Introduction
Synovial cell sarcoma, or synoviosarcoma, (SS) is a
mesenchymal malignancy that is termed SS since its histological
appearance is similar to that of the synovium. However, SS rarely
exhibits a synovial structure and is considered to originate from
pluripotent mesenchymal cells (1).
The characteristic biphasic pattern of SS is due to the two
morphologically distinct but histogenetically related cell types
that compose the sarcoma. Depending on the relative prominence of
the two cell populations and the degree of differentiation, these
tumors form a continuous histopathological spectrum of biphasic,
monophasic fibrous, monophasic epithelial and poorly differentiated
(round-cell) types (2). Since SS
can be slow-growing, appear to be benign on imaging studies, vary
in size and cause pain resembling that associated with trauma, SS
is the most commonly misdiagnosed soft tissue malignancy (3,4). The
diagnosis of SS is made on the basis of its relatively distinctive,
yet markedly variable, histopathological appearance in conjunction
with histochemical findings, immunohistochemistry, electron
microscopy and cytogenetic analysis, which have proved valuable in
confirming morphological diagnoses (5,6).
SS is a distinct soft tissue sarcoma that tends to
be located in the extremities (2).
The lower extremities account for ~70% of cases, whereas SS is
uncommon in the head and neck region, with only 3% of SS tumors
located there (7). Due to low
clinical morbidity, non-specific symptoms and heterogeneous
histopathological features, head and neck SS (HNSS) is often
misdiagnosed (8). As a result,
clinical diagnosis and treatment planning remain a challenge
(9). To the best of our knowledge,
there have been no controlled studies to define the optimal
management protocol for HNSS, and the treatment methods reported
include surgery, chemotherapy, radiotherapy and multiple treatment
modalities, with variable results. In addition, no specific
prognostic factors of HNSS have been reported to date. The aims of
the present study were to review the clinicopathological
characteristics of HNSS in head and neck patients, report and
compare the treatment options, and identify the prognostic factors
of mortality.
Materials and methods
Selection of studies
A systematic literature search was performed using
PubMed and Google Scholar. The search strategy was based on the
combination of text words: ‘Synoviosarcoma OR synovial sarcoma OR
synovial cell sarcoma’, ‘head and neck region’, ‘upper
aerodigestive tract’, ‘oral and maxillofacial region’, ‘sinonasal
region’ and ‘neck’. For the literature search in PubMed, no lower
date limit was utilized and the upper date limit was October 31,
2013. Despite the fact that no language restrictions were initially
imposed, the full-text review and the final analysis were limited
to studies published in English. The references of all the
retrieved studies were searched for additional relevant studies to
enlarge the scope of the literature search.
Eligible criteria
A study was included for analysis if it reported a
human study and histologically confirmed primary HNSS, provided a
clear description of any treatment, reported a definite follow-up
time of more than month, and provided the treatment outcome. The
study was excluded if it reported recurrent or metastatic HNSS, or
synchronous or metachronous multiple cancers in other organs or
diseases, and if the study was a case series providing a mean or
medium follow-up time.
Data extraction
A data extraction sheet was developed. The data
extracted for each patient consisted of the age, gender, tumor
history, tumor presentation, tumor size, tumor extension,
lymphadenopathy status, surgery type, surgical margins, presence of
neck dissection, histological grade, adjuvant therapy provided,
follow-up time and treatment outcome. Not all studies contained all
these pieces of data; however, they were included in the present
analysis if the treatment and outcome were provided. In certain
cases, the patients had more than one treatment and, thus, only the
final treatment received was included in the comparison of
treatments.
Statistical analysis
The χ2 or Fisher’s exact tests for
categorical variables were used for two-group comparisons of the
clinicopathological parameters. Differences in the numerical
variables were assessed using Student’s t-test or non-parametric
Wilcoxon test. Significant variables identified by univariate
analysis were then entered into binary logistic regression models
to identify independent predictors of mortality. The odds ratio and
95% confidence interval (CI) were reported for the logistic
regression model. For time-to-event analysis, Kaplan-Meier curves
were plotted and the log-rank test was used. Analysis of the effect
of prognostic factors on cause-specific survival was undertaken
using Cox proportional-hazards regression. When P<0.05, the
difference was regarded as statistically significant. All the
statistical tests were two-tailed and all the data were analyzed
using SPSS 18.0 software for Windows (SPSS, Inc., Chicago, IL,
USA).
Results
Patient demographics
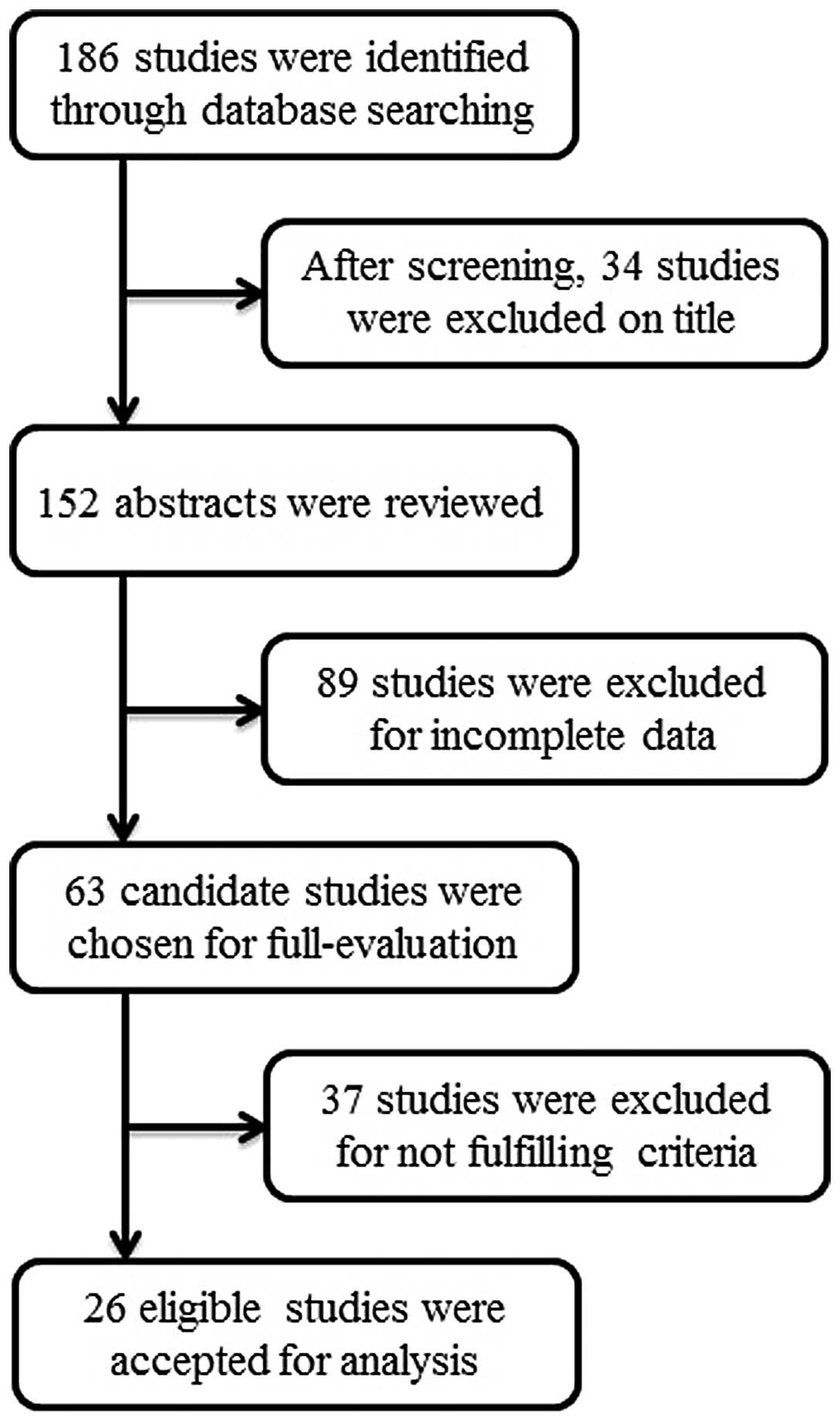
In total, 93 cases from 26 studies met the
eligibility criteria for inclusion in the present analysis
(8,10–34).
The details of the identification and selection of the studies are
presented in Fig. 1. The 93
patients consisted of 55 male and 38 female patients, providing a
male-to-female ratio of 1.44:1.
The median age at the time of diagnosis was 32.1
years (range, 4–76 years).
Tumor location, treatment and
follow-up
In total, 50.5% of the tumors were located in the
upper aerodigestive tract, 26.9% in the neck and 14.0% in the skull
base. The treatment modalities consisted of surgery (41.9%),
surgery plus radiotherapy (28.0%), surgery plus radiochemotherapy
(20.4%) and other treatments (9.7%), including surgery with
chemotherapy followed by radiotherapy. The median follow-up period
was 62.1 months (range, 1–373 months). The baseline characteristics
of the 93 HNSS patients are illustrated in Table I.
 | Table IBaseline characteristics, tumor site
distribution and treatment type of 93 patients with head and neck
synoviosarcoma. |
Table I
Baseline characteristics, tumor site
distribution and treatment type of 93 patients with head and neck
synoviosarcoma.
| Feature | Value |
|---|
| Age, years |
| Median | 32.1 |
| Range | 4–76 |
| Gender, n |
| Male | 55 |
| Female | 38 |
| Site, n |
| Upper aerodigestive
tract | 47 |
| Neck | 25 |
| Skull base | 13 |
| Other | 8 |
| Treatment type,
n |
| S | 39 |
| S+R | 26 |
| S+R+C | 19 |
| S+C+R | 3 |
| Other | 6 |
Differential analysis between
clinicopathological characteristics and outcome statuses
In order to identify the differences between the
clinicopathological features of HNSS patients with different
outcome statuses, the data of the 93 cases were categorized into
three outcome groups, local recurrence, distant metastasis and
survival. Each category was further divided into two groups, which
resulted in the recurrence, recurrence-free, metastasis,
metastasis-free, non-survival and survival groups (Table II). Significant differences in
tumor size were identified between the recurrence-free and
recurrence, metastasis-free and metastasis, and survival and
non-survival groups (P=0.001, P<0.001 and P<0.001,
respectively). In addition, significant differences were found in
the pathological differentiation between the recurrence-free and
the recurrence (P=0.008) and survival and non-survival groups
(P=0.026). The logistic regression model was performed to evaluate
the risk of recurrence, metastasis and mortality. The risk of tumor
recurrence, metastasis and mortality was higher in the patients
with a tumor >5.0 cm in diameter compared with those with a
tumor ≤5.0 cm in diameter (Table
III).
 | Table IIClinicopathological differences in
head and neck synoviosarcoma between different outcome
statuses. |
Table II
Clinicopathological differences in
head and neck synoviosarcoma between different outcome
statuses.
| Recurrence, n
(%) | | | Metastasis, n
(%) | | | Survival, n (%) | | |
|---|
|
| | |
| | |
| | |
|---|
| Characteristic | No | Yes | Total | P-value | Yes | No | Total | P-value | Yes | No | Total | P-value |
|---|
| Age, years | | | | 0.165 | | | | 0.256 | | | | 0.907 |
| ≤32 | 27 (65.9) | 14 (34.1) | 41 | | 32 (72.7) | 12 (27.3) | 44 | | 39 (73.6) | 14 (26.4) | 53 | |
| >32 | 26 (81.3) | 6 (18.8) | 32 | | 26 (83.9) | 5 (16.1) | 31 | | 29 (72.5) | 11 (27.5) | 40 | |
| Gender | | | | 0.186 | | | | 0.998 | | | | 0.919 |
| Male | 28 (66.7) | 14 (33.3) | 42 | | 34 (77.3) | 10 (22.7) | 44 | | 40 (72.7) | 15 (27.3) | 55 | |
| Female | 25 (80.6) | 6 (19.4) | 31 | | 24 (77.4) | 7 (22.6) | 31 | | 28 (73.7) | 10 (26.3) | 38 | |
| Tumor location | | | | 0.443 | | | | 0.537 | | | | 0.532 |
| Superficial | 17 (77.3) | 5 (22.7) | 22 | | 18 (85.7) | 3 (14.3) | 21 | | 20 (74.1) | 7 (25.9) | 27 | |
| Moderate | 10 (83.3) | 2 (16.7) | 12 | | 10 (71.4) | 4 (28.6) | 14 | | 12 (63.2) | 7 (36.8) | 19 | |
| Deep | 26 (66.7) | 13 (33.3) | 39 | | 30 (75.0) | 10 (25.0) | 40 | | 36 (76.6) | 11 (23.4) | 47 | |
| Tumor size, cm | | | | 0.001 | | | | <0.001 | | | | <0.001 |
| ≤5.0 | 28 (77.8) | 8 (22.2) | 36 | | 29 (82.9) | 6 (17.1) | 35 | | 35 (79.5) | 9 (20.5) | 44 | |
| >5.0 | 5 (29.4) | 12 (70.6) | 17 | | 4 (26.7) | 11 (73.3) | 15 | | 5 (26.3) | 14 (73.7) | 19 | |
| Tumor
extension | | | | 1.000 | | | | 0.516 | | | | 1.000 |
| No | 4 (100.0) | 0 (0.0) | 4 | | 3 (100.0) | 0 (0.0) | 3 | | 4 (100.0) | 0 (0.0) | 4 | |
| Yes | 12 (80.0) | 3 (20.0) | 15 | | 8 (66.7) | 4 (33.3) | 12 | | 13 (86.7) | 2 (13.3) | 15 | |
| Surgical
margins | | | | 0.228 | | | | 1.000 | | | | 1.000 |
| Negative | 14 (93.3) | 1 (6.7) | 15 | | 11 (84.6) | 2 (15.4) | 13 | | 14 (93.3) | 1 (6.7) | 15 | |
| Positive | 1 (50.0) | 1 (50.0) | 2 | | 1 (100.0) | 0 (0.0) | 1 | | 2 (100.0) | 0 (0.0) | 2 | |
| Neck
dissection | | | | 0.315 | | | | 0.101 | | | | 0.553 |
| No | 22 (84.6) | 4 (15.4) | 26 | | 22 (88.0) | 3 (12.0) | 25 | | 25 (92.6) | 2 (7.4) | 27 | |
| Yes | 5 (62.5) | 3 (37.5) | 8 | | 4 (57.1) | 3 (42.9) | 7 | | 7 (87.5) | 1 (12.5) | 8 | |
| Histology | | | | 0.008 | | | | 4.190 | | | | 0.026 |
| Monophasic | 26 (76.5) | 8 (23.5) | 34 | | 17 (77.3) | 5 (22.7) | 22 | | 19 (65.5) | 10 (34.5) | 29 | |
| Biphasic | 22 (84.6) | 4 (15.4) | 26 | | 22 (71.0) | 9 (29.0) | 31 | | 28 (66.7) | 14 (33.3) | 42 | |
| Unclassified | 5 (38.5) | 8 (61.5) | 13 | | 19 (86.4) | 3 (13.6) | 22 | | 21 (95.5) | 1 (4.5) | 22 | |
| Treatment type | | | | 0.828 | | | | 0.116 | | | | 0.803 |
| Surgery | 22 (75.9) | 7 (24.1) | 29 | | 24 (88.9) | 3 (11.1) | 27 | | 29 (74.4) | 10 (25.6) | 39 | |
| Surgery +
radiotherapy | 20 (80.0) | 5 (20.0) | 25 | | 33 (73.3) | 12 (26.7) | 45 | | 21 (80.8) | 5 (19.2) | 26 | |
| Surgery +
radiochemotherapy | 10 (71.4) | 4 (28.6) | 14 | | 57 (79.2) | 15 (20.8) | 72 | | 14 (73.7) | 5 (26.3) | 19 | |
 | Table IIILogistic regression analysis of risk
factors for head and neck synoviosarcoma. |
Table III
Logistic regression analysis of risk
factors for head and neck synoviosarcoma.
| Characteristic | Odds ratio (95%
CI) | P-value |
|---|
| Recurrence |
| Tumor size >5.0
cm | 8.400
(2.275–31.009) | 0.001 |
| Metastasis |
| Tumor size >5.0
cm | 13.292
(3.140–56.270) | <0.001 |
| Mortality |
| Tumor size >5.0
cm | 10.889
(3.099–38.261) | <0.001 |
Survival and Cox-regression analysis
In total, 20 cases relapsed following the first
treatment and the recurrence rate was 21.5%. The distant metastasis
and mortality rates were 18.3 and 26.9%, respectively. The
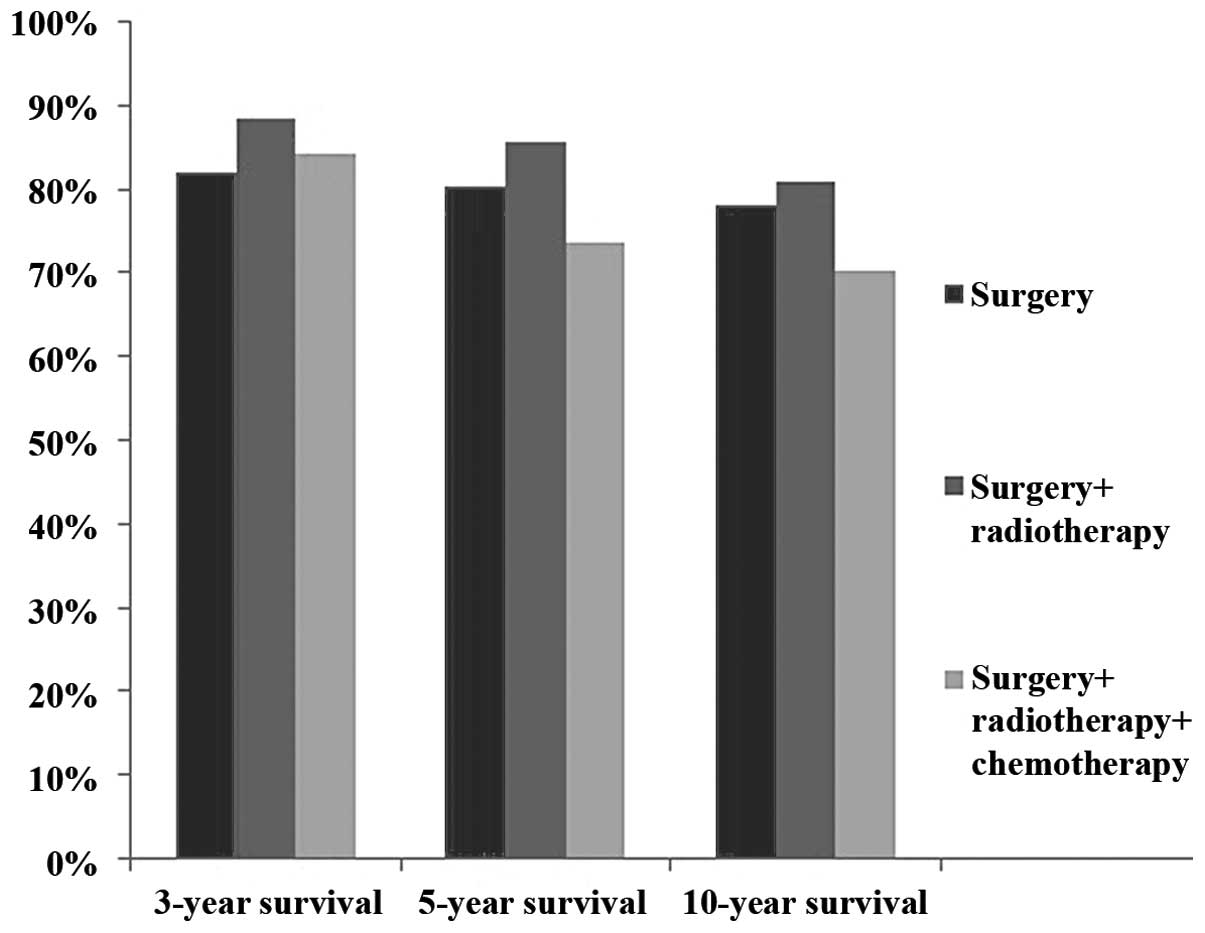
three-year survival rate was 82.1% for surgery alone, 88.5% for
surgery plus radiotherapy and 84.2% for surgery plus
radiochemotherapy. The five-year survival rate was 80.4% for
surgery alone, 85.5% for surgery plus radiotherapy and 73.7% for
surgery plus radiochemotherapy (Fig.
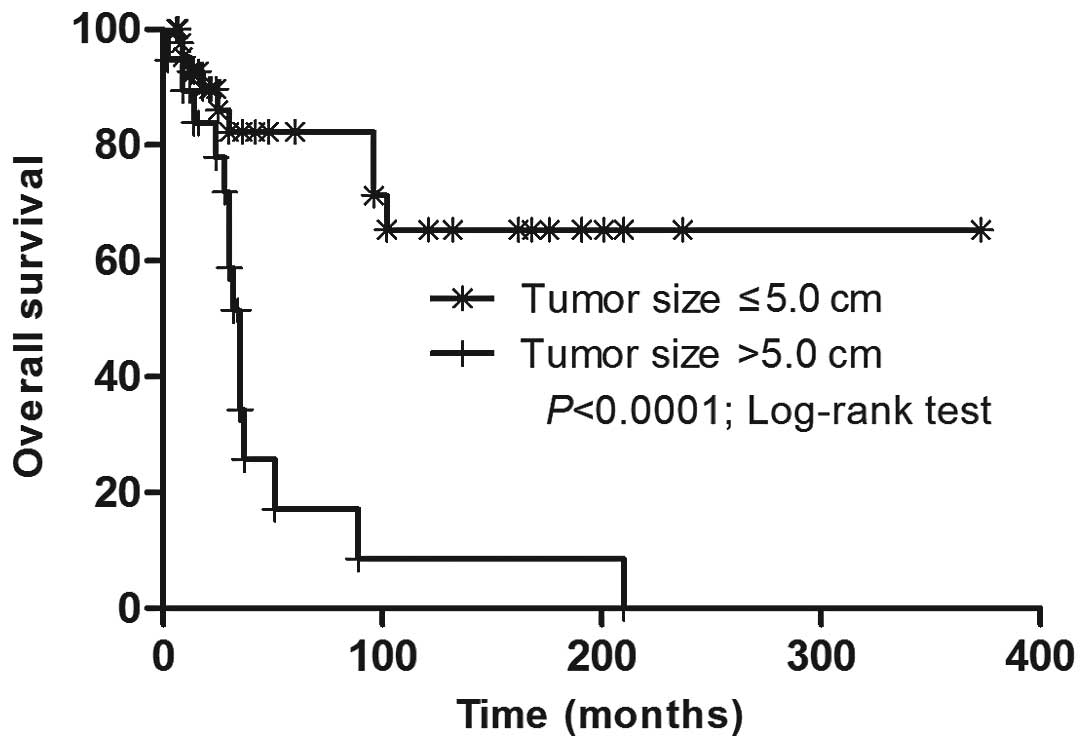
2). Marked tumor size-dependent differences in the overall
survival (OS) rate were revealed (Fig.
3). The Cox proportional-hazards model was utilized to predict
the independent prognostic factors for OS. A tumor >5.0 cm in
diameter was associated with a worse OS rate and the mortality risk
increased by 6.460-fold (95% CI, 2.206–18.917).
Discussion
To better elucidate whether the clinicopathological
characteristics and treatment were correlated with survival in
patients with HNSS and to find specific prognostic factors, a large
meta-analysis of 93 patients with histologically confirmed primary
HNSS was performed. Surgery is the major treatment for HNSS,
resulting in a good prognosis, while surgery-based combined
treatment modalities are not statistically superior to surgery
alone. In addition, the patients with tumors >5.0 cm in diameter
have a higher risk of local tumor recurrence, distant metastasis
and mortality than those with tumors ≤5.0 cm in diameter.
Importantly, the tumor size was the only independent adverse
prognostic factor for determining the OS.
Approximately half of the tumors in the 93 cases
were located in the upper aerodigestive tract. The upper
aerodigestive tract and neck are the most common originating sites
of HNSS and they account for 75% of HNSS. The tumor site determines
the clinical presentation of HNSS. Clinically, HNSS is a painless
and slow-growing mass, and is usually asymptomatic until it attains
a size sufficient to create pressure on the adjacent structures. As
a result, those in concealed locations, such as the infratemporal
fossa and skull base, which are inaccessible for the clinical
examination of a tumor in the early stages, grow unnoticed for a
considerable period and the tumors are commonly found at an
advanced stage.
Surgical excision is the mainstay of treatment for
HNSS, according to the present study. In total, 93% of the cases
were treated with surgery or surgery plus adjuvant therapy and
resulted in a three-year OS rate of 85.3%, five-year OS rate of
81.4% and 10-year OS rate of 78.3%. The results in the present
study were higher than those previously reported in the former
largest analysis with 40 consecutive cases, by the University of
Texas MD Anderson Cancer Center (Houston, TX, USA) (9). An explanation for this survival gap is
that 19 of 40 cases possessed recurrent disease with positive
surgical margins, and a robust association between negative margins
and local recurrence-free survival was observed.
The present meta-analysis results are influenced by
literature selection biases. However, existing data support the
role of adjuvant radiotherapy in improving the local control of
HNSS. The patients who received surgery plus radiotherapy achieved
good local control and higher survival rates than those treated
with surgery alone, although the difference was not statistically
significant (P=0.19). The group of patients who underwent surgery
plus radiochemotherapy possessed decreased five- and 10-year OS
rates compared with the other two treatment modalities, although it
is too soon to conclude that chemotherapy does not improve the OS
rate of HNSS since six of the 19 patients in the surgery plus
chemoradiotherapy group were diagnosed with advanced-stage disease,
either with an extremely large tumor size with extension to
adjacent structures, or the patients possessed multiple distant
metastasis already. It may be concluded that the early detection of
HNSS and total extirpation of the tumor, achieving negative
margins, is more effective than employing a salvaging approach at a
late stage of tumor development.
Another major interest of the present study was to
identify the prognostic factors for HNSS patients. Prognosis in SS
has been correlated with the patient age, tumor site, tumor size,
mitotic rate, presence of necrosis and histological subtype
(35–39). The present study confirms that the
tumor size is the only unfavorable prognostic factor for HNSS
survival. Certain early studies reported a more favorable outcome
in patients with biphasic tumors, whereas other groups found no
differences in survival between patients with monophasic tumors and
those with biphasic tumors (36,39,40).
The present results confirmed the lack of prognostic importance of
the histological subtype, even though there were significant
differences between the histological subtype and different outcome
statuses (Table II).
A few limitations of the present study must be
considered. Firstly, even though all the analyzed cases included
the treatment outcome and follow-up, certain pieces of important
information, including the pathological subtype, surgical margins
and tumor extension, were not clearly specified in several cases.
Missing these important clinicopathological parameters may
influence the results of the present study. Secondly, it is
extremely difficult to assemble single center or multicenter
prospective trials for an uncommon disease such as HNSS. Thus, the
retrospective data makes selection bias a possibility.
Despite its limitations, the present meta-analysis
comprehensively analyzed the clinicopathological features of HNSS
from the sporadic case reports in the peer-reviewed English
literature to date. Surgical excision is a mainstream treatment of
HNSS. Post-operative adjuvant radiotherapy is effective in local
tumor control and improves the OS rate of HNSS. However, the
effectiveness remains to be validated in further multicenter,
longitudinal, prospective, large cohort studies. In addition, the
present study confirmed that a tumor size >5.0 cm in diameter
was an independent adverse prognostic factor for OS.
Acknowledgements
This study was supported by the Shanghai Committee
of Science and Technology (grant no. 12DZ2260100).
References
|
1
|
Gurney JG, Young JL Jr, Roffers SD, et al:
Soft tissue sarcomas. Cancer Incidence and Survival Among Children
and Adolescents: United States SEER Program 1975–1995. Gloeckler
Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL Jr and
Bunin GR: National Cancer Institute, SEER program; Bethesda, MA,
USA: pp. 111–123. 1999
|
|
2
|
Bergh P, Meis-Kindblom JM, Gherlinzoni F,
et al: Synovial sarcoma: identification of low and high risk
groups. Cancer. 85:2596–2607. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ichinose H, Wickstrom JK, Hoerner HE and
Derbes VL: The early clinical presentation of synovial sarcoma.
Clin Orthop Relat Res. 185–189. 1979.PubMed/NCBI
|
|
4
|
Spillane AJ, A’Hern R, Judson IR, Fisher C
and Thomas JM: Synovial sarcoma: a clinicopathologic, staging, and
prognostic assessment. J Clin Oncol. 18:3794–3803. 2000.PubMed/NCBI
|
|
5
|
Kawai A, Woodruff J, Healey JH, et al:
SYT-SSX gene fusion as a determinant of morphology and prognosis in
synovial sarcoma. N Engl J Med. 338:153–160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Åkerman M, Ryd W and Skytting B:
Fine-needle aspiration of synovial sarcoma: Criteria for diagnosis:
Retrospective reexamination of 37 cases, including ancillary
diagnostics. A Scandinavian sarcoma group study. Diagn Cytopathol.
28:232–238. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cormier JN and Pollock RE: Soft tissue
sarcomas. CA Cancer J Clin. 54:94–109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Daraji W, Lasota J, Foss R and
Miettinen M: Synovial sarcoma involving the head: nalysis of 36
cases with predilection to the parotid and temporal regions. Am J
Surg Pathol. 33:1494–1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harb WJ, Luna MA, Patel SR, et al:
Survival in patients with synovial sarcoma of the head and neck:
association with tumor location, size, and extension. Head Neck.
29:731–740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barkan GA and El-Naggar AK: Primary
synovial sarcoma of the parotid gland. Ann Diagn Pathol. 8:233–236.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fisher RM and Spiro PC: Cervical synovial
sarcoma in a young boy. S Afr Med J. 48:2181–2182. 1974.PubMed/NCBI
|
|
12
|
Saydam L, Kizilay A, Kalcioglu MT, Mizrak
B and Bulut F: Synovial sarcoma of the pharynx: a case report. Ear
Nose Throat J. 81:36–39. 2002.PubMed/NCBI
|
|
13
|
Bertolini F, Bianchi B, Pizzigallo A,
Tullio A and Sesenna E: Synovial cell sarcoma of the neck. Case
report and review of the literature. Acta Otorhinolaryngol Ital.
23:391–395. 2003.
|
|
14
|
Agada FO, Murphy J, Sharma R, Karsai L and
Stafford ND: Biphasic synovial sarcoma of the posterior pharyngeal
wall: a case report. Ear Nose Throat J. 84:3023043062005.PubMed/NCBI
|
|
15
|
Yildirim A, Tosun F and Alaomeroglu M:
Synovial sarcoma of the nasal septum. Ann Otol Rhinol Laryngol.
114:84–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bukawa H, Kawabata A, Murano A, et al:
Monophasic epithelial synovial sarcoma arising in the
temporomandibular joint. Int J Oral Maxillofac Surg. 36:762–765.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orlandi E, Zonca G, Pignoli E, et al:
Postoperative radiotherapy for synovial sarcoma of the head and
neck during pregnancy: clinical and technical management and fetal
dose estimates. Tumori. 93:45–52. 2007.PubMed/NCBI
|
|
18
|
Lai V, Farrag TY, Cao D, et al: Synovial
sarcoma of the infratemporal fossa. Am J Otolaryngol. 28:444–447.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo CW, Liu CJ and Chang KM: Synovial
sarcoma of the temporomandibular joint area: report of a case. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 104:e62–e65. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jay A, Hutchison I, Piper K, Farthing PM
and Richards PS: Synovial sarcoma presenting as a parotid mass:
case report and review of literature. Head Neck. 30:1654–1659.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishiki H, Miyajima C, Nakao K, Asakage T,
Sugasawa M and Motoi T: Synovial sarcoma of the head and neck: rare
case of cervical metastasis. Head Neck. 31:131–135. 2009.
View Article : Google Scholar
|
|
22
|
Blankenburg S, Petersen I, Katenkamp D and
Chilla R: An unusual case of a synovial sarcoma of the parotid
gland in an elderly patient. Auris Nasus Larynx. 38:523–527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato T, Hasegawa H, Sugasawa M, et al:
Free jejunal transfer for a 15-year-old girl with synovial sarcoma
of the hypopharynx. J Plast Reconstr Aesthet Surg. 64:1100–1103.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rigante M, Visocchi M, Petrone G, Mulè A
and Bussu F: Synovial sarcoma of the parotid gland: a case report
and review of the literature. Acta Otorhinolaryngol Ital. 31:43–46.
2011.PubMed/NCBI
|
|
25
|
Dhawan A, Shenoy AM, Chavan P, Sandhu S
and Sriprakash D: Synovial sarcoma of the infratemporal fossa with
extension into the oral cavity - a rare presentation and literature
review. J Oral Maxillofac Surg. 70:2923–2929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khademi B, Mohammadianpanah M, Ashraf MJ
and Yeganeh F: Synovial sarcoma of the parapharyngeal space. Auris
Nasus Larynx. 34:125–129. 2007. View Article : Google Scholar
|
|
27
|
Bilgic B, Mete O, Oztürk SA, Demiryont M,
Keles N and Basaran M: Synovial sarcoma: a rare tumor of larynx.
Pathol Oncol Res. 9:242–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamarit Conejeros JM, Estrems Navas P,
Estellés Ferriol E and Dalmau Galofre J: Synovial sarcoma of the
infratemporal fossa. Acta Otorrinolaringol (English Edition).
61:389–391. 2010. View Article : Google Scholar
|
|
29
|
Kikuchi I, Anbo J, Nakamura S, et al:
Synovial sarcoma of the thyroid. Report of a case with aspiration
cytology findings and gene analysis. Acta Cytol. 47:495–500. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kartha SS and Bumpous JM: Synovial cell
sarcoma: diagnosis, treatment, and outcomes. Laryngoscope.
112:1979–1982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Zhang J, He X and Niu Y: Synovial
sarcoma in the oral and maxillofacial region: report of 4 cases and
review of the literature. J Oral Maxillofac Surg. 66:161–167. 2008.
View Article : Google Scholar
|
|
32
|
Capelli M, Bertino G, Morbini P, Proh M,
Falco CE and Benazzo M: CO2 laser in the treatment of laryngeal
synovial sarcoma: a clinical case. Tumori. 93:296–299.
2007.PubMed/NCBI
|
|
33
|
Meer S, Coleman H and Altini M: Oral
synovial sarcoma: a report of 2 cases and a review of the
literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
96:306–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alberty J and Dockhorn-Dworniczak B:
Monophasic synovial sarcoma of the neck in an 8-year-old girl
resembling a thyroglossal duct cyst. Int J Pediatr
Otorhinolaryngol. 63:61–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roth JA, Enzinger FM and Tannenbaum M:
Synovial sarcoma of the neck: a followup study of 24 cases. Cancer.
35:1243–1253. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cagle LA, Mirra JM, Storm FK, Roe DJ and
Eilber FR: Histologic features relating to prognosis in synovial
sarcoma. Cancer. 59:1810–1814. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rööser B, Willén H, Hugoson A and Rydholm
A: Prognostic factors in synovial sarcoma. Cancer. 63:2182–2185.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brodsky JT, Burt ME, Hajdu SI, Casper ES
and Brennan MF: Tendosynovial sarcoma. Clinicopathologic features,
treatment, and prognosis. Cancer. 70:484–489. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singer S, Baldini EH, Demetri GD, Fletcher
JA and Corson JM: Synovial sarcoma: prognostic significance of
tumor size, margin of resection, and mitotic activity for survival.
J Clin Oncol. 14:1201–1208. 1996.PubMed/NCBI
|
|
40
|
Krall RA, Kostianovsky M and Patchefsky
AS: Synovial sarcoma: a clinical, pathological, and ultrastructural
study of 26 cases supporting the recognition of a monophasic
variant. Am J Surg Pathol. 5:137–151. 1981. View Article : Google Scholar : PubMed/NCBI
|