Introduction
Wingless-type MMTV integration site, member 1
(Wnt-1), the first identified member of the Int-1
family, was named by Nusse in 1991 (1). The first Int-1 was termed
Wnt-1, as Int-1 and the Wnt gene family were
homologous. The Wnt-1 gene consists of 805 adenines, 1,523
cytosines, 1,320 guanines and 874 thymines. Current studies have
proposed that the Wnt-1 protein controls cell growth,
proliferation, secretion of key signaling molecules, mediates
information flow between cells and stem cells, and is important in
neural development. Wnt-1 gene expression has been found to
closely correlate with the development of numerous types of tumor
(1–7). You et al (8), as well as other studies, have also
indicated that Wnt-1-target genes may be potential cancer
therapy targets (9,10). Preliminary experiments in the
present study found that the Wnt-1 protein expression in human
glioma was significantly higher than in normal brain tissue, and is
closely associated with the degree of malignancy. Therefore, the
aim of the current study was to further investigate the role of
Wnt-1 in glioma by RNA interference (RNAi) targeting.
Materials and methods
Materials
U251 glioma cells were purchased from the Cell Bank
of the Shanghai Institute of Biochemistry and Cell Biology
(Shanghai, China). The vectors used were as follows: pGPU6/green
fluorescent protein (GFP)/Neo-short hairpin RNA (shRNA)-GAPDH
served as a positive control and pGPU6/GFP/Neo-shNC served as a
negative control. The pGPU6/GFP/Neo expression plasmid was
purchased from Wuhan Genesil Biotechnology Co., Ltd (Wuhan, China).
Transfections were performed using liposome
LipofectamineTM 2000 reagent and TRIzol that were
purchased from Life Technologies (Foster City, CA, USA). The
Wnt-1 primary antibody was obtained from Neomarkers Inc.
(Fremont, CA, USA), while the horseradish peroxidase-conjugated
monoclonal goat anti-rabbit secondary antibody was purchased from
ZhongShan Bio-Tech Co., Ltd. (Guangzhou, China). High glucose
Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Life
Technologies and fetal calf serum was purchased from Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou,
China). The restriction enzymes (BamHI, PstI and
BbsI), DNA markers, agarose gel DNA purification kit version
2.0, T4 DNA ligase Taq DNA polymerase and polymerase chain reaction
(PCR) primers were purchased from Takara Bio, Inc. (Shiga, Japan).
Little mention plasmid kit, and propidium iodide (PI) and MTT
reagents were purchased from Beyotime Institute of Biotechnology
(Nantong, China). M-MLV reverse transcriptase was purchased from
Promega Corporation (Madison, WI, USA).
Small interfering RNA (siRNA) target
sequence design
The full-length Wnt-1 gene sequence was
retrieved from GenBank (NM_005430) and the siRNA design principles
were obtained from Takara Biotechnology special siRNA design
software [Takara Biotechnology (Dalian) Co., Ltd., Dalian, China)
to select a 21-nt siRNA target template. The shRNA selected
TTCAAGAGA loop structure was maintained to avoid formation of a
termination signal and the shRNA transcription termination sequence
was composed of a T6 structure. CACC was added to the sense strand
of the template to form a BbsI complementary sticky end and
antisense strand template. GATC was added to the 5′ end and was
digested with BamHI to generate complementary sticky ends.
When the first siRNA base was not a G, then an additional CACC was
added after the G. The following primers were used: Sense,
5′-ACGGCGTTTATC TTCGCTATC-3′ and antisense, 3′-TGCCGCAAATAGAAG
CGATAG-5′ for Wnt-1. The following hairpin single-stranded
oligonucleotide sequence was then designed and synthesized: Sense,
5′-CACCGACGG CGTTTATCTTCGCTATCTTCAAGAGAGATAGCGAAG
ATAAACGCCGTTTTTTTG-3′ and antisense, 5′-GATCCA
AAAAAACGGCGTTTATCTTCGCTATCTCTCTTGAAG ATAGCGAAGATAAACGCCGTC-3′ for
Wnt-1. The oligonucleotides were synthesized by the Shanghai
Jima Company (Shanghai, China).
Construction of Wnt-1 targeting
pGPU6/GFP/Neo-Wnt-1 vector for RNAi
Synthetic single-stranded oligonucleotides were
dissolved in Tris-EDTA buffer (pH 8.0; 10mM Tris-HCl, 1 mM EDTA),
diluted to 100 μmol/l and annealed to form an shRNA template (5 μl
justice chain, 5 μl antisense strand, 5 μl annealing buffer and 35
μl double distilled (dd) H2O to a 50-μl total volume).
The shRNA template was annealed on a PCR instrument (ABI7900HT,
Invitrogen Applied Biosystems, Foster City, CA, USA) according to
the following procedure: 95°C for 5 min, 85°C for 5 min, 75°C for 5
min and 70°C for 5 min. The template was stored at 4°C to generate
a 10-μM concentration of shRNA. The template solution was diluted
500-fold to a final concentration of 20 nM for ligation.
Restriction digestions were performed with 2μg pGPU6/GFP/Neo,
according to the following conditions: 5 μl 10X buffer G (Shanghai
PureOne Biotechnology Co., Shanghai, China), 2 μl BbsI
enzyme, 2 μl BamHI enzyme, 2 μg pGPU6/GFP/Neo and
ddH2O to a total volume of 50 μl. Following digestion at
37°C for 1 h, the product concentrations were examined by agarose
gel electrophoresis, using the agarose gel DNA purification kit
version 2.0 and diluted to 50 ng/μl. Ligations were performed in 2
μl 10X T4 ligation buffer, 1 μl pGPU6/GFP/Neo (BbsI +
BamHI), 1 μl shRNA template, 0.5 μl T4 DNA ligase (5 Weiss
U/μl) and 30 μl ddH2O to a total volume of 20 μl at room
temperature for 2 h.
Plasmid identification
Competent Escherichia coli DH5α cells were
grown on lysis buffer medium plates containing kanamycin (Beyotime
Institute of Biotechnology) at 37°C for 18 h. Six colonies were
selected and used to inoculate the kanamycin-containing LB liquid
medium (50 μg/ml), which was agitated using a blender (Beijing
Tianshi Tianli Medical Device Technology Development Center,
Beijing, China) overnight. The plasmids were extracted by alkaline
lysis and digested sequentially with BamHI and PstI.
The positive recombinant vectors were digested with BamHI,
but not PstI. Two clones were selected for each vector and
sequenced by Invitrogen Applied Biosystems (Shanghai, China).
Cell culture
The U251 cell lines were grown at 37°C in a 5%
CO2 humidified atmosphere with high glucose DMEM medium
(containing 10% fetal bovine serum (FBS), 100 U/l penicillin, 100
mg/l streptomycin and 10% fetal calf serum).
Plasmid transfection
Transfected cells (3×105) were grown to
70–80% confluence for two days, and subsequently inoculated in
six-well plates (35 mm), with 2 ml 10% FBS (Chongqing Manuik
Technology Co., Ltd., Chongqing, China) per well. Prior to
transfection, the liposome/DNA was incubated for 10 min, and
replaced with 1 ml fresh serum-free 10% FBS (Chongqing Manuik
Technology Co., Ltd.). Based on the pretest results, the cells were
cultured with LipofectamineTM 2000 reagent (Life
Technologies) and plasmid at a ratio of 6 μl:2 μg, respectively,
according to the manufacturer’s instructions, and incubated at 37°C
in a 5% CO2 humidified atmosphere. After 5 h, 2–3 ml
fresh growth medium was added. The cells were collected the next
day and fresh 10% fetal calf serum was added. The cells were then
cultured for 48 h to observe any GFP expression. The cells were
divided into the following four transfection groups:
pGPU6/GFP/Neo-Wnt-1, pGPU6/GFP/Neo-shRNA-GAPDH (positive
control), pGPU6/GFP/Neo-shNC (negative control) and a
phosphate-buffered saline (PBS) control. GFP expression was used to
evaluate the transfection efficiency 48 h following transfection
and was measured by 488-nm excitation via fluorescence microscopy
(Olympus BX51; Olympus America Inc., Center Valley, PA, USA).
Reverse transcription (RT)-PCR detection
of Wnt-1 mRNA expression level
The primer design software Premier 5.0 (Premier
Biosoft, Palo Alto, CA, USA) was used to design the following
primers: Sense, 5′-CTGCCTCTCTTCTTCCCCTT-3′ and antisense,
5′-TCACAGCTGTTCAATGGCTC-3′ for Wnt-1 (251-bp product); and
sense, 5′-CCATGT TCGTCATGGGTGTGAACCA-3′ and antisense, 5′-GCCAGT
AGAGGCAGGGATGATGTTC-3′ for the internal reference, GAPDH (246-bp
product). Preamplification of GAPDH was used to assess the efficacy
of RT, with a semi-quantitative PCR reaction run in parallel, which
served as an internal reference. TRIzol was used to extract the
total cellular RNA and first-strand cDNA synthesis was performed
according to the manufacturer’s instructions. The total RNA (2 μg)
was used for RT, in addition, 0.1 μg oligo-dt, denatured at 65°C
for 10 min and placed immediately in an ice bath for 5 min, was
added to 5 μl 5X PCR buffer [Takara Biotechnology (Dalian) Co.,
Ltd.], 1 μl dNTP (10 mM each), 100 units RNAsin, 10 units M-MLV
reverse transcriptase and water, which was added to produce a total
volume of 25 μl. The samples were incubated at 42°C for 60 min
following a 5-min 95°C denaturalization step, subsequently placed
in an ice bath for 3 min and stored at −20°C to conserve the RNA
template for the PCR. The RNA template was then converted into cDNA
for use in the RT-PCR, which was performed in a total reaction
volume of 50 μl, containing 5 μl 10X buffer, 4 μl MgCl2
(25 mM), 1 μl dNTP (10 mM each), 0.5 μl primer (50 pmol/μl), 0.5 μl
downstream primer (50 pmol/μl), 0.3 μl Taq enzyme (5 U/μl), 2 μl
cDNA template and 30–50 μl paraffin oil. PCR reactions were
performed as follows: 95°C denaturation for 5 min; 30 cycles of
94°C for 30 sec, 50°C annealing for 30 sec and 72°C for 1 min; with
a final step at 72°C for 5 min. The products were subjected to 2%
agarose electrophoresis and ethidium bromide staining. Images were
captured using the IBM 586 computer-controlled Gel Doc 1000 imaging
system (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Western blot analysis was used to detect Wnt-1
protein expression prior to and following U251 transfection. Total
cellular protein was determined by SDS-PAGE gel electrophoresis.
The proteins were transferred to nitrocellulose membranes (Whatman
Inc., London, UK) for protein immunoassays and the primary
monoclonal mouse anti-human proto-onocogene Wnt-1 antibody (Boster
Biotechnology Co., Ltd., Wuhan, China) was used at a dilution of
1:100, with the secondary monoclonal rabbit anti-mouse antibody
(Boster Biotechnology Co., Ltd.) at 1:500.
MTT cell proliferation assay
U251-transfected cells were subjected to trypsin
digestion following 24 h. Single cell suspensions were prepared in
culture medium containing 10% fetal calf serum, transferred into
96-well plates at a final volume of 200 μl and grown at 37°C in a
5% CO2 humidified atmosphere for 48 h. Each MTT well
contained a 20-μl MTT solution (5 mg/ml), which was incubated for 4
h. At culture termination, the medium was carefully aspirated and
the supernatant discarded. Each well contained 150 μl dimethyl
sulfoxide and was agitated for 10 min to fully dissolve the
crystals. ELISA was used to record the absorbance at 490 nm and the
cellular proliferation rate was calculated as follows: Cellular
proliferation rate (%) = (experimental group/absorbance value of
blank control group) × 100.
Flow cytometry (FCM) detection of cell
cycle
Trypsinized cells were transfected with plasmids 48
h following digestion. The cell suspension was then centrifuged in
a clean centrifuge tube using a high speed centrifuge (Beijing Era
Beili Centrifuge Co., Ltd., Beijing, China) at 507 xg for 5 min.
The culture medium was discarded and washed three times with cold
PBS. The cells were resuspended by adding 75% alcohol, fixed for 30
min in ethanol and centrifuged at 1,917 xg for 5 min, followed by
washing three times with PBS. The cells were then resuspended in
100 μl PBS and 2.5 μl RNase (10 mg/ml), followed by 25 μl PI
pyridine (10 mg/ml) and staining for 30 min. The cells were
examined using a FACSAria flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) to determine the G1/G0,
G2/M and S phase fractions.
Statistical analysis
SPSS 10.0 (SPSS Inc., Chicago, IL, USA) was used for
analysis of variance. Data are presented as the mean ± standard
deviation. The means between two groups were compared using
Student’s t-test, while multiple comparisons were analyzed using
the F-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
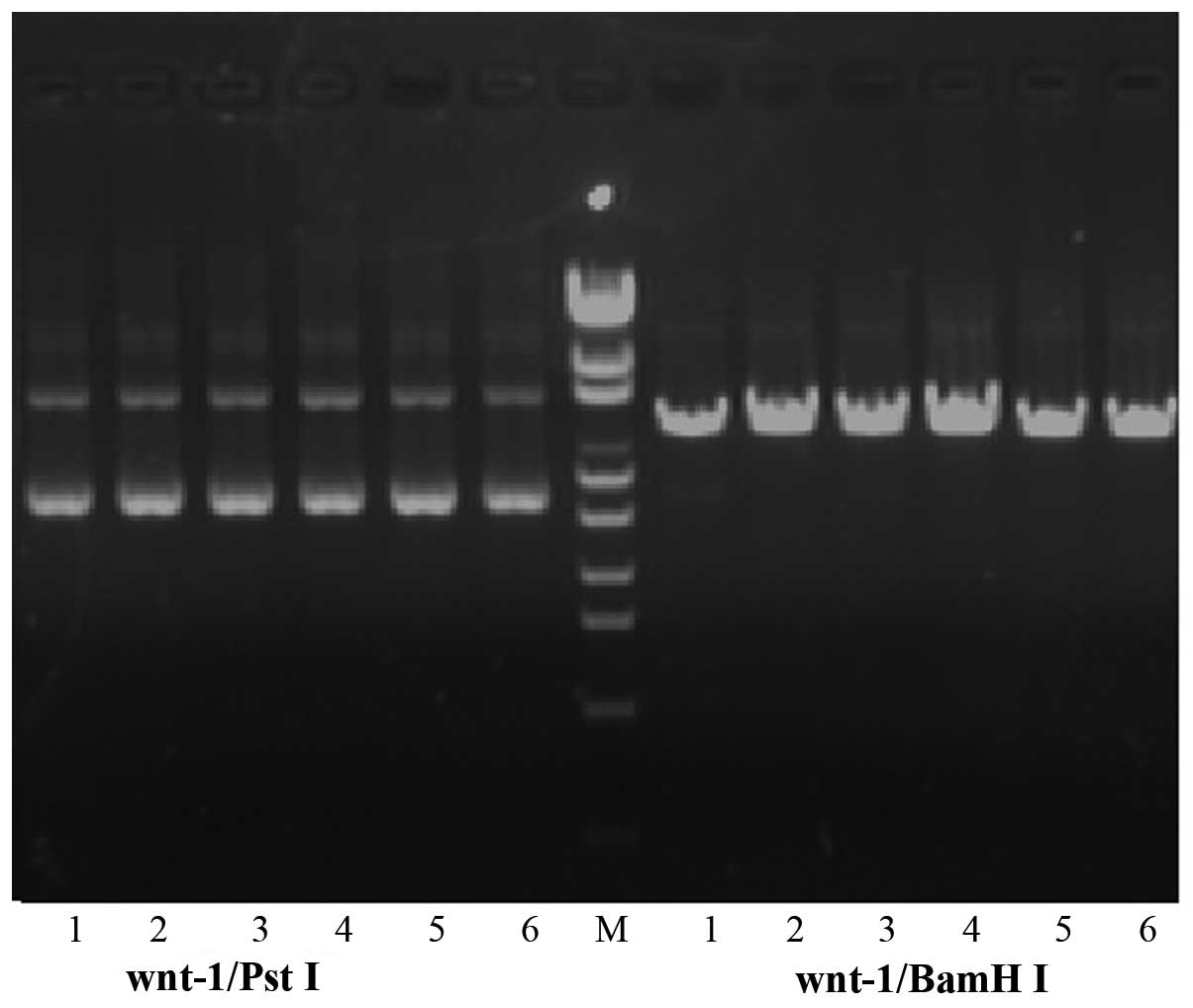
Plasmid verification
Successful digestion by BamHI, but not
PstI confirmed construction of the recombinant plasmid
vector (Fig. 1). Furthermore,
sequencing verified that the targeted Wnt-1 gene had been
inserted into the pGPU6/GFP/Neo complete shRNA vector.
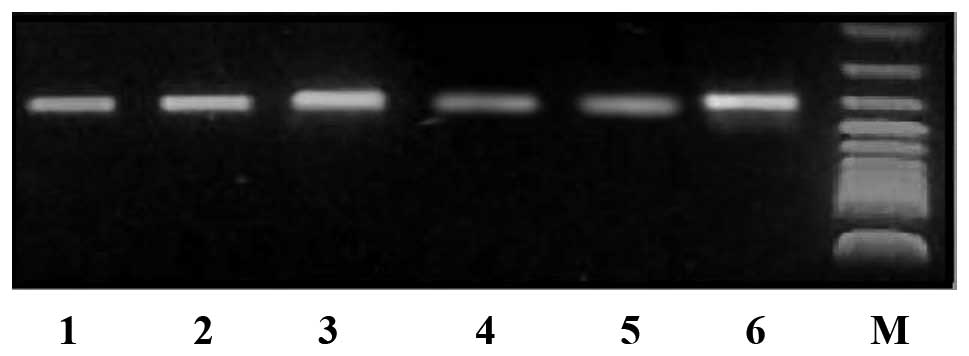
RT-PCR detection of Wnt-1 gene mRNA
expression level changes following RNAi
Transfected cells were characterized by
semi-quantitative RT-PCR 48 h following transfection to detect the
pGPU6/GFP/Neo-shRNA-Wnt-1-positive cells. The Wnt-1
mRNA content had decreased by 63% compared with the controls, while
the negative and positive control groups did not exhibit any
significant differences (Fig. 2).
Compared with the controls, no significant differences in Wnt-1
gene mRNA expression levels were observed between the negative and
positive control group (99 and 100%, respectively).
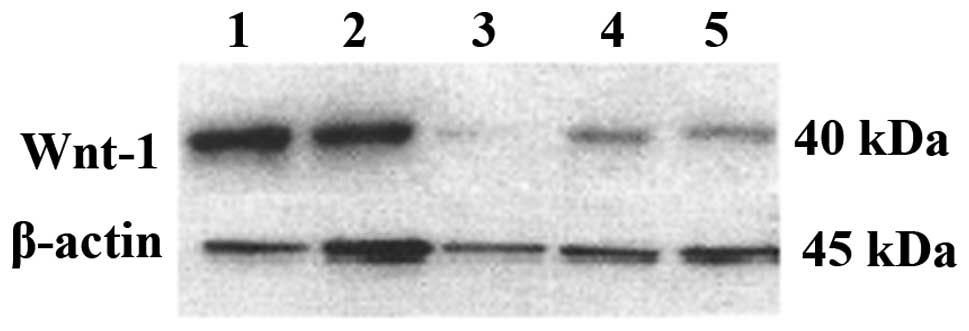
Knockdown of Wnt-1 protein expression by
plasmid transfection
Western blot analysis showed that the Wnt-1 protein
expression was significantly reduced following transfection.
Although the negative control did not change significantly, the
expression of the positive control decreased. This may be the
result of excessive cell death, which reduced the amount of
extracted protein (Fig. 3).
MTT cell proliferation assay
The results of the MTT assay indicated that the
positive and negative controls exhibited decreased cell growth
following pGPU6/GFP/Neo-shRNA-Wnt-1 transfection. The
positive controls that were transfected with
pGPU6/GFP/Neo-shRNA-GAPDH showed decreased cell survival compared
with the pGPU6/GFP/Neo-shRNA-Wnt-1 transfected samples,
however, survival at 48 h was not identified to be statistically
significant.
FCM test results
No statistically significant differences were
identified between the positive and negative control groups at 48 h
post-transfection (P>0.05), which indicated that the plasmid and
transfection reagents were not toxic. The positive controls
transfected with pGPU6/GFP/Neo-shRNA-Wnt-1 were
predominantly arrested at G0/G1 and the
number of cells in the S phase was decreased (P<0.05).
Furthermore, the number of replicating cells decreased, which
indicated that U251 proliferation was inhibited (Table I).
 | Table ICell cycle changes of each group
(mean ± standard deviation; n=4). |
Table I
Cell cycle changes of each group
(mean ± standard deviation; n=4).
| Group |
G0/G1 phase (%) | S phase (%) | G2/M
phase (%) |
|---|
| Transfection
pGPU6/GFP/Neo-shRNA-Wnt-1 | 79.5±0.6 | 16.8±1.0 | 3.7±0.5 |
| Positive
control | 78.5±0.4 | 11.6±0.6 | 10.0±0.6 |
| Negative
control | 67.2±1.6 | 26.5±0.6 | 5.6±1.1 |
| Blank control | 67.4±0.7 | 27.2±0.4 | 5.1±0.5 |
Discussion
The Wnt signaling pathways are important in
cellular differentiation and proliferation in a variety of species,
and are involved in the formation of certain types of tumor
(11–13). The Wnt/β-catenin signaling
pathway is considered to be a classical signaling pathway, which
has been investigated in-depth (14). Wnt signal transduction
mediates other processes, including Wnt/Ca2+
signaling, planar cell polarity, spindle orientation and asymmetric
cell growth (11,14). Wnt binding of the Frizzled
receptor inhibits the effect of glycogen synthase kinase 3-β, axin,
and adenomatous polyposis coli protein complexes that are involved
in β-catenin phosphorylation and degradation. Non-phosphorylated
β-catenin is not degraded, however, accumulates in the cytoplasm,
prior to translocation to the nucleus. β-catenin then binds to the
lymphocyte enhancer factor/T cell-specific transcription factor,
activating the target genes, c-myc, cyclin D and
MMP7, leading to cellular proliferation and tumor formation.
Therefore, certain studies have hypothesized that targeted therapy
of Wnt signaling may inhibit tumor formation and growth
(15).
Gliomas are the most common type of primary
intracranial tumor and more effective therapeutic strategies are
required to stop glioma progression. The Wnt/β-catenin
signaling cascade is an important signal transduction pathway in
human cancer (16). The human
Wnt family consists of 19 members that regulate cell
proliferation, differentiation, motility and fate during embryonic
development and tumorigenesis. Wnt members bind to the cell
surface receptors, Frizzled and low density lipoprotein
receptor-related protein, and transduce their signals through
β-catenin-dependent and -independent intracellular signaling
pathways (17). Wnt-1 was
one of the first signaling molecules to be identified and it is
considered to be the first step in the classical
Wnt/β-catenin signaling pathway (18,19).
Increasing evidence indicates that interplay between the
Wnt/β-catenin and PI3K/AKT signaling cascades is involved in
tumor development and progression. However, the mechanism of this
in glioma is not well understood (20). Although a number of studies have
identified a variety of tumors that exhibit abnormal Wnt-1
expression (1,10), abnormal expression is rare in
glioma. In our previous study, it was demonstrated that
Wnt-1 gene expression is increased in glioma, with little or
weak expression identified in normal brain tissue. Furthermore,
Wnt-1 expression showed a positive correlation with the
glioma grade (15). The present
study indicated that targeting Wnt-1 gene expression by RNAi
knockdown inhibits the growth of human glioma cells. This
demonstrates that glioma growth may be closely correlated with
Wnt-1 protein expression. Malignant glioma growth may involve
activation of the Wnt-1 signaling pathway (21), however, the specific underlying
mechanism requires further investigation (22).
Current RNAi methods are important for investigating
gene function (18,23,24)
and it is hypothesized that the Wnt signaling pathway may be
ideal for targeted drug development. However, few studies have used
Wnt-1 gene RNAi. Certain studies have used fragments of
chemically synthesized siRNA in transfected cells (25,26),
or plasmids lacking a reporter gene (27), which complicates the assessment of
transfection efficiency and the identification of stable cell
lines. A previous study described the transfection of siRNA
targeting Wnt-1 into SH-SY5Y neuroblastoma cells using
LipofectamineTM 2000 (28). Wnt-1 and β-catenin protein
expression decreased following transfection with siRNA, as did the
cell number, and was accompanied by abundant floating dead cells.
Additionally, the SH-SY5Y cells transfected with siRNA targeting
Wnt-1 showed less viability.
In conclusion, the current study used a
pGPU6/GFP/Neo carrier plasmid containing a GFP reporter, which
detects cellular expression efficiency and Kanamycin/G418
resistance, into stable cell lines. Therefore, siRNA targeting
Wnt-1 was used to investigate which region of the
Wnt-1 gene may be targeted as a novel hotspot (29). The results of the present study
revealed that miR-106a-5p is a tumor suppressor gene in
astrocytomas. The overexpression of miR-106a-5p inhibits
astrocytoma cell proliferation, migration, and invasion and
promotes apoptosis. In addition, FASTK was found to be a direct
target for miR-106a-5p. Although much remains to be elucidated in
terms of the role of miR-106a-5p in the pathogenesis of
astrocytomas, miR-106a-5p presents a novel potential therapeutic
target for the treatment of astrocytomas.
References
|
1
|
Benad P, Rauner M, Rachner TD and Hofbauer
LC: The anti-progestin RU-486 inhibits viability of MCF-7 breast
cancer cells by suppressing WNT1. Cancer Lett. 312:101–108. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benchabane H, Xin N, Tian A, et al:
Jerky/Earthbound facilitates cell-specific Wnt/Wingless signalling
by modulating β-catenin-TCF activity. EMBO J. 30:1444–1458. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu X, Sun PL, Li JZ, Jheon S, Lee CT and
Chung JH: Aberrant Wnt1/β-catenin expression is an independent poor
prognostic marker of non-small cell lung cancer after surgery. J
Thorac Oncol. 6:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakashima N, Huang CL, Liu D, Ueno M and
Yokomise H: Intratumoral Wnt1 expression affects survivin gene
expression in non-small cell lung cancer. Int J Oncol. 37:687–694.
2010.PubMed/NCBI
|
|
5
|
Oguma K, Oshima H and Oshima M:
Inflammation, tumor necrosis factor and Wnt promotion in gastric
cancer development. Future Oncol. 6:515–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fracalossi AC, de Silva MS, Oshima CT and
Ribeiro DA: Wnt/beta-catenin signalling pathway following rat
tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. Exp Mol
Pathol. 88:176–183. 2010. View Article : Google Scholar
|
|
7
|
Wang FL, Guo X, Yuan TZ, et al: Expression
and clinical significance of Wnt-1 and beta-catenin in
nasopharyngeal carcinoma. Ai Zheng. 28:72–75. 2009.PubMed/NCBI
|
|
8
|
You L, Kim J, He B, Xu Z, McCormick F and
Jablons DM: Wnt-1 signal as a potential cancer therapeutic target.
Drug News Perspect. 19:27–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Augustin I, Goidts V, Bongers A, et al:
The Wnt secretion protein Evi/Gpr177 promotes glioma
tumourigenesis. EMBO Mol Med. 4:38–51. 2012. View Article : Google Scholar :
|
|
10
|
Natsume A, Kinjo S, Yuki K, et al:
Glioma-initiating cells and molecular pathology: implications for
therapy. Brain Tumor Pathol. 28:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ille F and Sommer L: Wnt signaling:
multiple functions in neural development. Cell Mol Life Sci.
62:1100–1108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toledo EM, Colombres M and Inestrosa NC:
Wnt signaling in neuroprotection and stem cell differentiation.
Prog Neurobiol. 86:281–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han L, Yang Y, Yue X, et al: Inactivation
of PI3K/AKT signaling inhibits glioma cell growth through
modulation of β-catenin-mediated transcription. Brain Res.
1366:9–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Ferrari GV and Moon RT: The ups and
downs of Wnt signaling in prevalent neurological disorders.
Oncogene. 25:7545–7553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu C, Tu Y, Sun X, et al:
Wnt/beta-Catenin pathway in human glioma: expression pattern and
clinical/prognostic correlations. Clin Exp Med. 11:105–112. 2011.
View Article : Google Scholar
|
|
17
|
Kamino M, Kishida M, Kibe T, et al: Wnt-5a
signaling is correlated with infiltrative activity in human glioma
by inducing cellular migration and MMP-2. Cancer Sci. 102:540–548.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiele S, Rauner M, Goettsch C, et al:
Expression profile of WNT molecules in prostate cancer and its
regulation by aminobisphosphonates. J Cell Biochem. 112:1593–1600.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharma V, Dixit D, Koul N, Mehta VS and
Sen E: Ras regulates interleukin-1β-induced HIF-1α transcriptional
activity in glioblastoma. J Mol Med (Berl). 89:123–136. 2011.
View Article : Google Scholar
|
|
20
|
Chen L, Huang K, Han L, et al:
β-catenin/Tcf-4 complex transcriptionally regulates AKT1 in glioma.
Int J Oncol. 39:883–890. 2011.PubMed/NCBI
|
|
21
|
Wei W, Chua MS, Grepper S and So SK:
Blockade of Wnt-1 signaling leads to anti-tumor effects in
hepatocellular carcinoma cells. Mol Cancer. 24:762009. View Article : Google Scholar
|
|
22
|
Sareddy GR, Challa S, Panigrahi M and Babu
PP: Wnt/beta-catenin/Tcf signaling pathway activation in malignant
progression of rat gliomas induced by transplacental
N-ethyl-N-nitrosourea exposure. Neurochem Res. 34:1278–1288. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whangbo JS and Hunter CP: Environmental
RNA interference. Trends Genet. 24:297–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dillin A: The specifics of interfering RNA
specificity. Proc Nat Acd Sci USA. 100:6289–6291. 2003. View Article : Google Scholar
|
|
25
|
Mikami I, You L, He B, et al: Efficacy of
Wnt-1 monoclonal antibody in sarcoma cells. BMC Cancer. 5:532005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wieczorek M, Paczkowska A, Guzenda P,
Majorek M, Bednarek AK and Lamparska-Przybysz M: Silencing of Wnt-1
by siRNA induces apoptosis of MCF-7 human breast cancer cells.
Cancer Biol Ther. 7:268–274. 2008. View Article : Google Scholar
|
|
27
|
Polanec J, Pavelic ZP and Myers WL: Effect
of Wnt-1 antisense RNA on the outgrowth of a mammary adenocarcinoma
cell line expressing that oncogene. Clin Mol Pathol. 49:M166–M169.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Li K, Lv Z, Xiao X and Zheng J:
The effect on cell growth by Wnt1 RNAi in human neuroblastoma
SH-SY5Y cell line. Pediatr Surg Int. 25:1065–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi-Yanaga F and Sasaguri T: The
Wnt/beta-catenin signaling pathway as a target in drug discovery. J
Pharmacol Sci. 104:293–302. 2007. View Article : Google Scholar : PubMed/NCBI
|