Introduction
Nasopharyngeal carcinoma (NPC) is the most commonly
diagnosed type of head and neck cancer in Southeast Asia, with a
reported annual incidence of 30–80 cases per 100,000 individuals in
endemic regions (1). The p53
tumor suppressor gene is one of the most frequently studied genes.
p53 mutations or deletions occur with a high frequency in the
majority of tumor types and are associated with malignant
transformation and tumor progression (2,3). Mouse
double minute 2 homolog (MDM2) is an important negative regulator
of the p53 pathway and its overexpression has been associated with
tumor invasion and metastasis (4).
Eukaryotic translation initiation factor 4E (eIF4E), a
proto-oncogene, is important in translational regulation and its
overexpression selectively increases the mRNA translation of
proteins associated with tumor growth, invasion and metastasis.
eIF4E overexpression has been identified in various malignant
tumors, including cervical, ovarian, esophageal, lung and liver
cancer. Previous studies have shown that eIF4E is overexpressed in
~100% of head and neck cancers and has been found to correlate with
poor prognosis (5–7). The epidermal growth factor receptor
(EGFR), which is encoded by the c-erbB-1 proto-oncogene, is a
member of the ErbB family of protein tyrosine kinase receptors and
is involved in the regulation of cellular metabolism, growth,
migration and differentiation. EGFR is overexpressed in numerous
cancer types, including non-small cell lung, breast, prostate and
colorectal cancer (8). Therefore,
EGFR has become a key target for molecular-based therapies.
The aim of this retrospective study was to examine
p53, MDM2, eIF4E and EGFR expression, and their association with
clinical characteristics and survival rates in 96 cases of NPC.
Materials and methods
Patients and treatment
This study was approved by the Ethics Committee of
Sichuan Provincial Cancer Hospital (Chengdu, China). A total of 96
patients with complete medical records, who were treated at Sichuan
Provincial Cancer Hospital (Chengdu, China) between 2005 and 2009
were included in the study. The patients consisted of 74 male and
22 female individuals aged between 30 and 77 years (mean age ±
standard deviation, 49.9±10.92 years). NPC tissue samples were
obtained prior to radiotherapy and chemotherapy using an electronic
nasopharyngoscope. All tumor tissue samples were fixed in 10%
formalin, embedded in paraffin and cut into 4-μm-thick sections.
All patients received intensity-modulated radiotherapy on
nasopharyngeal areas, skull basal lesions and positive neck lymph
nodes. The following radiotherapy dosages were administered over 28
to 33 sessions: Gross tumor volume (GTV)nx, 66–76 Gy; GTVln, 60–70
Gy; clinical target volume (CTV)1, 60–66 Gy; CTV2, 54–60 Gy; and
CTVln, 50–55 Gy. The Cobalt-60 or 6 megavoltage X-ray conventional
anterior neck half-field technique was used with a dose of 46–50 Gy
for radiotherapy of the lower neck and supraclavicular target
regions (9). All patients also
underwent concurrent cisplatin-based chemotherapy. Induction
chemotherapy regimens included docetaxel (75 mg/m2 on
day 1) in combination with cisplatin (100 mg/m2 on days
1–3) and cisplatin (100 mg/m2 on days 1–3) in
combination with fluorouracil (750 mg/m2 on days 1–4).
Concurrent chemotherapy regimens included cisplatin alone (100
mg/m2 on days 1–3) and cisplatin (100 mg/m2
on days 1–3) in combination with fluorouracil (750 mg/m2
on days 1–4). Induction and concurrent chemotherapy comprised of
one cycle every 21 days. Supplementary chemotherapy regimens were
the same as those used for induction chemotherapy. Written informed
consent was obtained from all patients.
Patient follow-up
All patients were followed-up for between three and
six years. During the first year of treatment, patients had one
follow-up session every three months. During the first year of
treatment patients were followed up every three months. During the
second and thurd years following treatment, follow-up was performed
every six months. After the third year, follow-up was then
performed annually. The follow-up sessions involved indirect
nasopharyngoscopy, nasopharyngeal and neck computed
tomography/magnetic resonance imaging, abdominal B-mode
ultrasonography, chest radiography and blood tests.
Immunohistochemistry
p53, MDM2, EIF4E and EGFR expression were determined
by immunohistochemistry using the histostain-streptavidin
peroxidase kit (#95-9943; Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. Archived
paraffin-embedded sections of NPC tumor tissues (4 μm) were
deparaffinized and rehydrated. Antigen retrieval was performed
using 0.01 M citrate buffer solution (pH 9.0) under pressurized
steam for 10 min. The sections were then cooled, washed with
phosphate-buffered saline (PBS), blocked with 5% serum and
incubated with mouse anti-human monoclonal p53 (1:500 dilution),
mouse anti-human monoclonal MDM2 (1:1,000 dilution), rabbit
anti-human polyloclonal EIF4E (1:1,000 dilution) and mouse
anti-human monoclonal EGFR (1:1,000 dilution) primary antibodies
(Abcam, Cambridge, UK) ab1101, ab3110, ab1126, ab131498) overnight
at 4°C. After washing with PBS, the tissue samples were incubated
with biotinylated goat anti-mouse polyclonal IgG secondary antibody
for 60 min at 37°C. Next, the samples were washed with PBS, stained
with diaminobenzidine solution, counterstained with hematoxylin,
dehydrated and sealed. Positive NPC tissue sections were used as a
positive control and tissue sections incubated with PBS instead of
primary antibodies were used as negative controls. The percentage
of positive cells was quantitated by counting 100 cells in four
random microscopic fields (Olympus BH2; Olympus Corporation, Tokoy,
Japan). Immunostaining was categorized into four groups according
to the percentage of positive cells: (−), <5% positive cells;
(+), 5–25% positive cells; (++), 26–50% positive cells; and (+++),
≥51% positive cells. Negative expression was represented by (−) and
positive expression by (+) to (+++).
Statistical analysis
All statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Differences
in count data were analyzed using the χ2 test, and
Spearman’s rank correlation test was used for correlational
analysis. The Kaplan-Meier estimator was used to calculate survival
rates. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of p53, MDM2, EGFR, and eIF4E
in NPC
The expression of p53, MDM2, EGFR and eIF4E in NPC
was found to be localized in the nucleus. The expression levels of
p53, MDM2, EGFR and eIF4E were 65.6% (63/96), 79.16% (76/96), 89.5%
(86/96) and 77.08% (74/96), respectively.
Association between p53, MDM2, EGFR and
eIF4E expression and T stage, clinical stage and lymph node
metastasis
The expression levels of p53 and EGFR were
significantly associated with NPC T stage, according to the 2002
Union for International Cancer Control staging system (Tables I and II) (10).
p53 and EGFR expression levels were significantly lower in early T1
and T2 stages than in late T3 and T4 stages (p53;
χ2,20.322; P=0.001; EGFR: χ2, 8.337;
P=0.005). The expression levels of p53 and EGFR were also
significantly associated with clinical stage (Tables I and II). The expression levels of p53 and EGFR
in stage I+II NPC were significantly lower than that in stages
III+IV (p53: χ2,4.809; P=0.037; EGFR: χ2,
15.128; P=0.001). Furthermore, MDM2 expression levels were
significantly higher in NPC patients with lymph node metastasis
than in those without lymph node metastasis (χ2, 16.361;
P=0.001) (Table I). However T
stage, clinical stage and lymph node metastasis were not associated
with eIF4E expression.
 | Table IAssociation between p53 and MDM2
expression and the clinical characteristics of 96 nasopharyngeal
carcinoma patients. |
Table I
Association between p53 and MDM2
expression and the clinical characteristics of 96 nasopharyngeal
carcinoma patients.
| | p53 | | | MDM2 | | |
|---|
| |
| | |
| | |
|---|
| Clinical
characteristics | No. of cases | − | + to +++ | χ2
value | P-value | − | + to +++ | χ2
value | P-value |
|---|
| T stage | | | | 20.322 | 0.001 | | | 1.748 | 0.215 |
| T1+2 | 45 | 5 | 40 | | | 12 | 23 | | |
| T3+4 | 51 | 28 | 23 | | | 8 | 53 | | |
| Lymph node
metastasis | | | | 0.157 | 0.732 | | | 16.361 | 0.001 |
| No | 10 | 4 | 6 | | | 7 | 3 | | |
| Yes | 86 | 29 | 57 | | | 13 | 73 | | |
| Clinical stage | | | | 4.809 | 0.037 | | | 0.976 | 0.366 |
| I+II | 21 | 3 | 18 | | | 6 | 15 | | |
| III+IV | 75 | 30 | 45 | | | 14 | 61 | | |
 | Table IIAssociation between EGFR and eIF4E
expression and the clinical characteristics of 96 nasopharyngeal
carcinoma patients. |
Table II
Association between EGFR and eIF4E
expression and the clinical characteristics of 96 nasopharyngeal
carcinoma patients.
| | EGFR | | | eIF4E | | |
|---|
| |
| | |
| | |
|---|
| Clinical
characteristics | No. of cases | − | + to +++ | χ2
value | P-value | − | + to +++ | χ2
value | P-value |
|---|
| T stage | | | | 8.337 | 0.005 | | | 4.404 | 0.051 |
| T1+2 | 45 | 9 | 36 | | | 6 | 39 | | |
| T3+4 | 51 | 1 | 50 | | | 16 | 35 | | |
| Lymph node
metastasis | | | | 1.099 | 0.278 | | | 0.317 | 0.691 |
| No | 10 | 2 | 8 | | | 3 | 7 | | |
| Yes | 86 | 8 | 78 | | | 19 | 67 | | |
| Clinical stage | | | | 15.128 | 0.001 | | | 1.651 | 0.242 |
| I+II | 21 | 7 | 14 | | | 7 | 14 | | |
| III+IV | 75 | 3 | 72 | | | 15 | 60 | | |
Association between p53 and MDM2
expression in NPC
Spearman’s rank correlational analysis revealed a
significant negative correlation between the expression levels of
p53 and MDM2 (r, −3.24; P<0.05).
Association between p53, MDM2, EGFR and
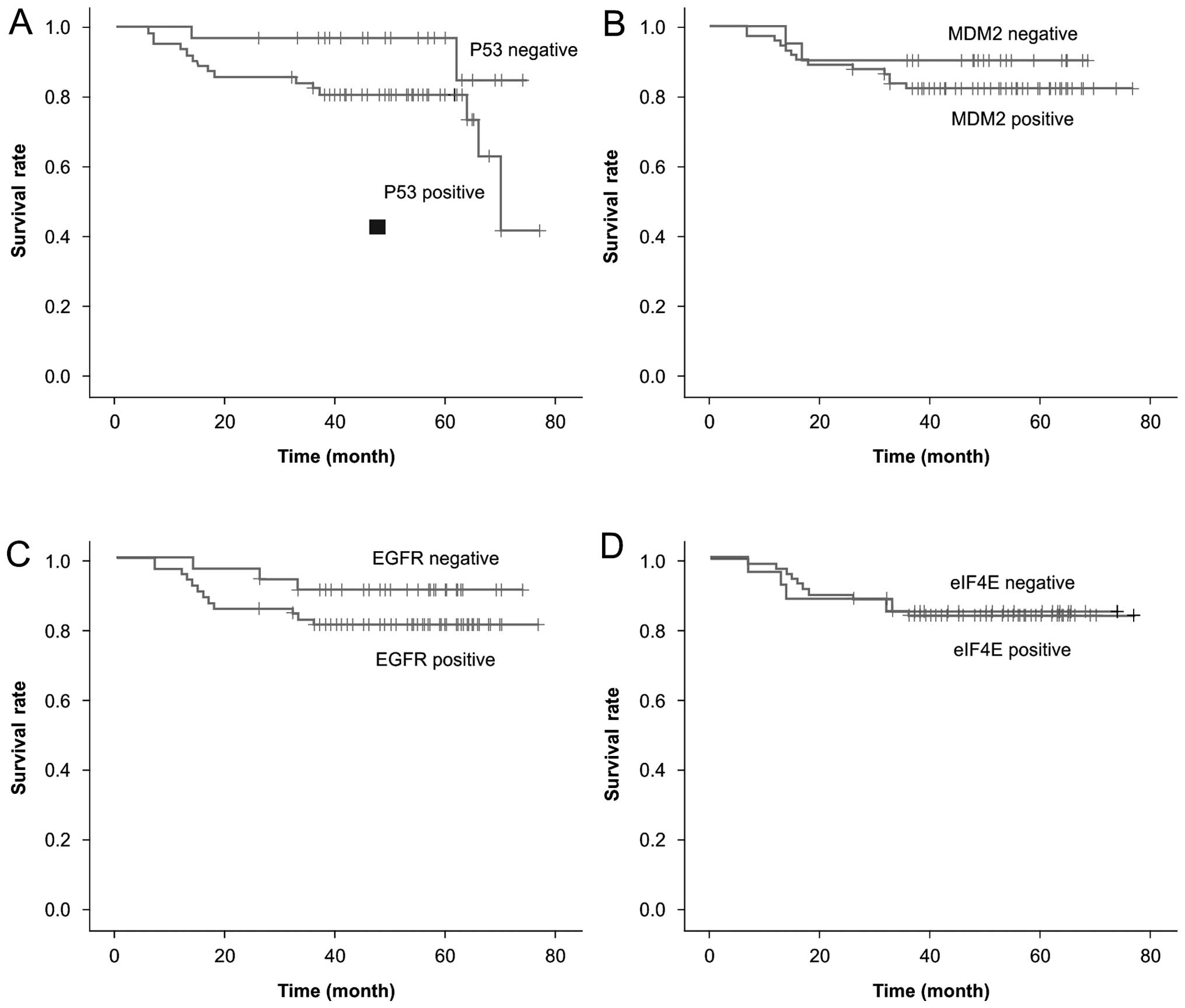
eIF4E expression and survival rate
The three-year disease-free survival and overall
survival (OS) rates for the patient population were 73.9 and 84.4%,
respectively. The three-year OS rates were 76.2 and 93.9% in
patients with p53-positive and -negative expression, respectively,
and 75.8% and 91.2% in patients with EGFR-positive and -negative
expression, respectively. Therefore, positive expression of p53
(χ2, 4.682; P=0.046) and EGFR (χ2, 5.682;
P=0.046) was associated with a poor prognosis (Fig. 1). Cox regression model multivariate
analysis revealed that p53 (β, −0.455; χ2, 5.491;
P=0.019) and EGFR (β, 3.93; χ2, 11.95; P=0.001) were
both independent prognostic factors for survival in NPC. By
contrast, MDM2 and eIF4E expression were not associated with
survival in NPC.
Discussion
NPC is a common malignant tumor in China and the
standard treatment is radiation therapy. Following radiotherapy,
the five-year survival rate is ~40–50% (11). Therefore, it is important to
investigate novel adjuvant treatments to improve the five-year
survival rates of patients with NPC. With the development of
biological therapies for tumors, molecular targeted therapy has
become a novel area for the study of tumor therapy. EGFR is
expressed in 88–100% of head and neck squamous cell carcinomas, and
is important in tumor cell growth, repair and survival.
Furthermore, its overexpression often indicates a poor prognosis,
early metastasis, chemotherapy resistance or a shorter survival
(12–14). Therefore, EGFR has become a key
target for cancer gene therapy. In the present study, EGFR was
highly expressed in NPC. EGFR-positive expression was observed in
86 out of 96 NPC patients and was found to be associated with
aggressive clinical behavior, including advanced T clinical stages.
Furthermore, EGFR expression was an independent prognostic factor
for survival. A worse survival was observed in patients with
positive EGFR expression when compared with patients with negative
EGFR expression.
The p53 pathway is one of the most important
pathways that regulates the cell cycle. p53 is activated in
response to various internal and external stresses and results in
an increase in the p21 transcription factor and its translational
products. p21 induces G1 cell cycle arrest and DNA repair by
inhibiting cyclin-dependent kinase 5 and increasing the expression
of the growth arrest and DNA damage inducible genes. When DNA
damage cannot be repaired, p53 induces apoptosis by increasing Bcl
2-associated protein X (BAX) expression and the formation of BAX
homodimers. Mutations in the p53 gene are one of the most common
genetic alterations found in human tumors. Approximately 50% of
human tumors exhibit p53 mutations (15–17).
In the present study, p53-positive expression was identified in
65.6% of the NPC cases. Positive p53 expression was associated with
poor clinical characteristics, including advanced T and clinical
stages. Furthermore, p53 expression was an independent prognostic
factor of survival. Patients with positive p53 expression exhibited
a worse three-year survival rate than those with negative p53
expression.
MDM2 inactivates p53 via direct binding or its E3
ubiquitin ligase activity, which targets p53 for proteasomal
degradation. Wild-type p53 is unstable, has a very short half-life
and is rapidly degraded by the ubiquitin pathway, whereas mutated
p53 is very stable and not easily degraded. In the present study,
p53 expression was found to negatively correlate with MDM2
expression. Recent studies have reported that MDM2 expression in
malignant tumors is associated with invasion, metastasis, poor
prognosis and chemotherapy resistance (18–21).
In the present study, high MDM2 expression was identified in NPC
(79.16%) and was found to significantly correlate with N stage.
MDM2 expression levels were significantly higher in NPC patients
with lymph node metastasis than in those without lymph node
metastasis. However, MDM2 expression was not associated with T
stage, clinical stage or survival in NPC.
eIF4E is a rate-limiting factor in protein synthesis
and is distributed throughout the cytoplasm and nucleus in
free-floating or multiprotein complex forms in almost all
eukaryotic cells. eIF4E expression is regulated by c-myc, RAS and
other oncogenes, and its overexpression selectively increases the
mRNA translation of proteins associated with tumor growth and
invasion, including fibroblast growth factor-2, transforming growth
factor-β, platelet-derived growth factor and vascular endothelial
growth factor (VEGF). eIF4E overexpression is considered to be a
valuable prognostic marker in various tumor types (22,23).
In the present study, positive eIF4E expression was present in 74
out of 96 NPC cases (77.08%). However, eIF4E expression was not
associated with T stage, clinical stage, lymph node metastasis or
survival in NPC.
A complex association has been identified between
p53, MDM2 and EGFR expression in a number of human tumors, whereby
p53 protein expression was significantly lower and the EGFR and
MDM2 expression were higher when compared with that of the normal
tissues (24). However, the
association between alterations in p53, MDM2, EGFR and the survival
of patients with anaplastic astrocytoma or glioblastoma remains
controversial (25). In comparison
with the initial tumor, recurrent lesions were characterized by a
reduced expression of p53 and the number of MDM2 and EGFR positive
specimens was reduced. Overexpression and deletion mutations of the
EGFR gene, as well as MDM2 overexpression, have been linked to the
absence of p53 gene mutations in human glioblastoma multiforme
(26). High expression of VEGF and
EGFR were independent adverse prognostic factors for long-term
outcomes in nonmetastatic NPC independent of clinical stage
(27). Mutations of p53 were
associated with the overexpression of EGFR and absence of MDM2 in
human esophageal carcinomas (28).
In vivo studies have revealed that wild-type p53 activates
the promoter of EGFR (28).
Furthermore, clinical studies have demonstrated that the expression
of p53, MDM2 and EGFR are prognostic of human cancers. Numerous
studies have reported that the altered expression of these three
genes were associated with poor survival and were prognostic of
glioblastoma patients (30–37). For example, Ruano et al
(37) reported that poor outcome in
primary glioblastoma multiforme patients is associated with
concurrent EGFR and p53 alterations. Furthermore, p53, EGFR and
MDM2 are also predictive markers in breast cancer (38), lung cancer (39), blastomatoid pulmonary carcinosarcoma
(40), Wilms’ tumor (41), anaplastic thyroid carcinoma
(42), bladder cancer (43) and prostate cancer (44). However, p53, MDM2, eIF4E and EGFR
have not been previously associated with clinical characteristics
in NPC. In this study, the expression of these proteins were
detected and their correlation with clinicopathological
characteristics and prognosis in NPC was investigated. The results
indicated that p53 and EGFR expression correlate with T stage,
whereas MDM2 expression correlates with lymph node metastasis. In
addition, p53 and EGFR expression were identified as independent
prognostic factors in NPC.
Acknowledgements
This study was funded by the Fundamental Applied
Scheme of Sichuan Province Science and Technology Department (grant
no. 2011JY0109).
Abbreviations:
|
CTV
|
clinical target volume
|
|
EGFR
|
epidermal growth factor receptor
|
|
eIF4E
|
eukaryotic translation initiation
factor 4E
|
|
GTV
|
gross target volume
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MDM2
|
mouse double minute 2 homolog
|
|
NPC
|
nasopharyngeal carcinoma
|
|
PBS
|
phosphate-buffered saline
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Ho FC, Tham IW, Earnest A, et al: Patterns
of regional lymph node metastasis of nasopharyngeal carcinoma: a
meta-analysis of clinical evidence. BMC Cancer. 12:982012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golubovskaya VM, Conway-Dorsey K, Edmiston
SN, et al: FAK overexpression and p53 mutations are highly
correlated in human breast cancer. Int J Cancer. 125:1735–1738.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olivier M, Eeles R, Hollstein M, et al:
The IARC TP53 database: new online mutation analysis and
recommendations to users. Hum Mutat. 19:607–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo I, Iida S, Takagi Y and Sugihara K:
MDM2 mRNA expression in the p53 pathway may predict the potential
of invasion and liver metastasis in colorectal cancer. Dis Colon
Rectum. 51:1395–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sunavala-Dossabhoy G, Palaniyandi S, Clark
C, et al: Analysis of eIF4E and 4EBP1 mRNAs in head and neck
cancer. Laryngoscope. 121:2136–2141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh J, Jayaraj R, Baxi S, Mileva M,
Curtin J and Thomas M: An Australian retrospective study to
evaluate the prognostic role of p53 and eIF4E cancer markers in
patients with head and neck squamous cell carcinoma (HNSCC): study
protocol. Asian Pac J Cancer Prev. 14:4717–4721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin X, Kim RH, Sun G, Miller JK and Li BD:
Overexpression of eukaryotic initiation factor 4E is correlated
with increased risk for systemic dissemination in node-positive
breast cancer patients. J Am Coll Surg. 218:663–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Von Pawel J: Gefitinib (Iressa, ZD1839): a
novel targeted approach for the treatment of solid tumors. Bull
Cancer. 91:E70–E76. 2004.PubMed/NCBI
|
|
9
|
Feng M, Wang W, Fan Z, Fu B, Li J, Zhang S
and Lang J: Tumor volume is an independent prognostic indicator of
local control in nasopharyngeal carcinoma patients treated with
intensity-modulated radiotherapy. Radiat Oncol. 8:2082013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greene FL, Page DL, et al: AJCC Cancer
Staging Manual. 6th edition. Springer; New York, NY: pp. 47–60.
2002
|
|
11
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berg M and Soreide K: EGFR and downstream
genetic alterations in KRAS/BRAF and PI3K/AKT pathways in
colorectal cancer: implications for targeted therapy. Discov Med.
14:207–214. 2012.PubMed/NCBI
|
|
13
|
Baselga J: The EGFR as a target for
anticancer therapy - focus on cetuximab. Eur J Cancer. 37:S16–S22.
2001. View Article : Google Scholar
|
|
14
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meek DW: Tumour suppression by p53: a role
for the DNA damage response? Nat Rev Cancer. 9:714–723.
2009.PubMed/NCBI
|
|
16
|
Chang LJ and Eastman A: Decreased
translation of p21 (waf1) mRNA causes attenuated p53 signaling in
some p53 wild-type tumors. Cell Cycle. 11:1818–1826. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seong HA and Ha H: Murine protein
serine/threonine kinase 38 activates p53 function through Ser15
phosphorylation. J Biol Chem. 287:20797–20810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin Y, Stephen CW, Luciani MG, et al: p53
stability and activity is regulated by mdm2-mediated induction of
alternative p53 translation products. Nat Cell Biol. 46:462–467.
2002. View
Article : Google Scholar
|
|
19
|
Camus S, Ménendez S, Fernandes K, et al:
The p53 isoforms are differentially modified by Mdm2. Cell Cycle.
11:1646–1655. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang L, Yan Z, Liao X, et al: The p53
inhibitors MDM2/MDMX complex is required for control of p53
activity in vivo. Proc Natl Acad Sci. 108:12001–12006. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicholson J and Hupp TR: The molecular
dynamics of MDM2. Cell Cycle. 9:1878–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zimmer SG, DeBenedetti A and Graff JR:
Translational control of malignancy: the mRNA cap-binding protein,
eIF-4E, as a central regulator of tumor formation, growth, invasion
and metastasis. Anticancer Res. 20:1343–1351. 2000.PubMed/NCBI
|
|
24
|
Stark AM, Hugo HH, Witzel P, et al:
Age-related expression of p53, Mdm2, EGFR and Msh2 in glioblastoma
multiforme. Zentralbl Neurochir. 64:30–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ushio Y, Tada K, Shiraishi S, et al:
Correlation of molecular genetic analysis of p53, MDM2, p16, PTEN,
and EGFR and survival of patients with anaplastic astrocytoma and
glioblastoma. Front Biosci. 1:281–288. 2003. View
Article : Google Scholar
|
|
26
|
Halatsch ME, Schmidt U, Unterberg A and
Vougioukas VI: Uniform MDM2 overexpression in a panel of
glioblastoma multiforme cell lines with divergent EGFR and p53
expression status. Anticancer Res. 26:4191–4194. 2006.
|
|
27
|
Pan J, Tang T, Xu L, et al: Prognostic
significance of expression of cyclooxygenase-2, vascular
endothelial growth factor, and epidermal growth factor receptor in
nasopharyngeal carcinoma. Head Neck. 35:1238–1247. 2013. View Article : Google Scholar
|
|
28
|
Esteve A, Lehman T, Jiang W, et al:
Correlation of p53 mutations with epidermal growth factor receptor
overexpression and absence of mdm2 amplification in human
esophageal carcinomas. Mol Carcinog. 8:306–311. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deb SP, Muñoz RM, Brown DR, et al:
Wild-type human p53 activates the human epidermal growth factor
receptor promoter. Oncogene. 9:1341–1349. 1994.PubMed/NCBI
|
|
30
|
Korkolopoulou P, Christodoulou P, Kouzelis
K, et al: MDM2 and p53 expression in gliomas: a multivariate
survival analysis including proliferation markers and epidermal
growth factor receptor. Br J Cancer. 75:1269–1278. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harada K, Kurisu K, Tahara H, et al:
Telomerase activity in primary and secondary glioblastomas
multiforme as a novel molecular tumor marker. J Neurosurg.
93:618–625. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Halatsch ME, Schmidt U, Botefur IC, et al:
Overexpression of deletion-mutant epidermal growth factor receptor
is associated with altered genotoxic stress-provoked p53 mRNA
induction in a human glioblastoma cell line. Anticancer Res.
21:189–195. 2001.PubMed/NCBI
|
|
33
|
Kleihues P and Ohgaki H: Primary and
secondary glioblastomas: from concept to clinical diagnosis. Neuro
Oncol. 1:44–51. 1999.
|
|
34
|
Stark AM, Hugo HH, Witzel P, et al:
Age-related expression of p53, Mdm2, EGFR and Msh2 in glioblastoma
multiforme. Zentralbl Neurochir. 64:30–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Houillier C, Lejeune J, Benouaich-Amiel A,
et al: Prognostic impact of molecular markers in a series of 220
primary glioblastomas. Cancer. 106:2218–2223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dehais C, Laigle-Donadey F, Marie Y, et
al: Prognostic stratification of patients with anaplastic gliomas
according to genetic profile. Cancer. 107:1891–1897. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruano Y, Ribalta T, de Lope AR, et al:
Worse outcome in primary glioblastoma multiforme with concurrent
epidermal growth factor receptor and p53 alteration. Am J Clin
Path. 131:257–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruiz C, Seibt S, Al Kuraya K, et al:
Tissue microarrays for comparing molecular features with
proliferation activity in breast cancer. Int J Cancer.
118:2190–2194. 2006. View Article : Google Scholar
|
|
39
|
Berghmans T, Mascaux C, Haller A, et al:
EGFR, TTF-1 and Mdm2 expression in stage III non-small cell lung
cancer: a positive association. Lung Cancer. 62:35–44. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schaefer IM, Sahlmann CO, Overbeck T, et
al: Blastomatoid pulmonary carcinosarcoma: report of a case with a
review of the literature. BMC Cancer. 12:4242012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vasei M, Modjtahedi H, Ale-Booyeh O, et
al: Amplification and expression of EGFR and ERBB2 in Wilms tumor.
Cancer Genet Cytogenet. 194:88–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wiseman SM, Masoudi H, Niblock P, et al:
Anaplastic thyroid carcinoma: expression profile of targets for
therapy offers new insights for disease treatment. Ann Surg Oncol.
14:719–729. 2007. View Article : Google Scholar
|
|
43
|
Baffa R, Letko J, McClung C, LeNoir J,
Vecchione A and Gomella LG: Molecular genetics of bladder cancer:
targets for diagnosis and therapy. J Exp Clin Cancer Res.
25:145–160. 2006.PubMed/NCBI
|
|
44
|
Bianco R, Caputo R, Caputo R, Damiano V,
De Placido S, Ficorella C, et al: Combined targeting of epidermal
growth factor receptor and MDM2 by gefitinib and antisense MDM2
cooperatively inhibit hormone-independent prostate cancer. Clin
Cancer Res. 10:4858–4864. 2004. View Article : Google Scholar : PubMed/NCBI
|