the tumor
areas. In this case, the intensity of the reaction was moderate
(score, 2) and the expression patterns were cytoplasmic or mixed
(Figs. 4C and 5).
Discussion
Expression of CK5 and CK14 was found to be specific
for mucosal areas and for tumors that originated from the oral
cavity, oropharynx, hypopharynx and larynx, and in the basal
actively mitotic cells of the squamous stratified epithelia
(8). Velluci et al (9) and Marley et al (10) observed a decreased expression of CK5
and CK14 along with malignant alterations; however, the expression
did not cease completely. The presence of K5 expression was
observed in all of the cases of squamocellular carcinoma that were
included in the present study.
Kaufmann et al (12) demonstrated a high expression of CK5
and CK6 in 81% of squamocellular carcinomas that were included in
their study, with an intense immunoexpression, which was diffuse in
the majority of the tumoral cells. In comparison to squamocellular
carcinomas, Kaufmann et al observed that only 14.2% of the
non-squamocellular carcinomas expressed CK5 and CK6, with
distribution in a reduced number of tumor cells. In the present
study, all the cases of squamocellular carcinomas originating from
the oral and maxillofacial areas expressed CK5 in the tumor cells,
with scores ranging from 1 to 3. Whereas, the variation between the
intensity scores for CK5/6 and p63 was relatively small in a
previous study (12). Crook et
al (13) highlighted that p63
expression in squamocellular carcinomas is not dependent on the
tumoral grade, as it was identified to be strongly expressed in all
cases of poorly-differentiated carcinoma originating from the
nasopharynx. The same expression was recorded for CK5 and CK6. In
the present study, a decrease of CK5 expression was observed in two
of the poorly-differentiated squamocellular carcinoma cases; one
originating from the tongue and the other from the submandibular
area, with scores of 1 (between 10–30% of positive tumoral cells).
An intermediate score of 2 was observed in the case originating
from the gingival sulcus. Maintenance of the maximal score of 3 was
observed in the case that originated from squamocellular carcinomas
located in the upper jaw. Malzahn et al (14) and Tot (15) reported differences between p63, and
CK5 and CK6 expression in breast carcinoma, where CK5 and CK6 were
positive in 61% of the cases, compared with p63 that was expressed
in 11% of the cases that were included in the study.
According to Chung et al (16) specific Ks, such as K14 and K15, are
associated the molecular classification of squamocellular
carcinomas based on gene analysis, however, the expression of CK5
and CK6 have not been analyzed. The present study promotes the
introduction of CK5 in classifying the subtypes of squamocellular
carcinomas.
In conclusion, CK5 was expressed in all of the types
of squamocellular carcinoma that were included in the present
study, with scores varying from 1 to 3 and a higher expression
observed in the poorly-differentiated carcinomas as follows: A
score of 3 for those originating from the jaw, 2 for the one
originating from the gingival sulcus, and 1 for carcinomas of the
tongue and submandibular area. The expression was present for well-
and moderately-differentiated histopathological grades with a
maximal score of 3 for all of the cases, with the exception of one
carcinoma of the larynx where the score was 2. The present study
confirms the role of CK5 in the definition of the differentiation
of squamocellular carcinoma of head and neck revealing a
differential expression depending on the anatomic site of the
primary tumour. On these bases, additional studies on a larger
series of patients are required.
References
|
1
|
Franke WW, Schmid E, Osborn M and Weber K:
Intermediate-sized filaments of human endothelial cells. J Cell
Biol. 81:570–580. 1979.
|
|
2
|
Schweizer J, Bowden PE, Coulombe PA,
Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA,
Rogers MA and Wright MW: New consensus nomenclature for mammalian
keratins. J Cell Biol. 174:169–174. 2006.
|
|
3
|
Cheang MC, Voduc D, Bajdik C, Leung S,
McKinney S, Chia SK, et al: Basal-like breast cancer defined by
five biomarkers has superior prognostic value than triple-negative
phenotype. Clin Cancer Res. 14:1368–1376. 2008.
|
|
4
|
Yamamoto Y, Ibusuki M, Nakano M, Kawasoe
T, Hiki R and Iwase H: Clinical significance of basal-like subtype
in triple-negative breast cancer. Breast Cancer. 16:260–267.
2009.
|
|
5
|
Moll R, Divo M and Langbein L: The human
keratins: biology and pathology. Histochem Cell Biol. 129:705–733.
2008.
|
|
6
|
Woodcock-Mitchell J, Eichner R, Nelson WG
and Sun TT: Immunolocalization of keratin polypeptides in human
epidermis using monoclonal antibodies. J Cell Biol. 95:580–588.
1982.
|
|
7
|
McDonald LA, Walker DM and Gibbins JR:
Cervical lymph node involvement in head and neck cancer detectable
as expression of a spliced transcript of type II keratin K5. Oral
Oncol. 34:276–283. 1998.
|
|
8
|
Lersch R, Stellmach V, Stocks C, Giudice G
and Fuchs E: Isolation, sequence, and expression of a human keratin
K5 gene: transcriptional regulation of keratins and insights into
pairwise control. Mol Cell Biol. 9:3685–3697. 1989.
|
|
9
|
Vellucci VF, Germino FJ and Reiss M:
Cloning of putative growth regulatory genes from primary human
keratinocytes by subtractive hybridization. Gene. 166:213–220.
1995.
|
|
10
|
Marley JJ, Robinson PA and Hume WJ:
Expression on human cytokeratin 14 in normal, premalignant and
malignant oral tissue following isolation by plaque differential
hybridization. Eur J Cancer B Oral Oncol. 30B:305–311. 1994.
|
|
11
|
Stanzione M, Petillo O, Calarco A,
Valarezo E, Napoli M, Longo P, Riccitiello F, Vittoria V and Peluso
G: Enhanced in vitro antitumor activity of a titanocene complex
encapsulated into polycaprolactone (PCL) electrospun fibers. J Appl
Biomater Funct Mater. 11:61–70. 2013.
|
|
12
|
Kaufmann O, Fietze E, Mengs J and Dietel
M: Value of p63 and cytokeratin 5/6 as immunohistochemical markers
for the differential diagnosis of poorly differentiated and
undifferentiated carcinomas. Am J Clin Pathol. 116:823–830.
2001.
|
|
13
|
Crook T, Nicholls JM, Brooks L, et al:
High level expression of deltaN-p63: a mechanism for the
inactivation of p53 in undifferentiated nasopharyngeal carcinoma
(NPC)? Oncogene. 19:3439–3444. 2000.
|
|
14
|
Malzahn K, Mitze M, Thoenes M and Moll R:
Biological and prognostic significance of stratified epithelial
cytokeratins in infiltrating ductal breast carcinomas. Virchows
Arch. 433:119–129. 1998.
|
|
15
|
Tot T: The cytokeratin profile of
medullary carcinoma of the breast. Histopathology. 37:175–181.
2000.
|
|
16
|
Chung CH, Zhang Q, Hammond EM, Trotti AM
III, Wang H, et al: Integrating epidermal growth factor receptor
assay with clinical parameters improves risk classification for
relapse and survival in head-and-neck squamous cell carcinoma. Int
J Radiat Oncol Biol Phys. 81:331–338. 2011.
|