Introduction
Cholangiocarcinoma is a malignant tumor that
originates in the intra- and extrahepatic biliary epithelium and is
commonly found in the elderly, being more frequent in males than
females. In the early stage of neoplastic transformation,
cholangiocarcinoma lacks specific clinical manifestations. Certain
common symptoms, including jaundice, usually do not present until
late in the course of the disease, at which time they are
relatively resistant to chemotherapeutic agents. As such, these
symptoms are difficult to treat and exhibit a poor prognosis
(1–4). A previous study has reported the
overall five-year survival rate following radical resection to be
15–50%, as cholangiocarcinoma cells easily metastasize, resulting
in relapse (5). The formation of
cholangiocarcinoma often proceeds through several steps, including
interaction among the cancer-promoting environmental factors,
oncogene activation and tumor-suppressor gene inactivation. The
relative balance controlling these processes is broken following
chronic injury to the cholangiocytes. During the tumorigenesis of
cholangiocarcinoma, certain growth factors, receptors and signaling
pathway molecules may be involved (1).
Raf kinase inhibitor protein (RKIP), also known as
human phosphatidylethanolamine binding protein 1, is a soluble
basic protein with a molecular weight of 21–23 kD and an
isoelectric point of ~8.6. RKIP is a highly conserved small
molecular protein that has been well preserved during evolution
(6). RKIP is mainly located in the
cytoplasmic organelles and on the plasma membrane. Previous studies
have found that RKIP affects various cellular processes. The
protein can inhibit MAP kinase (Raf-MEK-ERK), G protein-coupled
receptor (GPCR) kinase (GRK) and nuclear factor-κB (NFκB) signaling
cascades. Non-phosphorylated RKIP interferes with Raf-1 activity,
disrupts the interaction between Raf and MEK, and prevents the
activation of MEK and its downstream components, resulting in the
negative regulation of the Raf-MEK-ERK pathway. By contrast,
phosphorylated RKIP dissociates from Raf-1, but binds and inhibits
G-protein coupled receptor kinase (GRK)-2, resulting in sustained
G-protein signaling (6–8).
RKIP is implicated in several types of cell
behavior, including cell growth, apoptosis, invasion and migration
(8). It has previously been
indicated that RKIP expression is decreased in metastatic prostate
and breast cancer. Furthermore, the overexpression of RKIP in
metastatic prostate and breast cancer can decrease the invasive
ability of these cells. RKIP overexpression is induced in breast
and prostate cancer cells when they are treated with
chemotherapeutic agents, predisposing the cells to apoptosis
(9). It has been proposed that RKIP
expression can be used as a prognostic marker for renal cell
carcinoma patients (10). Another
study has found that RKIP can inhibit breast tumor metastasis
through the enhancement of microRNA let-7 processing (11). Additionally, the level of RKIP
expression is low in lung, bladder and cervical cancers and
nasopharyngeal carcinoma (12). Our
previous study found that RKIP may prevent liver fibrosis, which is
a pre-cancerous lesion, through its inhibitory effects on hepatic
stellate cell (HSC) proliferation (13). We have also previously demonstrated
an association between lymph node or distant metastasis in
decreased RKIP expression and esophageal cancer tissues. The study
demonstrated that RKIP downregulates the expression of GRK-2, Lin28
and matrix metalloproteinase (MMP)-14 (14). However, the association between RKIP
expression and the progression of cholangiocarcinoma, and the
effect of its regulation on the biological behavior of
cholangiocarcinoma cells is not yet clear. Therefore, the present
study investigated the association between RKIP expression and the
prognosis of cholangiocarcinoma, and the effects of the protein on
cholangiocarcinoma cell growth, apoptosis, invasion and
metastasis.
Materials and methods
Subjects
In total, 30 extrahepatic cholangiocarcinoma tumor
and adjacent uninvolved peritumoral tissues were obtained at the
time of surgery from patients in the Second Hospital of Hebei
Medical University (Shijiazhuang, Hebei, China). The tissues were
fixed in 10% neutral-buffered formalin overnight and were embedded
in paraffin in a tissue processor. The present study was approved
by the Ethics Committee of the Second Hospital of Hebei Medical
University, in line with ethics requirements. Written informed
consent was obtained from all patients.
Immunohistochemical staining
The paraffin-embedded tissue specimens were
sectioned to a 4-μm thickness and mounted on Adhesion Microscope
slides (Citotest Labware Manufacturing Co., Ltd., Haimen, China).
The tissue sections were deparaffinized, rehydrated and stained by
hematoxylin and eosin (HE) or used for immunohistochemistry (IHC)
with mouse monoclonal anti-human cytokeratin (CK)19 (1:1, Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China) or
rabbit anti-human polyclonal RKIP (1:200; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) antibodies.
The sections were stained and the RKIP staining
results were scored by a previously described method (14,15).
Tissues with a final score that exceeded the median score were
classed as having high RKIP expression and tissues with a final
score equal to or below the median exhibited downregulated RKIP
expression. The correlations between RKIP expression in the
cholangiocarcinoma tissue and age, gender, differentiation,
pathological stage and lymph node or distant metastases were
investigated.
Design and cloning of short hairpin RNA
constructs
Two 21-nucleotide (nt) small-interfering (si)RNA
sequences of Homo sapiens (Genbank accession, NM_002567)
were designed using the GenScript web-based program (http://www.genscript.com/siRNA_service.html, GenScript
USA Inc., Piscataway, NJ, USA). The specificity of the siRNA
sequences was verified by a Basic Local Alignment Search Tool
search (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The siRNA
sequence was 5′-CGAGCAGCTGTCTGGGAAGTA-3′. A non-related 19-nt
sequence, 5′-TTCTCCGAACGTGTCACGT-3′, was used as an siRNA-negative
control. To employ viral delivery of the double-stranded siRNA, the
adenoviral vector, pDC316-siRNA (Shanghai Genechem Co., Ltd.,
Shanghai, China), was used. The successful pDC316-siRNA recombinant
vector [RKIP-RNA interference (RNAi)-AD] production was confirmed
by sequencing. A negative siRNA control with green fluorescent
protein (GFP; NC-RNAi-GFP-AD) was also used.
Preparation of the RKIP-overexpressing
vector
To induce RKIP overexpression in vitro, an
adenoviral vector expressing RKIP (RKIP-AD; Genbank accession,
NM_002567) and driven by a mCMV promoter was constructed. This was
accomplished by placing the complementary (c)DNA for RKIP, excised
by AgeI from plasmid pDC315-enhanced GFP (EGFP; Shanghai
Genechem Co., Ltd.), downstream of the mCMV gene promoter.
Recombinant virus from a single plaque was identified by DNA
analysis, expanded in NIH293 cells (American Type Culture
Collection, Manassas, VA, USA), and twice purified by an Adeno-XTM
Virus Purification kit (BD Biosciences, Clontech, San Jose, CA,
USA). The viral titers were determined by median tissue culture
infective dose (TCID50) assays. The titers determined by
the TCID50 assays were used in subsequent experiments.
No contamination was detected in the viral preparations used in the
experiments. The adenoviral vector pDC315-EGFP (GFP-AD) was used as
a negative control.
Infection of the human cholangiocarcinoma
RBE cell line
The human cholangiocarcinoma RBE cell line was
provided by the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The RBE cells were cultured in RPMI-1640,
supplemented with 10% heat-inactivated fetal bovine serum (FBS),
100 IU/ml penicillin, 100 μg/ml streptomycin (Biochrom KG, Berlin,
Germany) and 4 mmol/l L-glutamine under a humidified atmosphere of
5% CO2 and 95% air at 37°C, and subcultured when 90%
confluent. In total, 1×106 RBE cells were infected for
48 h with pretreated viral particles (RKIP-RNAi-AD, NC-RNAi-GFP-AD,
RKIP-AD and GFP-AD) at a multiplicity of infection (MOI) of 400.
The RKIP protein and mRNA expression was determined by western blot
analysis and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), respectively.
Western blot analysis
Subsequent to being rinsed three times with
phosphate-buffered saline (PBS), the RBE cells were lysed with
lysis buffer and extracted on ice (13). The samples were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis and
electroblotted onto polyvinylidene fluoride membranes (Millipore,
Bedford, MA, USA). The membranes were then incubated with primary
antibodies at 4°C overnight. The primary antibodies were rabbit
anti-human polyclonal RKIP (1:400) and rabbit anti-human polyclonal
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1,000; Santa
Cruz Biotechnology, Inc.). Subsequent to being washed with
Tris-buffered saline with Tween 20, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies (Santa
Cruz Biotechnology, Inc.) for 2 h at room temperature. The antigens
were detected by enhanced chemiluminescence (Santa Cruz
Biotechnology, Inc.). For protein quantification, the bands were
scanned and quantified by NIH ImageJ 1.38 software (National
Institutes of Health, Bethesda, MD, USA), using GAPDH as the
internal control. The results were reported as the mean of
triplicate assays.
RT-qPCR assay
The total RNA from the RBE cells was isolated using
TRIzol reagent (Tiangen Biotech (Beijing) Co., Ltd., Beijing,
China), according to the manufacturer’s instructions (14). Overall, ~200 ng of total RNA was
converted to first-strand cDNA using a SuperScript-II RT system
(Life Technologies, Carlsbad, CA, USA). qPCR was performed in a
total volume of 20 μl in the presence of SYBR Green PCR master mix
[Tiangen Biotech (Beijing) Co., Ltd] on an ABI Stepone Plus
Real-Time PCR Systems device (Applied Biosystems, Foster City, CA,
USA). The primers used to amplify RKIP, MMP-9, tissue inhibitor of
metalloproteinase 4 (TIMP-4) and GAPDH were as follows: RKIP
forward, 5′-AGACCCACCAGCATTTCGTG-3′ and reverse,
5′-GCTGATGTCATTGCCCTTCA-3′; MMP-9 forward,
5′-CACCGCCAACTACGACCGGG-3′ and reverse, 5′-GGTGGTAGCGCACCAGAGGC-3′;
TIMP-4 forward, 5′-AAGAGCCTCGGGTCCTGCCTC-3′ and reverse, 5′-CAA
GGCCGTTGTGCCCCTCG-3′; and GAPDH forward,
5′-GAACGGGAAGCTCACTGGCATGGC-3′ and reverse,
5′-TGAGGTCCACCACCCTGTTGCTG-3′. The PCR conditions were as follows:
10 min incubation phase at 95°C, 40 cycles of 95°C for 10 sec, 60°C
for 30 sec, and 72°C for 20 sec. Results in triplicate were
expressed as the ratio of the cycle threshold value for the target
gene cDNA concentration relative to that for GAPDH, using the
2−ΔΔCt method.
Cell proliferation assay
RBE cells in the logarithmic growth phase were
trypsinized and seeded in a 96-well plate, 200 μl (5×104
cells/ml) per well, and incubated with RKIP-RNAi-AD,
NC-RNAi-GFP-AD, RKIP-AD, GFP-AD or PBS for 24, 48, 72 or 96 h. Cell
proliferation was evaluated with a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich, St. Louis, MO, USA). The absorbance was
determined at a 492-nm wavelength, with a reference wavelength of
630 nm. The results are presented as the average absorbance of 6
wells in one experiment, and the assays were performed in
triplicate.
Apoptosis assay
The flow cytometry data of the RBE cells were
collected following adenoviral-mediated transfer, centrifugation at
700 × g for 5 min and resuspension in PBS at a concentration of
5×105 cells/ml. Annexin V-phycoerythrin was added and
the cells were kept away from light for 15 min at room temperature
until the analysis. The DNA content was analyzed using a flow
cytometer (Coulter Epics XL; Beckman Coulter, Inc., Brea, CA, USA).
Cell apoptosis was analyzed using the WinMDI software program
(Scripps Research Institute, La Jolla, CA, USA).
Transwell invasion assay
RBE cells (5×105 per well) at 48 h
post-transfection were resuspended in 200 μl serum-free medium
containing 1% bovine serum albumin and were seeded on the top
chamber of the 8-μm pore Transwell, using 6.5-mm polycarbonate
Transwell filters (Corning, Inc., New York, NY, USA). The bottom
chamber was supplied with 600 μl of medium containing 10% FBS.
After a 48-h incubation period in CO2 at 37°C, the
non-invading cells were removed from the upper surface of the
membrane by gentle scrubbing with a cotton swab. The cells that had
invaded the bottom chamber were fixed with methanol and stained
with crystal violet solution. The number of cells on the lower
surface of the filters was counted by two researchers blinded to
the sample groups. A total of five representative fields were
counted for each Transwell filter.
Wound healing assay
RBE cells (1×106 cells/well) at 12 h
post-transfection were seeded in six-well plates and allowed to
adhere for 36 h. Confluent monolayer cells were scratch wounded
using a sterile plastic micropipette tip and then washed three
times with PBS to clear any cell debris and suspended cells. Fresh
serum-free medium was added and the cells were allowed to close the
wound for 24 h. Images were captured at 0, 12 and 24 h at the same
wound position. Morphometric analyses of the digital images were
then performed with NIH ImageJ 1.38 software. The percentage of
wound closure was revealed by the change in the area of the wound
that remained open at each time-point. Curve fitting and
statistical analyses were carried out by GraphPad Prism software
(GraphPad Software, Inc., La Jolla, CA, USA).
Statistical analysis
Statistical analyses were performed by SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). The metrological data are presented
as the mean ± standard deviation. The χ2,
Kruskal-Wallis, Spearman’s rank correlation coefficient, one-way
analysis of variance and Fisher’s least significant difference
tests were used as appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
RKIP expression is decreased in
cholangiocarcinoma tissues
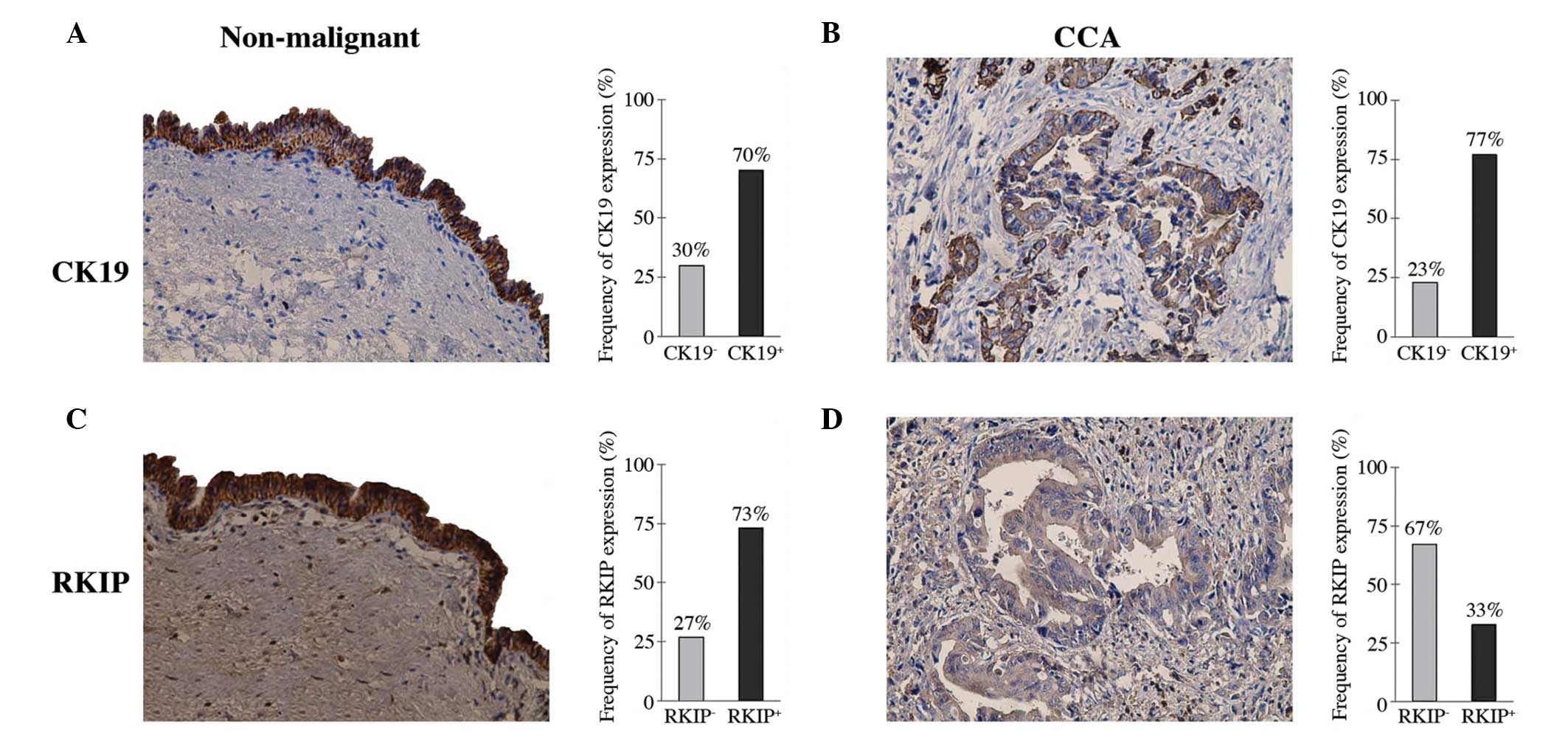
HE staining of the specimens obtained from 30
patients with cholangiocarcinoma revealed evident differences
between the specimens of the extrahepatic cholangiocarcinoma tumor
and adjacent uninvolved peritumoral tissues, for which
representative images from single similar experiments are provided.
CK19 was constitutively expressed in the normal cholangiocytes and
cholangiocarcinoma cells and there was no significant difference
between the two groups (χ2 test, P>0.05; Fig. 1A and B).
Immunohistochemical staining of the RKIP polyclonal
antibody revealed high RKIP expression in the normal
cholangiocytes, with abundant yellowish-brown particle sediment
appearing in the cytoplasm. However, RKIP expression was lower in
the cholangiocarcinoma cells, with no evident yellowish-brown
particle sediment being observed. The frequency of RKIP-positive
expression was 73 vs. 33% in the normal cholangiocytes and
cholangiocarcinoma, respectively (Fig.
1C and D). These results indicated that the RKIP expression was
significantly lower in the cholangiocarcinoma cells compared with
the normal cholangiocytes (χ2 test; P<0.01).
RKIP expression is associated with cell
differentiation and the lymph node or distant metastasis of
cholangiocarcinoma
The age of the cholangiocarcinoma patients, 12 male
and 18 female, ranged between 45 and 75 years old. The 30
cholangiocarcinoma tumors were all identified as adenocarcinoma by
HE staining. In total, 8 patients presented with
well-differentiated cholangiocarcinoma, 9 with
moderately-differentiated cholangiocarcinoma and 13 with
poorly-differentiated cholangiocarcinoma. Of the 30 patients with
regional lymph node involvement, 17 exhibited early-stage (I–II)
disease and 13 exhibited advanced-stage (III–IV) disease. In
addition, 13 patients presented with lymph node or distant
metastases.
RKIP expression was negatively correlated with cell
differentiation (P<0.001) and lymph node or distant metastasis
(P<0.01). However, RKIP expression did not correlate with the
age or gender of the patient or the pathological stage of the tumor
(Table I).
 | Table ICorrelations between
clinicopathological characteristics and RKIP expression. |
Table I
Correlations between
clinicopathological characteristics and RKIP expression.
| | RKIP
expression | |
|---|
| |
| |
|---|
| Parameters | Total (n=30) | High | Low | P-value |
|---|
| Mean age ± SD,
years | 59±10 | 58±8 | 60±12 | 0.451a |
| Gender, n | | | | 0.654b |
| Male | 12 | 7 | 5 | |
| Female | 18 | 9 | 9 | |
| Differentiation,
n | | | | <0.001b |
| Good | 8 | 8 | 0 | |
| Moderate | 9 | 6 | 3 | |
| Poor | 13 | 2 | 11 | |
| pStage, n | | | | 0.961b |
| I–II | 17 | 9 | 8 | |
| III–IV | 13 | 7 | 6 | |
| Lymph or distant
metastasis, n | | | | 0.004b |
| Yes | 13 | 3 | 10 | |
| No | 17 | 13 | 4 | |
RKIP overexpression or downregulation in
RBE cells infected by adenoviral vectors does not affect RBE cell
proliferation and apoptosis
To further elucidate the role of RKIP, the RBE cells
were infected with RKIP siRNA or RKIP-overexpressing adenoviral
vector. The adenoviral infection efficiency in the different groups
was first determined using flow cytometry. The infection rates of
RKIP-RNAi-AD, NC-RNAi-GFP-AD, RKIP-AD and GFP-AD in the RBE cells
were 87.9, 91.5, 94.5 and 89.4%, respectively, at 48 h
post-transfection (data not shown). Next, western blot analysis was
used to further confirm that the RKIP vector was transiently
transfected into the RBE cells. The data revealed that RKIP
expression in the RKIP-AD group was significantly increased
compared with the GFP-AD group (2.77±0.95 vs. 0.85±0.44;
P<0.05). Compared with the NC-RNAi-GFP-AD group, RKIP expression
was significantly decreased in the RKIP-RNAi-AD group (0.26±0.17
vs. 0.88±0.37; P<0.05; Fig. 2A and
B).
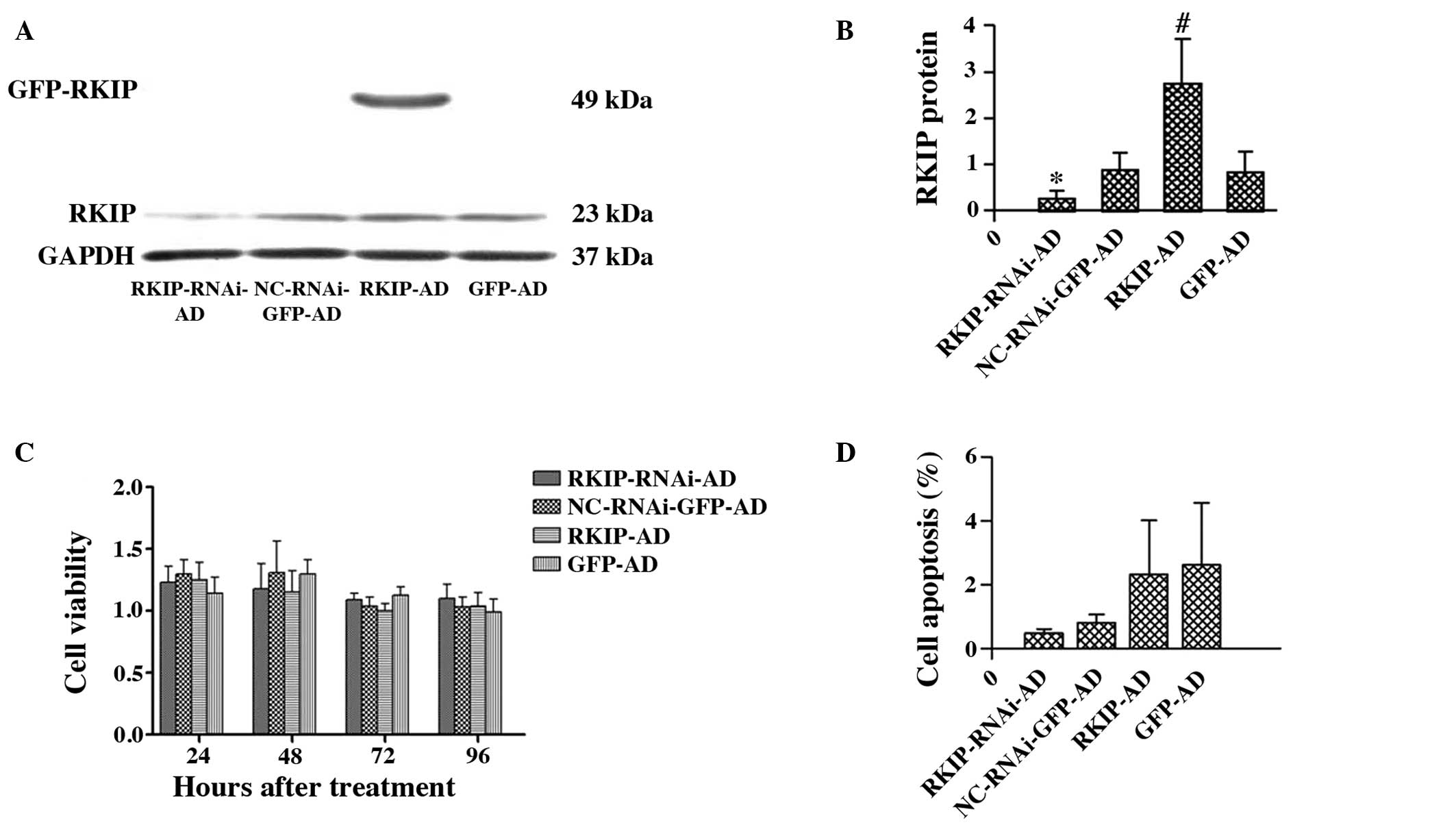 | Figure 2Western blot analysis to detect RKIP
and GAPDH protein expression in RBE cells 48 h after adenoviral
infection. (A and B) RKIP expression is decreased in the
RKIP-RNAi-AD treatment group compared with the NC-RNAi-GFP-AD group
(*P<0.05; n=3). In the RKIP-AD group, a large amount
of RKIP is expressed as an exogenous protein compared with the
GFP-AD group (#P<0.05; n=3). (C) Cell viability of
the RBE cells exposed to recombinant adenovirus at different
time-points, assessed by MTT assay. The RBE cells were transfected
with recombinant adenovirus for 24, 48, 72 or 96 h, respectively.
These cells were infected with RKIP-RNAi-AD, NC-RNAi-GFP-AD,
RKIP-AD or GFP-AD. The differences were not significant in either
of the two groups (P>0.05; n=3). (D) Apoptosis of the RBE cells
at 48 h after recombinant adenovirus infection. Apoptosis of the
RBE cells transfected with RKIP-RNAi-AD, NC-RNAi-GFP-AD, RKIP-AD
and GFP-AD, respectively, was detected by phycoerythrin-labeled
flow cytometry. The differences were not significant in either of
the two groups (P>0.05; n=3). RKIP, Raf kinase inhibitor
protein; RKIP-RNAi-AD, siRNA recombinant vector; NC-RNAi-GFP-AD,
siRNA-negative control with green fluorescent protein (GFP);
RKIP-AD, adenoviral vector expressing RKIP; GFP-AD, adenoviral
negative control. |
Adenovirus-mediated gene transfection at an MOI of
400 was used for the subsequent experiments. MTT assays revealed
that RKIP-RNAi-AD or RKIP-AD adenoviral infection for 24, 48, 72
and 96 h had no effect on the viability of the RBE cells
(P>0.05; Fig. 2C). Flow
cytometry demonstrated that there was no significant difference in
the apoptotic rate of the RBE cells between the RKIP-RNAi-AD
(0.47±0.15%) and NC-RNAi-GFP-AD groups (0.80±0.26%) (P>0.05).
Similarly, there was no significant difference in the apoptotic
rate of the RBE cells between the RKIP-AD (2.3±1.71%) and GFP-AD
groups (2.6±1.96%) (P>0.05; Fig.
2D).
RKIP inhibits RBE cell invasion
Next, the effect of RKIP on the invasive ability of
the RBE cells was investigated (Fig.
3A–E). There was an increase in the number of invasive cells in
the RKIP-RNAi group compared with the NC-RNAi-GFP-AD group
(115.00±18.30 vs. 52.67±16.62; P<0.05), while there was a
decrease in the number of invasive cells in the RKIP-AD group
compared with the GFP-AD group (17.00±6.48 vs. 60.67±22.02;
P<0.05). However, the cell invasion assay revealed that there
was no significant difference between the GFP-AD and NC-RNAi-GFP-AD
groups (60.67±22.02 vs. 52.67±16.621; P>0.05; Fig. 3E).
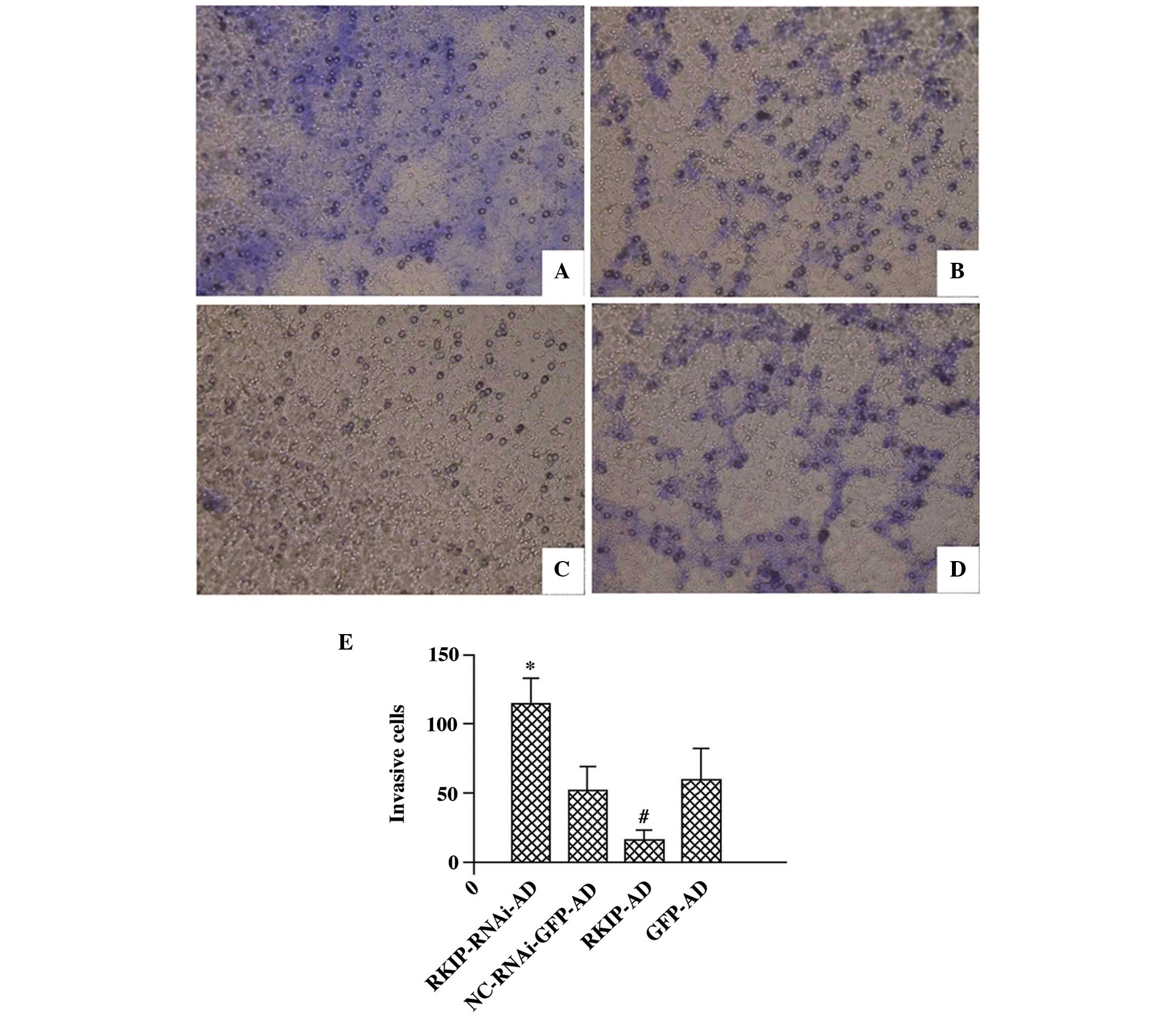 | Figure 3Invasive ability of RBE cells 48 h
after recombinant adenoviral infection, as detected by invasion
assay (magnification, ×200). The RBE cells were infected with (A)
RKIP-RNAi-AD, (B) NC-RNAi-GFP-AD, (C) RKIP-AD and (D) GFP-AD,
respectively. (E) Number of cells on the lower Transwell filter for
each condition, revealing that RKIP inhibits the invasion of RBE
cells (*P<0.05 vs. NC-RNAi-GFP-AD;
#P<0.05 vs. GFP-AD; n=6 for each group). RKIP, Raf
kinase inhibitor protein; RKIP-RNAi-AD, siRNA recombinant vector;
NC-RNAi-GFP-AD, siRNA-negative control with green fluorescent
protein (GFP); RKIP-AD, adenoviral vector expressing RKIP; GFP-AD,
adenoviral negative control; RNAi, RNA interference. |
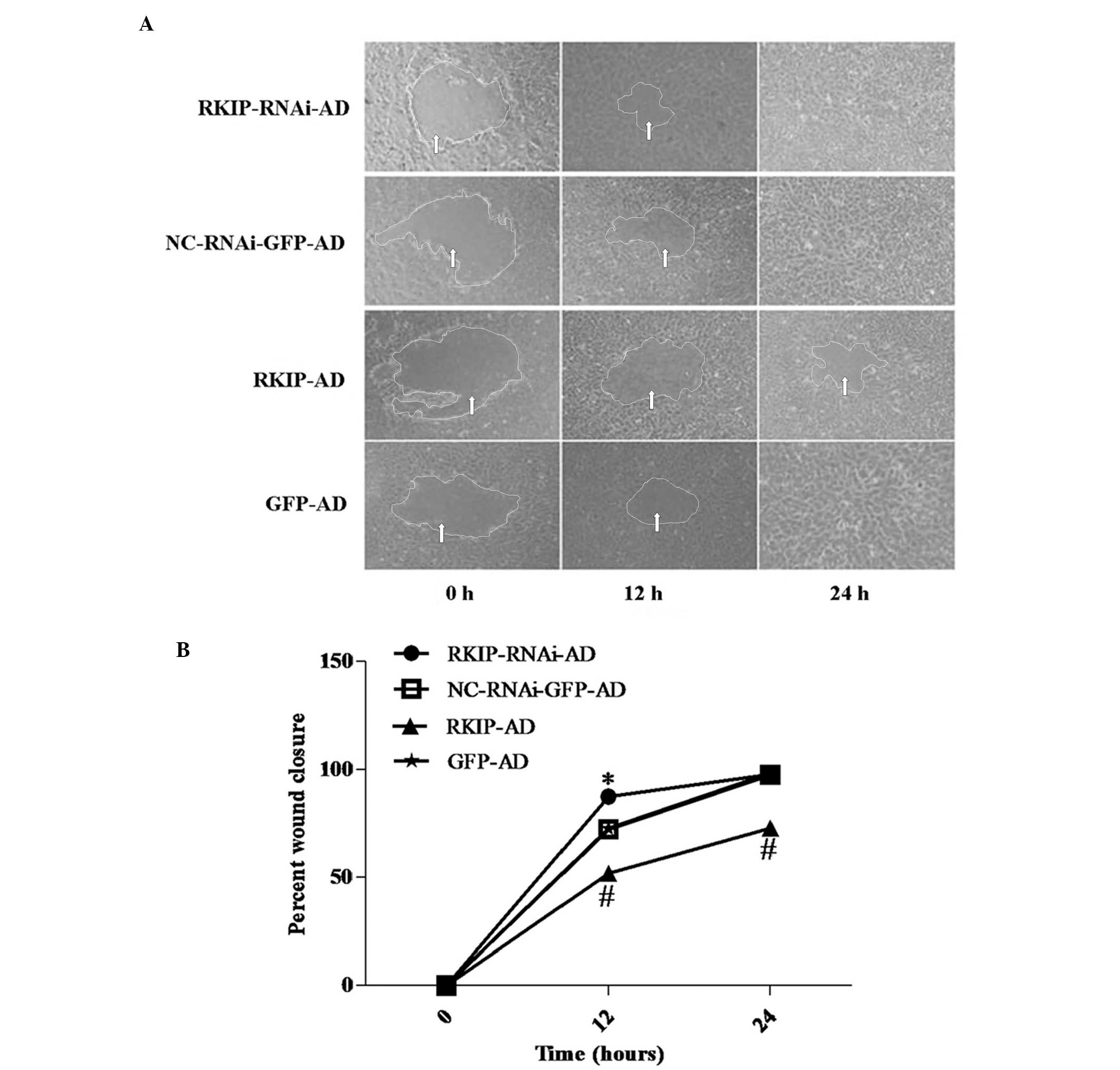
RKIP inhibits RBE cell migration
The wound closure assay revealed that at 12 h, the
RBE cells in the RKIP-RNAi-AD group grew to close 87.6% of the
wound, while the cells in the NC-RNAi-GFP-AD group only closed
72.3%; the difference between the two groups was statistically
significant (P<0.05). RKIP overexpression significantly
inhibited the wound closure rate of the RBE cells in the RKIP-AD
group, with only 52.2% of the wound being closed, while 72.8% was
closed in the GFP-AD group (P<0.05; Fig. 4). At 24 h, there was no significant
difference between the RKIP-RNAi-AD and NC-RNAi-GFP-AD groups.
However, compared with GFP-AD, RKIP-AD significantly inhibited RBE
cell migration (98.4 vs. 73.1%; P<0.01; Fig. 4B).
RKIP downregulates MMP-9, but upregulates
TIMP-4 mRNA expression
Following the silencing or overexpression of RKIP by
RKIP siRNA or adenoviral vectors, respectively, RT-qPCR was
performed to analyze the mRNA expression levels of RKIP, MMP-9 and
TIMP-4, using a quantitative method. When RKIP-RNAi-AD (0.22±0.04)
was compared with NC-RNAi-GFP-AD (1.00±0.00), a significant
decrease in RKIP mRNA expression levels was revealed (P<0.01,
Fig. 5A). However, RKIP mRNA
expression was significantly higher in the RKIP-AD group
(67.22±2.49) compared with the GFP-AD group (1.00±0.00; P<0.01)
(Fig. 5B).
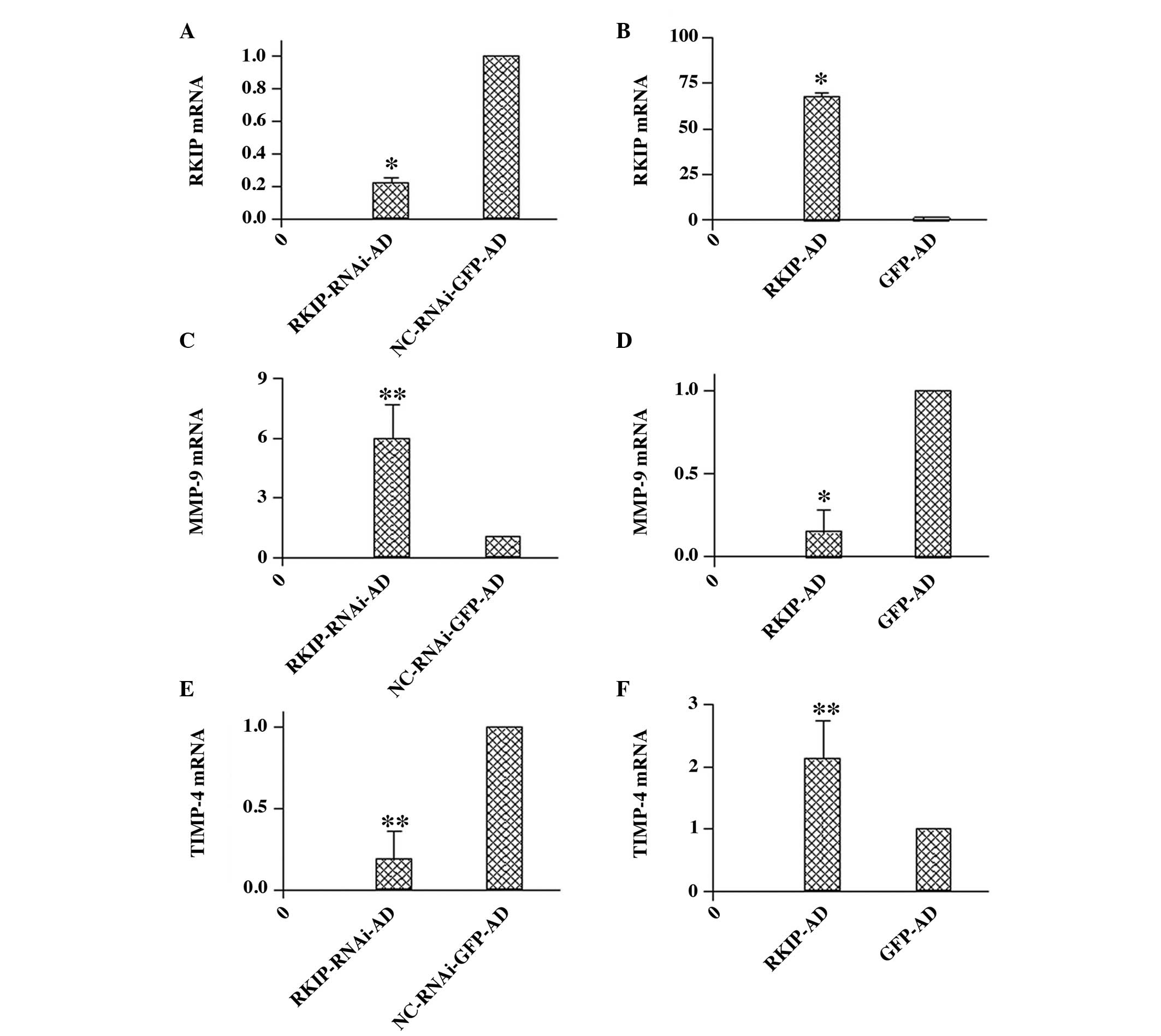 | Figure 5Effect of RKIP RNAi and overexpression
on the mRNA expression of RKIP, MMP-9 and TIMP-4 in RBE cells.
RKIP, MMP-9 and TIMP-4 mRNA expression was assessed by quantitative
polymerase chain reaction. The expression was normalized as a ratio
using GADPH as a reference gene. A value of 1 for this ratio was
arbitrarily assigned to the data obtained from the control. (A)
RKIP mRNA expression was significantly suppressed in the
RKIP-RNAi-AD group in RBE cells (*P<0.01) and (B)
RKIP mRNA expression was significantly increased in the RKIP-AD
group in the RBE cells (*P<0.01). (C) The expression
of the MMP-9 transcripts was increased by RKIP RNAi
(**P<0.05), (D) and the expression of the MMP-9
transcripts was significantly reduced in the RKIP-AD group
(*P<0.01). (E) The expression of the TIMP-4
transcripts was reduced by RKIP RNAi (**P<0.05) and
(F) the expression of TIMP-4 transcripts was significantly
increased in the RKIP-AD group (**P<0.05). All the
comparisons in (A), (C) and (E) were made between RBE cells
transfected with RKIP-RNAi-AD and those with the vector,
NC-RNAi-GFP-AD. All the comparisons in (B), (D) and (F) were made
between RBE cells transfected with RKIP-AD and those with the
vector GFP-AD. All the data are expressed as the mean ± standard
deviation from three individual experiments (n=3). RNAi, RNA
interference; RKIP, Raf kinase inhibitor protein; MMP, matrix
metalloproteinase; TIMP, tissue inhibitor of metalloproteinase;
RKIP-RNAi-AD, siRNA recombinant vector; NC-RNAi-GFP-AD,
siRNA-negative control with green fluorescent protein (GFP);
RKIP-AD, adenoviral vector expressing RKIP; GFP-AD, adenoviral
negative control. |
The mRNA expression of MMP-9 was significantly
higher in the RKIP-RNAi-AD group (5.96±1.70) compared with the
NC-RKIP-RNAi-AD group (1.00±0.00) (P<0.05; Fig. 5C). However, the MMP-9 mRNA
expression was significantly lower in the RKIP-AD group (0.15±0.13)
compared with the GFP-AD group (1.00±0.00) (P<0.01; Fig. 5D). In addition, the mRNA expression
of TIMP-4 was significantly lower in the RKIP-RNAi-AD group
(0.19±0.17) compared with the NC-RKIP-RNAi-AD group (1.00±0.00)
(P<0.05; Fig. 5E), and higher in
the RKIP-AD group (2.13±0.60) compared with the GFP-AD group
(1.00±0.00) (P<0.05; Fig.
5F).
Discussion
The current study provides evidence that RKIP
expression is decreased in cholangiocarcinoma tissues, and that it
negatively correlates with cholangiocarcinoma cell differentiation
and lymph node or distant metastasis. In the present study, RKIP
was revealed to inhibit the invasion and metastasis of
cholangiocarcinoma cells by downregulating MMP-9, but upregulating
TIMP-4 mRNA expression.
Cholangiocarcinoma are rare, malignant tumors that
originate in the biliary tract epithelia. Based on their anatomical
location, cholangiocarcinomas are classified as either intrahepatic
or ductal cholangiocarcinoma. Liver fluke infestations, chronic
viral hepatitis, hepatolithiasis, choledochal cysts and primary
sclerosing cholangitis can all predispose patients to developing
cholangiocarcinoma (16). The
tumor-node-metastasis classification for biliary tract cancers is
not useful in clinical practice, as the T classification does not
differentiate between the prognosis, for example, of T2 and T3
tumors. Surgery is the main treatment for resectable, localized
cholangiocarcinoma. However, it is extremely easy for
cholangiocarcinoma cells to metastasize and cause relapse. The
number of lesions and the presence of vascular invasion have been
found to be important prognostic factors, whereas tumor size has
not (17,18).
RKIP is a multifaceted kinase modulator that is
conserved between species. RKIP inhibits the Raf-MEK-ERK, GPCR
kinase and NFκB signaling cascades. RKIP can be phosphorylated at
serine 153 following protein kinase C stimulation. Residues 127–146
of RKIP are critical for dimer formation. The formation of the
dimer is an important mechanical feature in the switch in RKIP
association from Raf1 to GRK2 (19–21).
Several studies have demonstrated that RKIP is closely associated
with numerous tumors and participates in their occurrence and
development. RKIP expression is decreased in numerous tumors and
regulates the growth, apoptosis, invasion and metastasis of tumor
cells (8–12,14).
CK19 is a highly sensitive cholangiocyte marker and
is also commonly overexpressed in cholangiocarcinoma cells
(22). In the present study, CK19,
as a specific diagnostic marker, was stained in the extrahepatic
cholangiocarcinoma tumor and adjacent uninvolved peritumoral
tissues. Immunohistochemical staining revealed that RKIP expression
was decreased in cholangiocarcinoma tissues, which is similar to
the findings of a previous study, which revealed that RKIP
expression contributes to invasion and metastasis in carcinoma of
the ampulla of Vater (23). The
current study further demonstrated that reduced RKIP expression in
cholangiocarcinoma cells is negatively correlated with cell
differentiation and lymph node or distant metastasis. Therefore,
positive RKIP expression in cholangiocarcinoma cells may indicate a
good prognosis for patients.
To further explore the role of RKIP in
cholangiocarcinoma growth, in the present study, the protein was
either overexpressed through an RKIP adenoviral vector or silenced
by RKIP siRNA. It was found that neither overexpressed nor
downregulated RKIP affected cholangiocarcinoma cell proliferation
in vitro. At the same time, neither RKIP overexpression nor
RKIP-knockdown enhanced cell apoptosis.
RKIP has been identified as a member of a novel
class of metastasis suppressors, with evidence from prostate
cancer, breast cancer, malignant melanoma, insulinoma, colorectal
cancer, hepatocellular carcinoma and esophageal cancer (14,24).
However, the role of RKIP in cholangiocarcinoma metastasis requires
elucidation. Immunostaining for RKIP in the present study indicated
that reduced RKIP expression promotes cholangiocarcinoma cell
metastasis. In order to evaluate the effect of RKIP on
cholangiocarcinoma metastasis, the cholangiocarcinoma cell line,
RBE, was infected by RKIP-overexpressing vectors in vitro.
Cell invasion and wound closure assays revealed that RKIP not only
inhibits RBE cell invasion, but that it also suppresses cell
migration. By contrast, the RKIP-targeting siRNA vector promoted
RBE cell invasion and cell migration. A lack of RKIP expression can
make the cholangiocarcinoma cells susceptible to invasion and
migration.
Several prognostic factors are involved in the
evaluation of cholangiocarcinoma. Among these, lymph node and
distant metastasis is one of the most important prognostic factors
(25–27). Metastasis is a multi-step process
that involves the spread of cancer cells from the primary site to a
secondary location. During this process, the cancer cells must
invade the surrounding tissue, penetrate the blood or lymphatic
vessels and form a novel tumor mass at a distant site. During
invasion, the cancer cells produce MMPs and then degrade the
extracellular matrix (ECM) and basement membrane to generate space
for the cells to migrate out of the original site. The TIMPs play
an important role in maintaining the balance between ECM synthesis
and the degradation caused by MMPs. TIMPs are usually downregulated
in cancer cells. In addition, it has been determined that MMPs-2,
-7 and -9 and TIMPs-1, -2 and -3 are candidate cancer-promoting or
suppressor genes in the progression of cholangiocarcinoma (28–32).
RKIP has been revealed to prevent the invasion of cancer cells by
controlling the gene expression of MMPs, particularly MMP-1 and
MMP-2 (12). RKIP also inhibits
esophageal cancer cell invasion via the downregulation of MMP-14
expression (13). The present study
demonstrated that RKIP overexpression significantly inhibits MMP-9
expression, indicating that RKIP reduces the invasiveness of
cholangiocarcinoma RBE cells by downregulating MMP-9 expression. In
addition, RKIP significantly enhanced TIMP-4 gene expression,
playing a complementary role in suppressing cholangiocarcinoma cell
invasion.
In conclusion, the present results suggested that
reduced RKIP expression is associated with cholangiocarcinoma
metastasis. Positive RKIP expression in cholangiocarcinoma cells
may be be predictive of a better prognosis. RKIP inhibits
cholangiocarcinoma cell invasion and migration via downregulating
MMP-9 expression and upregulating TIMP-4 expression.
Acknowledgements
This study was supported in part by grants from the
Hebei Major Medical Scientific Research Project, the National
Natural Science Foundation of China (grant number, 81200311), the
Project of Scientific Technology Research and Development Program
in Hebei Province (grant no. 14277757D) and the Hebei Medical
Research Key Project (grant number, 20110353).
References
|
1
|
Francis H, Alpini G and DeMorrow S: Recent
advances in the regulation of cholangiocarcinoma growth. Am J
Physiol Gastrointest Liver Physiol. 299:G1–G9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Marsh RW, Alonzo M, Bajaj S, et al:
Comprehensive review of the diagnosis and treatment of biliary
tract cancer 2012. Part I: diagnosis-clinical staging and
pathology. J Surg Oncol. 106:332–338. 2012. View Article : Google Scholar
|
|
3
|
von Hahn T, Ciesek S, Wegener G, et al:
Epidemiological trends in incidence and mortality of hepatobiliary
cancers in Germany. Scand J Gastroenterol. 46:1092–1098. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marzioni M, Saccomanno S, Candelaresi C,
et al: Clinical implications of novel aspects of biliary
pathophysiology. Dig Liver Dis. 42:238–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciombor KK and Goff LW: Current therapy
and future directions in biliary tract malignancies. Curr Treat
Options Oncol. 14:337–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeung K, Seitz T, Li S, et al: Suppression
of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature.
401:173–177. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeung KC, Rose DW, Dhillon AS, et al: Raf
kinase inhibitor protein interacts with NF-kappaB-inducing kinase
and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol.
21:7207–7217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deiss K, Kisker C, Lohse MJ and Lorenz K:
Raf kinase inhibitor protein (RKIP) dimer formation controls its
target switch from Raf1 to G protein-coupled receptor kinase (GRK)
2. J Biol Chem. 287:23407–23417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chatterjee D, Bai Y, Wang Z, et al: RKIP
sensitizes prostate and breast cancer cells to drug-induced
apoptosis. J Biol Chem. 279:17515–17523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon A, Park JY, Sung JY, Park YK and Kim
YW: Reduced expression of Raf-1 kinase inhibitory protein in renal
cell carcinoma: a significant prognostic marker. Pathology.
44:534–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yun J, Frankenberger CA, Kuo WL, et al:
Signalling pathway for RKIP and Let-7 regulates and predicts
metastatic breast cancer. EMBO J. 30:4500–4514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beshir AB, Ren G, Magpusao AN, et al: Raf
kinase inhibitor protein suppresses nuclear factor-κB-dependent
cancer cell invasion through negative regulation of matrix
metalloproteinase expression. Cancer Lett. 299:137–149. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Li F, Liu L, et al: Raf kinase
inhibitor protein inhibits cell proliferation but promotes cell
migration in rat hepatic stellate cells. Liver Int. 29:567–574.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao D, Ma J, Shi J, et al: Raf kinase
inhibitor protein inhibits esophageal cancer cell invasion through
downregulation of matrix metalloproteinase expression. Oncol Rep.
30:304–312. 2013.PubMed/NCBI
|
|
15
|
Birner P, Jesch B, Schultheis A and
Schoppmann SF: RAF-kinase inhibitor protein (RKIP) downregulation
in esophageal cancer and its metastases. Clin Exp Metastasis.
29:551–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braconi C and Patel T: Cholangiocarcinoma:
new insights into disease pathogenesis and biology. Infect Dis Clin
North Am. 24:871–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nathan H, Aloia TA, Vauthey JN, et al: A
proposed staging system for intrahepatic cholangiocarcinoma. Ann
Surg Oncol. 16:14–22. 2009. View Article : Google Scholar
|
|
18
|
Ribero D, Nuzzo G, Amisano M, et al:
Comparison of the prognostic accuracy of the sixth and seventh
editions of the TNM classification for intrahepatic
cholangiocarcinoma. HPB (Oxford). 13:198–205. 2011.
|
|
19
|
Lorenz K, Lohse MJ and Quitterer U:
Protein kinase C switches the Raf kinase inhibitor from Raf-1 to
GRK-2. Nature. 426:574–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deiss K, Kisker C, Lohse MJ and Lorenz K:
Raf kinase inhibitor protein (RKIP) dimer formation controls its
target switch from Raf1 to G protein-coupled receptor kinase (GRK)
2. J Biol Chem. 287:23407–23417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Escara-Wilke J, Yeung K and Keller ET: Raf
kinase inhibitor protein (RKIP) in cancer. Cancer Metastasis Rev.
31:615–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maeda T, Kajiyama K, Adachi E, Takenaka K,
Sugimachi K and Tsuneyoshi M: The expression of cytokeratins 7, 19,
and 20 in primary and metastatic carcinomas of the liver. Mod
Pathol. 9:901–909. 1996.PubMed/NCBI
|
|
23
|
Kim HS, Lee SH, Won KY, et al: Expression
of Raf-1 kinase inhibitory protein in carcinoma of the ampulla of
Vater. Virchows Arch. 460:61–68. 2012. View Article : Google Scholar
|
|
24
|
Al-Mulla F, Bitar MS, Taqi Z and Yeung KC:
RKIP: much more than Raf kinase inhibitory protein. J Cell Physiol.
228:1688–1702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lódi C, Szabó E, Holczbauer A, et al:
Claudin-4 differentiates biliary tract cancers from hepatocellular
carcinomas. Mod Pathol. 19:460–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thummarati P, Wijitburaphat S, Prasopthum
A, et al: High level of urokinase plasminogen activator contributes
to cholangiocarcinoma invasion and metastasis. World J
Gastroenterol. 18:244–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SY, Kim JH, Won HJ, Shin YM and Kim
PN: Radiofrequency ablation of hepatic metastases after curative
resection of extrahepatic cholangiocarcinoma. AJR Am J Roentgenol.
197:W1129–W1134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Tang H, Yin S and Dong C:
Downregulation of microRNA-138 enhances the proliferation,
migration and invasion of cholangiocarcinoma cells through the
upregulation of RhoC/p-ERK/MMP-2/MMP-9. Oncol Rep. 29:2046–2052.
2013.PubMed/NCBI
|
|
29
|
Leelawat K, Sakchinabut S, Narong S and
Wannaprasert J: Detection of serum MMP-7 and MMP-9 in
cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC
Gastroenterol. 9:302009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
French JJ, Midwinter MJ, Bennett MK, Manas
DM and Charnley RM: A matrix metalloproteinase inhibitor to treat
unresectable cholangiocarcinoma. HPB (Oxford). 7:289–291. 2005.
View Article : Google Scholar
|
|
31
|
Jo Chae K, Rha SY, Oh BK, et al:
Expression of matrix metalloproteinase-2 and -9 and tissue
inhibitor of metalloproteinase-1 and -2 in intraductal and
nonintraductal growth type of cholangiocarcinoma. Am J
Gastroenterol. 99:68–75. 2004. View Article : Google Scholar
|
|
32
|
Selaru FM, Olaru AV, Kan T, et al:
MicroRNA-21 is overexpressed in human cholangiocarcinoma and
regulates programmed cell death 4 and tissue inhibitor of
metalloproteinase 3. Hepatology. 49:1595–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|