Introduction
The definition of multiple primary malignant
neoplasms (MPMNs) was determined by Warren and Gates in 1932
(1) and since then, an increasing
number of MPMNs have been diagnosed and reported. MPMN patients
usually undergo surgery or receive chemotherapy for treatment
(2,3). Additionally, with the increasing
lifespan of humans, the prevalence of MPMN has increased (4). Prior to diagnosis, a number of
patients with this condition have masses of unknown malignancy, and
the method to treat these lesions is controversial. One study has
proposed the observation of such lesions instead of biopsy
(5). The present study reports the
case of a patient who was diagnosed with rectal and bladder cancer,
and later presented with hepatocellular carcinoma, which developed
from an unexplained mass in the liver during surveillance following
surgery, and provides a promising method of treatment. This case
provides valuable insight for further research in this field. The
patient provided written informed consent.
Case report
A 62-year-old male presented to the West China
Hospital of Sichuan University (Chengdu, China) due to blood in the
feces and weight loss that had been occurring for approximately one
month. A proctoscopy indicated a rectal adenoma. At day seven
post-admission, the patient underwent Dixon’s rectectomy. During
the surgery, a 5×4-cm neoplasm was observed in the rectum and
diagnosed as hepatic cirrhosis. Following the surgery, the
microscopic examination confirmed the neoplasm to be a rectal
adenoma. The patient received four cycles of post-operative
chemotherapy composed of a 5-Fu infusion (250 mg/day from days one
to three). However, due to intolera- ble rashes, the treatment was
changed to 200 mg tegafur (three times per day on days four to 10)
for the first cycle, while for the following three cycles the
tegafur was administered at the same dose and frequency but for 10
days. The patient also received 41 doses of T-cell therapy (20 ml
infusion ever two or three days). There were no complications in
the procedure. Additionally, a hepatitis B virus (HBV) test showed
that the patient was positive for the HB surface, envelope and core
antigens. The HBV DNA content was 2.85×106/ml. The
patient received glutathione (1,200 mg/day during hospitalization)
and bifendate (15 mg, three times per day until the HBV-DNA levels
had ret- urned to normal) as liver treatment. The patient achieved
a complete response following these treatments. Approximately seven
months later, the patient required hospitalization due to the chief
complaint of painless gross hematuria persisting for 1 week.
Ultrasonography showed a mass in the urinary bladder, which did not
move with the change of body position. At day four post-admission,
the patient received a transurethral resection of the bladder
tumor. Following the surgery, bladder instillation was performed
for treatment with doxorubicin. The post-operative biopsy of the
neoplasm, with hematoxylin and eosin staining revealed that the
pathological type was a bladder transitional cell carcinoma,
following assessment by a pathologist from the West China Hospital.
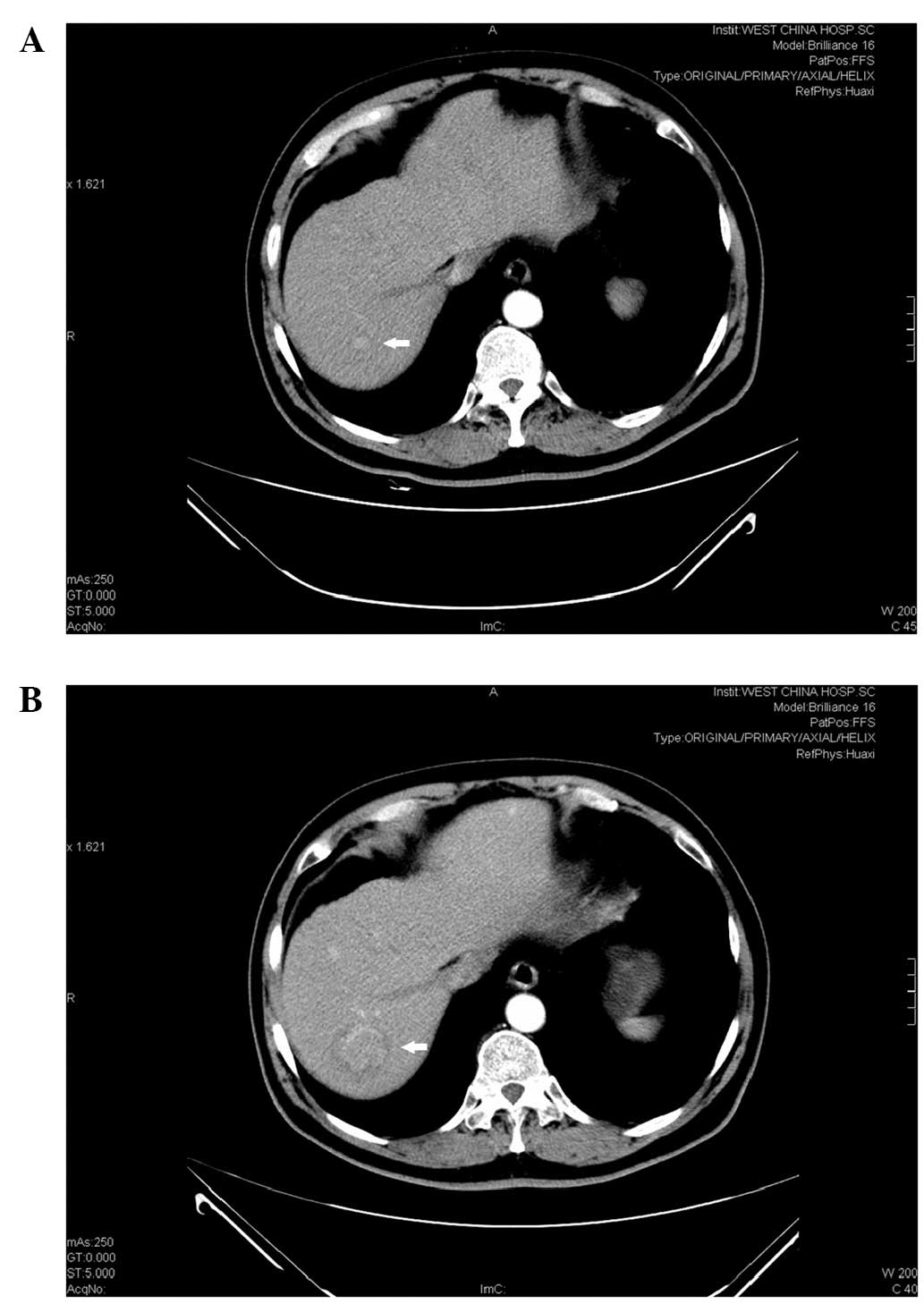
Eight years after the second surgery, a mass of 0.9 cm in diameter
was discovered in the liver by contrast computed tomography
(Fig. 1A). Approximately one year
later, this mass developed into hepatic cancer in the right
posterior lobe of the upper section of the liver (Fig. 1B). Subsequently, the patient
received a partial liver resection, the hepatic mass was stained
with hematoxylin and eosin and was confirmed as hepatocellular
carcinoma by pathological analysis, by a pathologist from the West
China Hospital. After this last surgery, the patient recovered well
and was disease-free with an Eastern Cooperative Oncology Group
score of 1.
Discussion
The description of MPMN provided by Warren and Gates
in 1932 is the generally accepted diagnostic standard for this
disease: Each of the tumors must have a definite element of
malignancy; each one must be distinct; and the probability of one
being a metastasis of other tumors must be excluded (1). The concept of metachronous cancers is
described as two or more tumors detected six months following the
primary tumor (6). According to the
aforementioned criteria, the present patient suffered from
metachronous MPMN. The prevalence of MPMN is not high, varying
between 0.341 and 5.464% (7–12). It
has been reported that the extended life span of humans and the
continuous persistence of carcinogens are significant factors for
the tumorigenesis of MPMN. A previous study has shown that, among
the patients with brain malignant tumors, the incidence of MPMN has
two peaks. The first is in the third decade of life, and the second
is after 50 years of age (4). A
point of high-risk for the development of a second primary tumor
occurs following a 10-year gap from the first (13). Although Evans et al (14) concluded that the risk of developing
a subsequent cancer in older patients is lower than expected, the
study did indicate that this may result from the incomplete design
of the experiment and the lack of data.
More significantly, the present study observed a
0.9-cm mass in the right lobe of the liver. When considering the
histological type prior to biopsy, three possibilities persisted: A
benign lesion, another primary cancer or a metastasis of a resected
tumor (1). Generally, for single
abdominal masses of <1 cm, they can be kept under surveillance
for dynamic observations (5).
However, the mass in the present case developed into hepatocellular
carcinoma 1 year later. Hence, to monitor patients with an
oncological history, particularly of colorectal cancer, and an
unexplained mass in the liver, it is necessary to screen the
α-fetoprotein (AFP) level. However, the level of AFP in certain
patients with HCC and those of biliary tract cancers does not
become elevated (15,16). Due to the unraised AFP level, a
biopsy should be taken. If malignancy is confirmed, surgery should
be performed as soon as possible to minimize the risk of invasion
and metastasis of the carcinoma.
In conclusion, older patients have a predisposition
to developing MPMN. Active biopsies for suspected hepatic disease
found upon imaging in patients with a history of cancer is a
prospective method for the early diagnosis of emerging
carcinoma.
Acknowledgements
The study was supported by the Technology Support
Program of Science and Technology Department of Sichuan Province,
China (grant no. 2012SZ0038).
References
|
1
|
Warren S and Gates O: Multiple primary
malignant tumors: a survey of the literature and a statistical
study. Am J Cancer. 16:1358–1414. 1932.
|
|
2
|
Koichi S, Takeo M, Kiyotaka Y, et al: A
case of triple synchronous cancers occurring in the gallbladder,
common bile duct, and pancreas. J Gastroenterol. 38:97–100.
2003.
|
|
3
|
Yakushiji H, Mukai S, Matsukura S, et al:
DNA mismatch repair deficiency in curatively resected sextuple
primary cancers in different organs: a molecular case report.
Cancer Lett. 142:17–22. 1999.
|
|
4
|
Nagane M, Shibui S, Nishikawa R, et al:
Triple primary malignant neoplasms including a malignant brain
tumor: report of two cases and review of the literature. Surg
Neurol. 45:219–229. 1996.
|
|
5
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011.
|
|
6
|
Demandante CG, Troyer DA and Miles TP:
Multiple primary malignant neoplasms: case report and a
comprehensive review of the literature. Am J Clin Oncol. 26:79–83.
2003.
|
|
7
|
Lohsiriwat V, Vongjirad A and Lohsiriwat
D: Incidence of synchronous appendiceal neoplasm in patients with
colorectal cancer and its clinical significance. World J Surg
Oncol. 7:512009.
|
|
8
|
Inskip PD: Multiple primary tumors
involving cancer of the brain and central nervous system as the
first or subsequent cancer. Cancer. 98:562–570. 2003.
|
|
9
|
Omür O, Ozcan Z, Yazici B, et al: Multiple
primary tumors in differentiated thyroid carcinoma and relationship
to thyroid cancer outcome. Endocr J. 55:365–372. 2008.
|
|
10
|
Antakli T, Schaefer RF, Rutherford JE and
Read RC: Second primary lung cancer. Ann Thorac Surg. 59:863–867.
1995.
|
|
11
|
Evans HS, Lewis CM, Robinson D, et al:
Incidence of multiple primary cancers in a cohort of women
diagnosed with breast cancer in southeast England. Br J Cancer.
84:435–440. 2001.
|
|
12
|
Van Westreenen HL, Westerterp M, Jager PL,
et al: Synchronous primary neoplasms detected on 18F-FDG PET in
staging of patients with esophageal cancer. J Nucl Med.
46:1321–1325. 2005.
|
|
13
|
Brown SR, Finan PJ, Hall NR and Bishop DT:
Incidence of DNA replication errors in patients with multiple
primary cancers. Dis Colon Rectum. 41:765–769. 1998.
|
|
14
|
Evans HS, Møller H, Robinson D, et al: The
risk of subsequent primary cancers after colorectal cancer in
southeast England. Gut. 50:647–652. 2002.
|
|
15
|
Sato Y, Nakata K, Kato Y, et al: Early
recognition of hepatocellular carcinoma based on altered profiles
of alpha-fetoprotein. N Engl J Med. 328:1802–1806. 1993.
|
|
16
|
Tsai JF, Chang WY, Jeng JE, et al:
Frequency of raised alpha-fetoprotein level among Chinese patients
with hepatocellular carcinoma related to hepatitis B and C. Br J
Cancer. 69:1157–1159. 1994.
|