Introduction
Glycogen storage disease type Ia (GSD-Ia; also
termed von Gierke disease) is an inherited metabolic disorder that
is caused by a deficiency of glucose-6-phosphatase, which leads to
the limited production of free glucose, and an excessive
accumulation of glycogen in the liver, kidney and intestinal
mucosa. The major short-term manifestations of the disease include
hypoglycemia, lactic acidemia and hepatomegaly. By contrast, the
development of hepatocellular adenoma presents a common long-term
complication of the disease (1,2). As a
result of these complications, liver transplantation is considered
to be the most effective treatment for GSD-Ia patients (3). To the best of our knowledge, no cases
of de novo gastric cancer in GSD-Ia patients following liver
transplantation have been reported. In the current study, the rare
case of a 29-year-old male who developed de novo gastric
cancer, following an orthotopic liver transplantation (OLT) twelve
years ago for the treatment of GSD-Ia is presented. Written
informed consent was obatined from the patient’s family.
Case report
In June 2001, a 29-year-old male underwent a
modified piggy-back liver transplantation for von Gierke disease.
Following the surgery, the patient’s quality of life markedly
improved. Tacrolimus (3 mg, every 12 h for one year), mycophenolate
mofetil (1000 mg, every 12 h for six months), and prednisone (5 mg,
every 12 h for two months) were administered for immunosuppression
following surgery and currently, the patient receives 50 mg
Ciclosporin in the morning and 75 mg at night, daily. In August
2013, the patient was admitted to Nanjing First Hospital (Nanjing,
China) due to melena that had been occurring for three days. The
melena disappeared following conservative treatment, which included
hydration with water, glucose, electrolytes and amino acids,
fasting and the administration of Losec, a proton-pump inhibitor. A
gastric endoscopy was subsequently performed and revealed a deep
ulcer with a necrotic gray area in the base, which was located in
the posterior wall of the fundus region of the stomach, accompanied
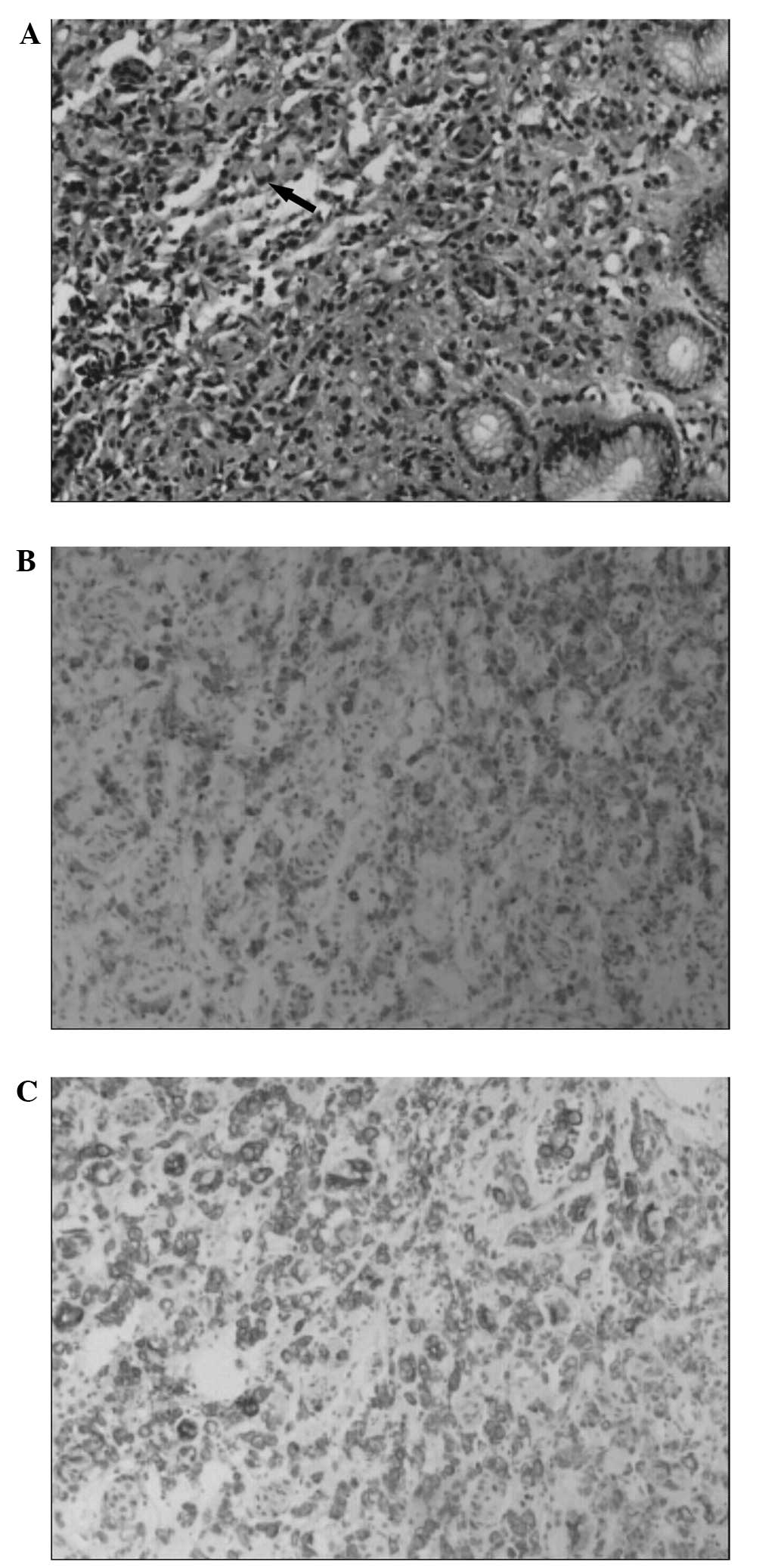
by edema in the immediately adjacent mucosa. Histological
examination of the biopsied specimen revealed signet-ring cells in
the muscularis propria (Fig. 1A)
and immunochemical staining was positive for epithelial membrane
antigen (Fig. 1B) and cytokeratin
(Fig. 1C) expression, which
indicated the diagnosis of signet-ring cell carcinoma. Enhanced
computed tomography scanning and magnetic resonance imaging of the
upper abdomen revealed a thickened stomach wall. In addition,
pre-operative serological examination revealed that the
α-fetoprotein (3.46 ng/ml; normal range, <20 ng/ml),
carcinoembryonic antigen (2.37 ng/ml; normal range, <10 ng/ml)
and carbohydrate antigen 19-9 (12.58 U/ml; normal range, <37
U/ml) levels were normal. Following the diagnosis of de novo
gastric cancer, surgery was performed in September 2013. During the
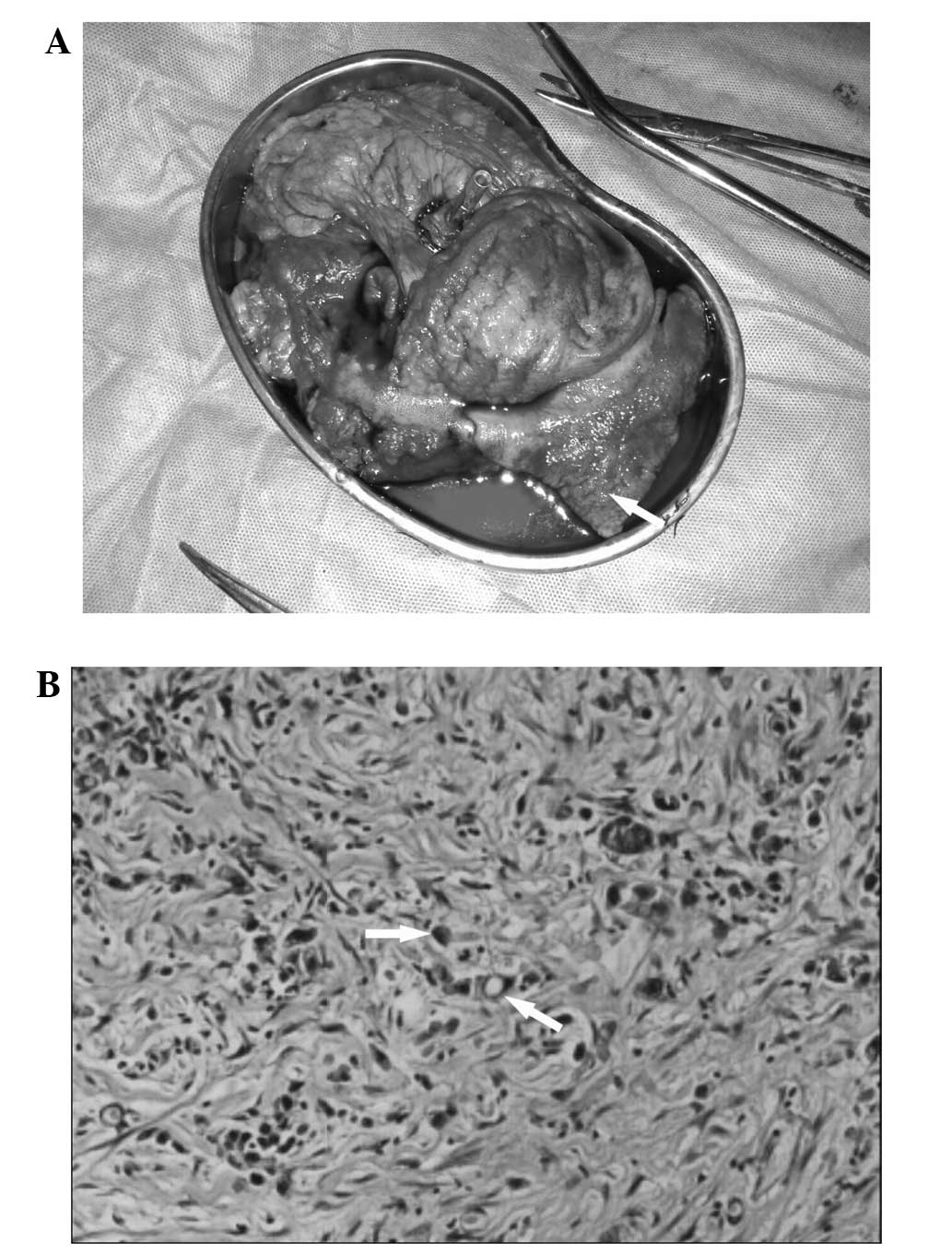
laparotomy, a broad region of the gastric posterior wall was
identified to be rigid with irregular margins (Fig. 2A). The tumor measured ~9.5×8.5 cm in
diameter. Subsequently, a total gastrectomy with lymph node
dissection was performed. Reconstruction was performed using the
Rous-en-Y esophagojejunostomy method and a naso-intestinal feeding
tube was inserted into the distal bowel. Following surgery, routine
nutritional support and immunosuppressive medications were
administered. Histopathological examination of the resected stomach
tissue revealed poorly differentiated adenocarcinoma with a number
of partially signet-ring carcinoma cells (Fig. 2B); the invasion penetrated the wall
via the serosa with perigastric lymph node metastasis (1/11 lymph
nodes removed from the lesser curvature and 0/4 lymph nodes from
the greater curvature were pathologically confirmed to be
metastatic). The tumor was staged as pT4N1M0 with no distant
metastasis. The patient recovered well and was discharged on
postoperative day 10 without any complications.
Discussion
De novo stomach cancer development following
liver transplantation is relatively rare. However, the incidence of
de novo malignancy following OLT is significantly higher in
patients that have undergone OLT, than that in the general
population. Park et al (4)
retrospectively reviewed 1,952 patients following OLT and found
that 44 patients exhibited de novo malignancies following a
mean post-transplantation period of 41 months. Among them, 11
patients were diagnosed with gastric cancer. The relative risk of
malignancy following OLT was 7.5-fold higher than that of the
general population. However, the majority of patients with
recurrent malignancies underwent OLT for hepatocellular carcinoma
(HCC), rather than for benign liver diseases.
Independent risk factors for the development of a
de novo malignancy include individual pre-transplant disease
status, immunosuppressive therapy and the time that elapses
following OLT. With regard to pre-transplant disease status,
numerous patients are diagnosed with HCC prior to OLT (5). Previous studies have indicated that
individuals with a primary carcinoma are more susceptible to the
development of additional tumors than healthy individuals, which
may be a result of a genetic predisposition to malignancy (6–8).
Furthermore, OLT recipients must undergo lifelong immunosuppression
following OLT. The immune system is important in tumor surveillance
in vivo and suppression of immune system activity may
promote tumorigenesis. Notably, immunosuppressive agents exhibit
direct carcinogenic effects, which may induce cancer progression
(9). Thus, it is biologically
plausible that immunosuppression is associated with a higher
incidence of malignancy when compared with the immunocompetent
population. Finally, due to advances in the medical field, the
survival time following transplantation has been prolonged
significantly and thus, the risk of developing malignancy is
increased. Previous studies have reported that the longest interval
observed between transplantation and occurrence of de novo
gastric cancer was 70 months (10–12).
In the present case, the de novo malignancy occurred 12
years following transplantation and, therefore, is the longest
interval reported thus far.
Previous studies have reported that smoking and
alcohol consumption may also contribute to the development of de
novo gastric cancer (9,12,13).
Acute alcohol intoxication is hypothesized to decrease natural
killer cell activity and promote tumor metastasis (12). In the present study, the patient’s
irregular eating habits, including irregular eating patterns and
consuming ‘unhealthy’ food, were considered to increase the risk of
developing de novo malignancy as this may destroy the
gastric muscousal membrane, leading to malignancy (14,15).
Furthermore, primary stomach diseases, including chronic gastritis,
stomach ulcers and the Helicobacter pylori infection may
also be pathogenic factors. In the present case, the patient was
diagnosed with chronic gastritis following a gastric endoscopy and
was found to be positive for the H. pylori infection.
At present, no standard treatment regimen has been
recommended for de novo gastric cancer in the literature or
guidelines; thus, clinicians usually follow the treatment
principles for gastric cancer. Numerous factors require particular
attention with regard to the treatment of patients; further
investigation is required regarding the immunosuppressive agents
that are administered, particularly whether they should be
sustained during the perioperative period. In the current case, the
patient received continuous low-dose immunosuppressive medications
and no postoperative complications, such as infection or disunion
incisions were identified. The adoption of adjuvant chemotherapy
remains controversial, as chemotherapy may harm the liver. In
addition, the patient’s medical history, including the specific
transplantation method, must be investigated prior to surgery for
gastric cancer so that the appropriate approach is selected to
reduce the negative impact of surgery. In the present case, a
bilateral subcostal incision with midline extension toward the
xiphoid was performed during the transplantation. Access to the
abdominal cavity was via a median incision to avoid tissue
adherence. During surgery, the anatomical structures of the hepatic
hilum and hepatogastric region must be considered. Furthermore,
when dissecting the lymph nodes, surgeons must consider the
reconstructive vessels and bile ducts to avoid iatrogenic injury.
In addition, liver function must be monitored following surgery.
Enteral nutrition must be administered at an early stage using an
intestinal feeding tube, while immunosuppressants may be
administered via a nasogastric tube.
In conclusion, OLT recipients exhibit a higher risk
of developing malignancies following a transplant. In this study,
although GSD-Ia is a benign disease, with a good prognosis
following effective treatment, the patient developed gastric cancer
12 years following OLT. De novo malignancies usually develop
quickly and are associated with a poor prognosis. Surveillance
protocols are required for these patients in order to detect de
novo tumors at an early stage. Subsequently, an appropriate
surgical procedure must be performed so that the survival rate is
improved.
References
|
1
|
Rake JP, Visser G, Labrune P, Leonard JV,
Ullrich K and Smit GP; European Study on Glycogen Storage Disease
Type I (ESGSD I). Guidelines for management of glycogen storage
disease type I - European Study on Glycogen Storage Disease Type I
(ESGSD I). Eur J Pediatr. 161(Suppl 1): S112–S119. 2002.
|
|
2
|
Rake JP, Visser G, Labrune P, Leonard JV,
Ullrich K and Smit GP: Glycogen storage disease type I: diagnosis,
management, clinical course and outcome. Results of the European
Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr.
161(Suppl 1): S20–S34. 2002.
|
|
3
|
Franco LM, Krishnamurthy V, Bali D, et al:
Hepatocellular carcinoma in glycogen storage disease type Ia: a
case series. J Inherit Metab Dis. 28:153–162. 2005.
|
|
4
|
Park HW, Hwang S, Ahn CS, et al: De novo
malignancies after liver transplantation: incidence comparison with
the Korean cancer registry. Transplant Proc. 44:802–805. 2012.
|
|
5
|
Senkerikova R, Frankova S, Sperl J, et al:
Incidental hepatocellular carcinoma: risk factors and long-term
outcome after liver transplantation. Transplant Proc. 46:1426–1429.
2014.
|
|
6
|
Lee JW, Kim JW and Kim NK: Clinical
characteristics of colorectal cancer patients with a second primary
cancer. Ann Coloproctol. 30:18–22. 2014.
|
|
7
|
Kinoshita O, Okamoto K, Konishi H, et al:
A case of metastatic gastric cancer secondary to pancreatic
neuroendocrine tumor fifteen years after distal pancreatectomy.
Nihon Shokakibyo Gakkai Zasshi. 110:1281–1287. 2013.(In
Japanese).
|
|
8
|
Fujibuchi T, Matsumoto S, Shimoji T, et
al: Cytogenetic study of secondary malignancy in giant cell tumor.
J Orthop Sci. Aug 9–2013.(Epub ahead of print).
|
|
9
|
Chak E and Saab S: Risk factors and
incidence of de novo malignancy in liver transplant recipients: a
systematic review. Liver Int. 30:1247–1258. 2010.
|
|
10
|
Pruthi J, Medkiff KA, Esrason KT, et al:
Analysis of causes of death in liver transplant recipients who
survived more than 3 years. Liver Transpl. 7:811–815. 2001.
|
|
11
|
Herrero JI, Lorenzo M, Quiroga J, et al:
De Novo neoplasia after liver transplantation: an analysis of risk
factors and influence on survival. Liver Transpl. 11:89–97.
2005.
|
|
12
|
Zanus G, Carraro A, Vitale A, et al:
Alcohol abuse and de novo tumors in liver transplantation.
Transplant Proc. 41:1310–1312. 2009.
|
|
13
|
Herrero JI: De novo malignancies following
liver transplantation: impact and recommendations. Liver Transpl.
15(Suppl 2): S90–S94. 2009.
|
|
14
|
Chen MJ, Chiou YY, Wu DC and Wu SL:
Lifestyle habits and gastric cancer in a hospital-based
case-control study in Taiwan. Am J Gastroenterol. 95:3242–3249.
2000.
|
|
15
|
Denova-Gutiérrez E, Hernández-Ramírez RU
and López-Carrillo L: Dietary patterns and gastric cancer risk in
Mexico. Nutr Cancer. 66:369–376. 2014.
|