Introduction
Astrocytomas, which are the most common primary
tumors of the central nervous system, account for ~one-third of the
intrinsic neoplasms of the central nervous system (CNS) (1). Despite recent advances in diagnosis
and multimodal therapies, including surgery, radiation,
chemotherapy and immunotherapy, the treatment of astrocytomas
remains a considerable challenge for neurosurgeons as recurrence is
common, however, the underlying mechanism of such remains unclear,
and a significant improvement in the survival of patients with
high-grade astrocytomas has not yet been achieved (1–5). The
recurrence and progression of astrocytomas and the subsequent
increase in malignancy accounts for the poor prognosis in patients.
These processes are mediated by numerous complex pathways and
various regulatory molecules, as well as the tumor microenvironment
(6–11). Thus, the molecular mechanisms of the
progression of astrocytomas and treatment strategies targeting
critical components of astrocytomas have stimulated wide
interest.
Wnt-1 inducible signaling pathway protein-2
(WISP-2), also known as CCN5, is a 29-kDa secreted protein that is
a member of the connective tissue growth factor (CTGF) and
nephroblastoma over-expressed gene family of matricellular
proteins, and is critical in growth factor mediated cell
proliferation (12–14). This family exhibits conserved
multi-modular domains with diverse biological functions, including
angiogenesis, stem cell differentiation and carcinogenesis
(15). WISP-2 has functions towards
the promotion and arrest of cell growth, depending on the cell type
and the surrounding microenvironment (16,17).
For example, the mitogenic action of estrogen-receptor-positive,
non-invasive breast tumor cells relies on the overproduction of
WISP-2 by epidermal growth factor or insulin-like growth factor
(IGF-1) (18,19). Furthermore, WISP-2 also acts as a
growth arrest-specific gene in vascular smooth muscle and prostate
cancer cells (20). In addition, it
is hypothesized that WISP-2 is significant in preventing the
progression of pancreatic cancer, as it is involved in
morphological alterations during the mesenchymal to epithelial
transition of pancreatic adenocarcinoma and breast cancer cells
(21,22). However, the clinical significance of
the expression of WISP-2 in astrocytomas of different grades has
not been previously investigated. Such studies may aid with the
identification of novel targets that enable the development of
effective prognostic and therapeutic strategies for the treatment
of astrocytoma.
The current study determined the levels of WISP-2
expression in astrocytomas and normal brain tissues using reverse
transcription (RT)-polymerase chain reaction (PCR) and
immunohistochemistry. In addition, the correlation between WISP-2
expression and progression-free survival (PFS), as well as overall
survival (OS) was investigated to obtain a greater insight into the
importance of WISP-2 in tumor invasion and recurrence.
Materials and methods
Tissue samples
A total of 47 fresh tumor samples were surgically
resected from treatment-naive astrocytoma patients (25 males and 22
females, mean age, 38.4 years; range, 2–71 years) with consent
obtained prior to treatment at the Department of Neurosurgery,
Xiangya Hospital (Changsha, China). For comparison, 10 normal brain
tissue samples were isolated from patients undergoing internal
decompression surgery following brain trauma. None of the 10
patients exhibited any evidence of cancer or mydriasis.
Tissue slides
A total of 154 astrocytoma and 14 normal brain
paraffin-embedded samples were obtained from the Human Brain Glioma
Bank and Brain Tissues Bank, Department of Pathology, Xiangya
Hospital (Changsha, China). All patients were admitted to Xiangya
Hospital and underwent microsurgical treatment between 2002 and
2008. All of the patients’ follow-up data were available and
medical records were reviewed to obtain the following data for each
patient: Age, gender, tumor size, extent of tumor removal,
pathological diagnosis, PFS and OS. The patient characteristics are
shown in Table I.
 | Table IThe patient characteristics and the
expression of WISP-2 protein in 154 astrocytoma and 15 normal brain
tissues. |
Table I
The patient characteristics and the
expression of WISP-2 protein in 154 astrocytoma and 15 normal brain
tissues.
| | WISP-2 (iOD) |
|---|
| |
|
|---|
| Clinical
parameter | Patients, n | Mean ± SD | P-value |
|---|
| Tissue type | | | 0.000 |
| Astrocytomas | 154 | 0.986±0.138 | |
| NBT | 15 | 0.157±0.059 | |
| Gender | | | 0.874 |
| Male | 80 | 0.687±0.434 | |
| Female | 74 | 0.666±0.467 | |
| Age (years) | | | 0.618 |
| <40 | 50 | 0.642±0.452 | |
| ≥40 | 104 | 0.708±0.445 | |
| Tumor size
(cm) | | | 0.107 |
| <4 | 89 | 0.654±0.408 | |
| ≥4 | 65 | 0.766±0.417 | |
| Pathological
grade | | | 0.000 |
| WHO I | 23 | 0.362±0.212 | |
| WHO II | 49 | 0.513±0.347 | |
| WHO III | 57 | 0.769±0.414 | |
| WHO IV | 26 | 0.893±0.473 | |
All specimens were collected and handled according
to the protocols approved by the Ethics Committee of Xiangya
Hospital. In this study, written informed consent signed either by
the patients themselves or their guardians was obtained for all
patients.
RT-PCR
Total RNA was isolated from human brain tumors and
normal tissue using Trizol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). The first strand of cDNA was synthesized from 1
μg of total RNA by using an Oligo (dT) 18 primer and M-MuLV RT
(Thermo Fisher Scientific, Waltham, MA, USA). The PCR was performed
under the following conditions: 95°C for 5 min, 30 cycles of 95°C
for 30 sec, 56°C for 30 sec, 72°C for 30 sec and a final extension
step at 72°C for 10 min, using the Taq DNA polymerase kit (Takara
Biotechnology Co., Ltd., Dalian, China). The primer sequences used
were as follows: Forward, 5′-TTTCTGGCCTTGTCTCTTCC-3′ and reverse,
5′-GTGTGTGTAGGCAGGGAGTG-3′, for human WISP-2 cDNA; forward,
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse, 5′-AGGGGAGCTTCAGTGTGGTG-3′
for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). GAPDH was
used as an internal control. The lengths of the WISP-2 and GAPDH
amplicons were 155 and 400 bp, respectively. Following PCR, the PCR
products were electrophoresed in 1.5% agarose gels. Densitometric
analysis was carried out using the Gelpro4.0 image analysis
software (Media Cybernetics, Inc., Rockville, MD, USA). The
expression of WISP-2 was calculated relative to that of GAPDH in
the same sample.
Immunohistochemistry
Sections of 5-μm were cut from formalin-fixed
tissues embedded in paraffin blocks and mounted onto polylysine
coated slides. The sections were dewaxed in xylene (Sigma-Aldrich,
St. Louis, MO, USA), rehydrated in solutions of descending alcohol
concentrations and blocked with endogenous peroxidase (3%
H2O2). The antigen retrieval was conducted by
microwave heating in 1 mM ethylene diaminetetraacetic acid for 10
min. The sections were incubated overnight at 4°C with the primary
rabbit anti-human WISP2 polyclonal antibody (1:100; ab38317; Abcam,
Cambridge, UK). Immunoreactive complexes were detected using the
streptavidin peroxidase system (Thermo Fisher Scientific) and
visualized with 3,3′-diaminobenzidine. All tissues were stained
with hematoxylin and eosin to confirm the diagnosis histologically.
The sections that were not probed with the primary antibody were
considered as negative controls. Staining data were obtained for at
least two sections per tissue and reviewed by two independent
investigators, blinded to all clinical data.
A semi-quantitative approach was based on the
intensity of staining, (0, negative; 1+, weak; and 2+, strong) and
the percentage of positively stained malignant cells, (0, 0–4%; 1,
5–24%; 2, 25–49%; 3,50–74%; and 4, 75–100%). The final
immunohistochemistry scores were obtained using the following
formula: Final immunohistochemistry score = values of intensity ×
percentage counts.
Long term patient follow up
The detailed case history and intra-operative
observations of all the patients were recorded. Long-term
follow-up, with regard to long-term survival and tumor recurrence
was conducted from 2002 until 2010. The mean duration of follow up
was 31.3±17.4 months, range, 3–60 months.
Statistical analysis
Statistical analyses were performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. The differences in the
variables between the groups were tested using one-way analysis of
variance or Student’s t-test when the data were normally
distributed. The correlation analysis between the WISP-2
immunohistochemical score and the clinical variables (pathological
grade, age and gender) was performed using the Kruskal-Wallis H
test. The association between PFS or OS and WISP-2 expression was
analyzed using log-rank tests and presented as Kaplan-Meier plots.
Furthermore, a multivariate analysis was performed using the Cox
proportional hazards regression to determine the prognostic effect
of WISP-2 expression and potential clinical variables [age, gender,
tumor size, extent of resection and World Health Organization (WHO)
grade (23)] on PFS and OS.
P<0.05 was considered to indicate a statistically significant
difference.
Results
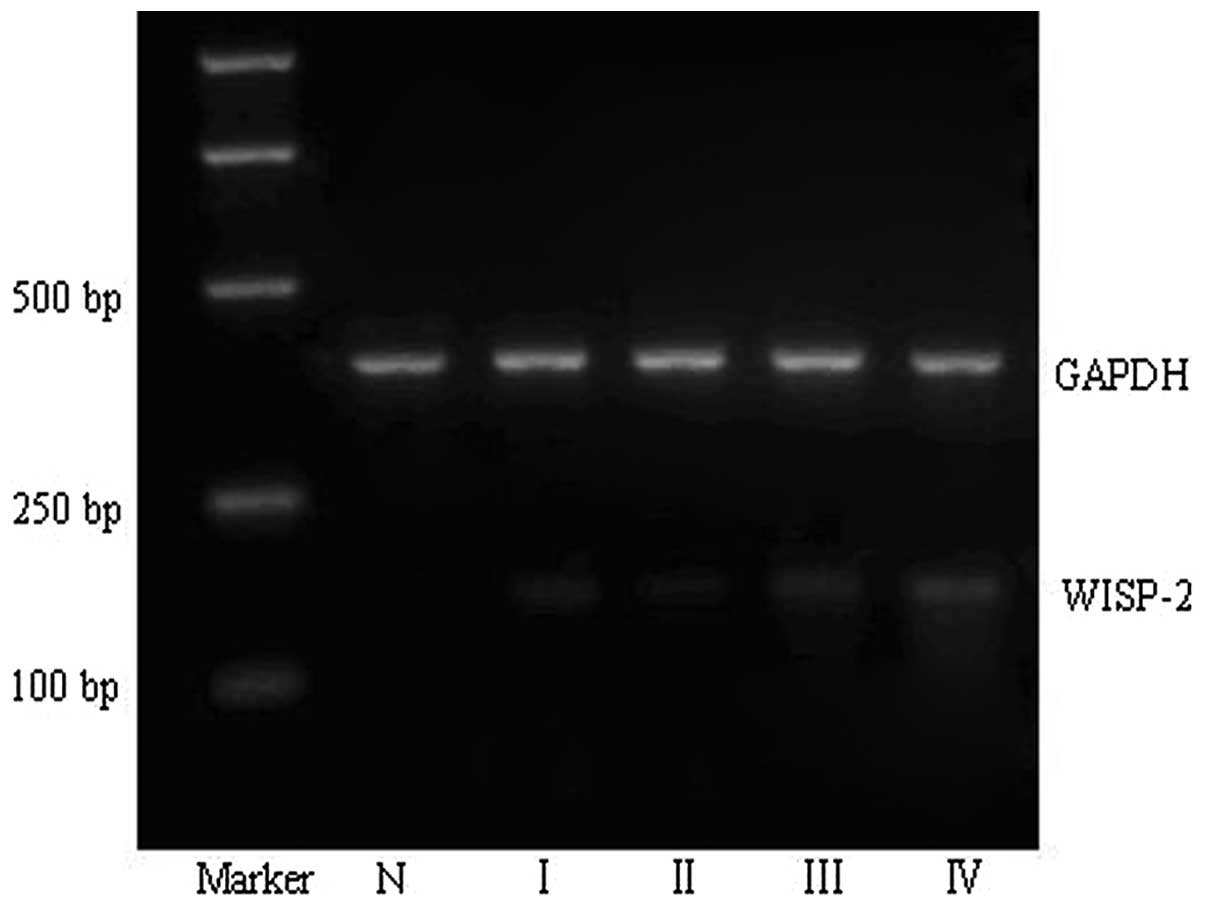
Expression of WISP-2 mRNA was found to
correlate with astrocytoma grade
The semi-quantitative RT-PCR assay demonstrated that
the mRNA levels of WISP-2 in glioma tissues (0.677±0.445) were
significantly higher than that in the normal brain tissues (0.172 ±
0.059; P<0.05). Additionally, increased mRNA expression of
WISP-2 was found to positively correlate with a higher pathological
grade of astrocytoma (P<0.05) (Fig.
1 and Table II).
 | Table IIWISP-2 mRNA expression levels in
normal brain and astrocytomas tissues. |
Table II
WISP-2 mRNA expression levels in
normal brain and astrocytomas tissues.
| | WISP-2 mRNA
(iOD) |
|---|
| |
|
|---|
| Clinical
parameter | Patients, n | Mean ± SD | P-value |
|---|
| Tissue type | | | 0.000 |
| Astrocytomas | 47 | 0.677±0.445 | |
| NBT | 10 | 0.172±0.059 | |
| Pathological
grade | | | 0.000 |
| WHO I | 9 | 0.417±0.276 | |
| WHO II | 17 | 0.634±0.334 | |
| WHO III | 15 | 0.829±0.441 | |
| WHO IV | 6 | 0.941±0.513 | |
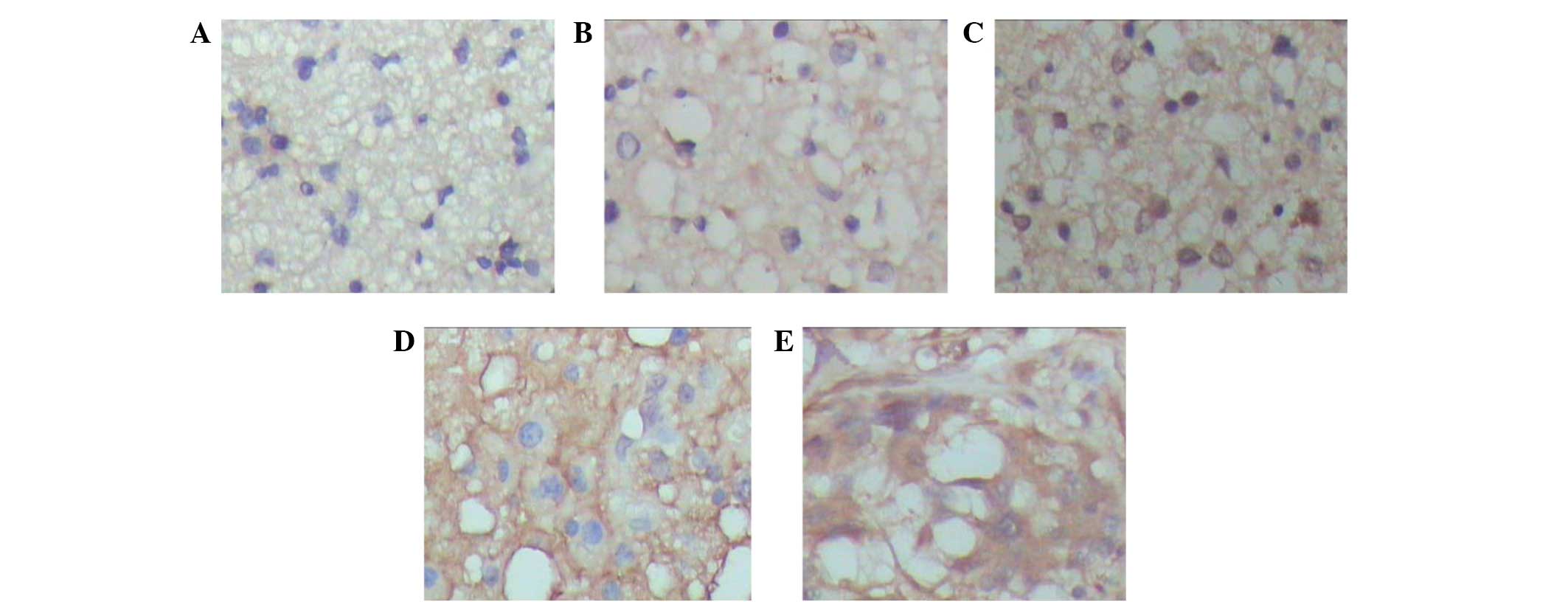
Expression of WISP-2 protein correlates
with astrocytoma grade
The expression of the WISP-2 protein was assessed by
immunohistochemistry in paraffin sections in a panel of 154
astrocytomas of various WHO grades and 15 normal brain tissues. In
the majority of astrocytomas, diffusive and prominent expression of
WISP2 in the cytoplasm of the tumor cells was detected (Fig. 2). The positive expression levels of
WISP-2 in the astrocytomas were significantly higher those that in
the normal brain tissues. For semi-quantification, WISP-2
expression was significantly associated with pathological grade,
however, no association was observed with age, gender or tumor
size. The detailed results are shown in Table I.
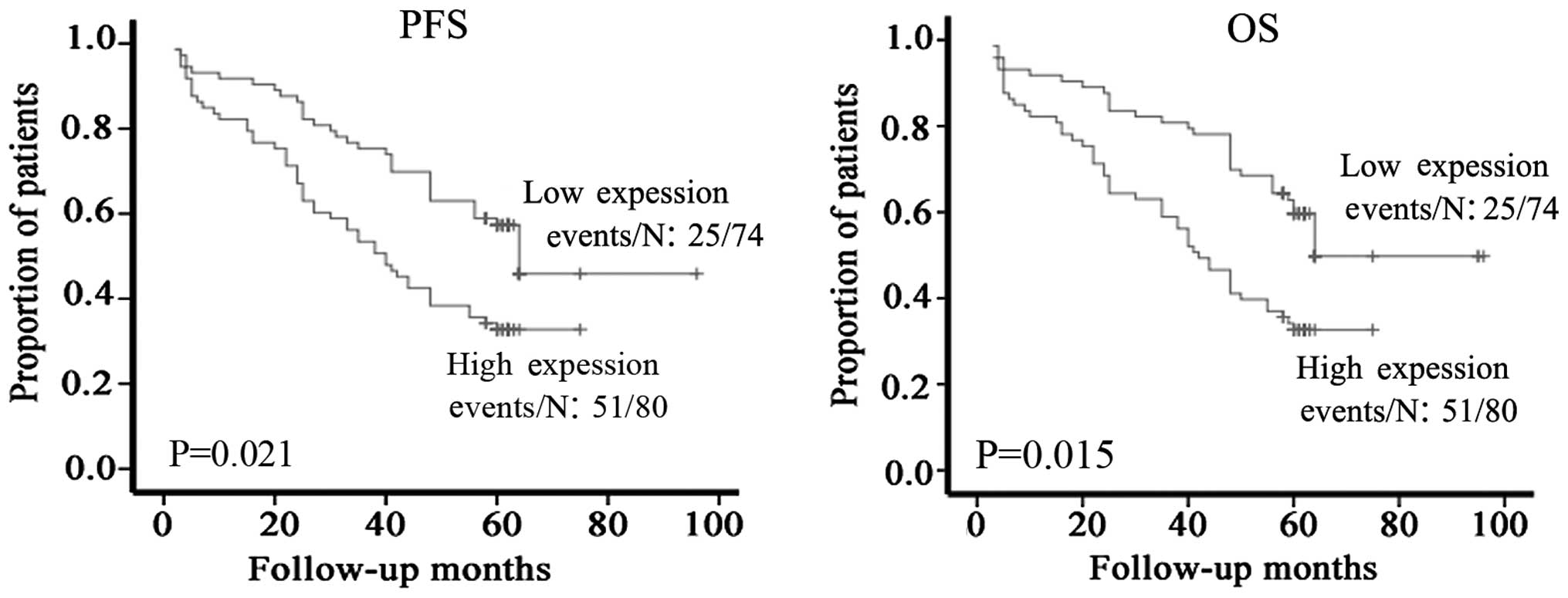
Correlation between WISP-2 and patient
survival
The prognostic value of WISP-2 protein expression
was also evaluated. Kaplan-Meier analysis was performed to
investigate the correlation between WISP-2 expression and patient
PFS and OS. The Kaplan-Meier analysis revealed that high WISP-2
expression was significantly associated with a shorter PFS and OS
(Fig. 3; P=0.021 and 0.015,
respectively). These results indicated that the protein expression
levels of WISP-2 may serve as important and independent predictors
of survival in astrocytoma patients.
In addition, multivariate Cox’s analysis of the OS
and PFS was performed, using WISP-2 expression, tumor size, age,
gender and pathological grade as categorical variables. The WISP-2
protein expression level and tumor pathological grade were found to
be independent prognostic indicators for patient PFS and OS
(P=0.013 and 0.019, Table III,
respectively).
 | Table IIICox regression analyses of the
factors associated with progression-free and overall survival in
astrocytoma patients. |
Table III
Cox regression analyses of the
factors associated with progression-free and overall survival in
astrocytoma patients.
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender | 1.822 | 0.850–3.906 | 0.223 | 1.814 | 0.841–3.914 | 0.216 |
| Age | 1.000 | 0.978–1.023 | 0.990 | 0.996 | 0.974–1.019 | 0.726 |
| WISP-2
expression | 1.865 | 1.225–2.115 | 0.013 | 1.971 | 1.201–3.166 | 0.019 |
| Tumor size | 0.953 | 0.925–0.985 | 0.805 | 1.021 | 0.901–1.168 | 0.406 |
| Pathological
grade | 1.344 | 0.810–2.231 | 0.003 | 1.618 | 0.863–2.428 | 0.001 |
Discussion
Astrocytomas, which are a type of glioma, account
for the largest group of primary CNS tumors. The current standard
management of glioma is a combined treatment based on microsurgery
and including chemotherapy, radiotherapy, immunotherapy and
anti-angiogenesis. Malignant astrocytomas are associated with poor
prognosis due to their pathological characteristics, including
rapid proliferation and diffuse brain invasion (24). To date, a substantial improvement in
the treatment of patients with glioma has not been achieved.
Therefore, identifying key regulatory molecules in tumor invasion
and progression has gained wide interest as they are crucial for
the understanding tumor progression and the development of novel
interventions (25).
The upregulation of the WISP-2 gene was initially
observed in C57MG cells transformed by the Wnt-1 retrovirus
(26). Although Wnt family members
are critical for numerous developmental processes, and components
of the Wnt signaling pathway have been linked to tumorigenesis, the
involvement of WISP-2 in mammalian carcinogenesis is not well
defined. WISP-2 has been hypothesized to exhibit oncogenic and
tumor suppressor activities. The wild type p53 protein has been
demonstrated to protect against cancer (27), and the elimination of cells with
mutagenic tendencies via apoptosis is critical to its
anti-carcinogenic properties. When one or more mutations are
present in specific regions of this gene, the tumor suppressor
function of the p53 protein is impaired (28). Overexpression of p53 is frequently
observed in numerous cancers and is dependant upon the synthesis of
mutated forms of the p53 protein. It has also been found to be
associated with the malignant progression of various cancers,
including procaspase activating compound (29,30).
Dhar et al (20)
demonstrated that the overexpression of oncogenically mutated forms
of the p53 gene may be associated with the silencing of WISP-2
during the progression of pancreatic cancer. Furthermore,
p53-mutant-induced invasive phenotypes may be mimicked by blocking
WISP-2 expression via RNAi. Fritah et al (31) found that inducing the expression of
WISP-2 or supplementing the WISP-2 protein reduces the rate of
proliferation, migration and invasion in WISP-2 (−) invasive human
breast cancer cells. Previous studies have also shown that the
inhibition of miR-10b expression in breast cancer cells induced by
WISP-2 is critical for the anti-invasive function of this gene and
is mediated via the inhibition of the JNK-HIF-1α-TWIST1 signaling
cascades (20,30,32–42).
Kouzu et al (43)
demonstrated that WISP-2 is a reliable independent marker and that
downregulation or loss of the WISP-2 gene may be associated with
the development of salivary gland tumors.
WISP-2 expression is required for breast tumor cells
proliferation in estrogen receptor (ER)-positive human breast
cancers. Dhar et al (19)
reported that IGF-1 induces WISP-2/CCN5 expression via a number of
molecular cross-talks and is crucial for the mitogenic switch by
the IGF-1 axis in ER-positive breast tumor cells (19). Collectively, the contrasting
pathobiological roles of WISP-2, including participation in
steroid- and growth factor-induced proliferation and the protection
of cells from EMT, migration and invasion, under different
microenvironments indicates that WISP2 may be a bifunctional cancer
gene and that the major role of WISP-2, under culture conditions,
is to protect the cells from adopting invasive phenotypes.
Although several studies have been conducted in
different cancer types to elucidate the role of WISP-2 in
carcinogenesis and its impact on prognosis, the contribution of
WISP-2 in astrocytoma has not been previously investigated.
In the current study, the expression profile of
WISP2 was determined at the mRNA and protein levels. WISP-2
expression was found to be significantly upregulated in astrocytoma
tissues when compared with normal brain tissues. Furthermore, a
significant correlation was identified between WISP-2 protein
expression and PFS, as well as OS, which exhibited a highly
significant, linear distribution in the Kaplan-Meier analysis
(P<0.01). Notably, increased levels of WISP-2 protein expression
significantly correlated with a shorter PFS and OS in astrocytoma
patients. Although the molecular mechanism requires further study,
these results indicate that WISP-2 may be involved in the
pathogenesis and progression of astrocytomas. Future studies, using
tumor cell lines, such as U251 cells, and WISP-2 gene silencing may
be of value with regard to eludicating the association between
WISP-2 expression and the proliferation and apoptosis of human
astrocytoma cells.
Acknowledgements
This study was supported by the Construct Program of
the Key Discipline in Hunan Province, the Planned Science and
Technology Project of Hunan Province, China (grant no. 2012SK2020)
and the postgraduate degree thesis innovation projects of Central
South University (grant no. 2011ssxt207).
References
|
1
|
Eroes CA, Zausinger S, Kreth FW,
Goldbrunner R and Tonn JC: Intramedullary low grade astrocytoma and
ependymoma. Surgical results and predicting factors for clinical
outcome. Acta Neurochir (Wien). 152:611–618. 2010. View Article : Google Scholar
|
|
2
|
Wade A, Hayhurst C, Amato-Watkins A,
Lammie A and Leach P: Cerebellar pilocytic astrocytoma in adults: a
management paradigm for a rare tumour. Acta Neurochir (Wien).
155:1431–1435. 2013. View Article : Google Scholar
|
|
3
|
Schuurman PR, Troost D, Verbeeten B Jr and
Bosch DA: 5-year survival and clinical prognostic factors in
progressive supratentorial diffuse “low-grade” astrocytoma: a
retrospective analysis of 46 cases. Acta Neurochir (Wien). 139:2–7.
1997. View Article : Google Scholar
|
|
4
|
Sturiale CL, Sabatino G, Albanese A, et
al: Subcutaneous malignant melanoma of the scalp surgical flap
after brain irradiation for anaplastic astrocytoma. J Neurooncol.
106:203–207. 2012. View Article : Google Scholar
|
|
5
|
Wick W, Platten M, Meisner C, et al:
Temozolomide chemotherapy alone versus radiotherapy alone for
malignant astrocytoma in the elderly: the NOA-08 randomised, phase
3 trial. Lancet Oncol. 13:707–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsutani T, Nagai Y, Mine S, et al:
Akt/protein kinase B overexpression as an accurate prognostic
marker in adult diffuse astrocytoma. Acta Neurochir (Wien).
151:263–268. 2009.discussion 268. View Article : Google Scholar
|
|
7
|
Imaizumi T, Murakami K, Ohta K, et al:
MDA5 and ISG56 mediate CXCL10 expression induced by toll-like
receptor 4 activation in U373MG human astrocytoma cells. Neurosci
Res. 76:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El Hindy N, Bankfalvi A, Herring A, et al:
Correlation of aquaporin-1 water channel protein expression with
tumor angiogenesis in human astrocytoma. Anticancer Res.
33:609–613. 2013.PubMed/NCBI
|
|
9
|
Barton VN, Donson AM, Birks DK, et al:
Insulin-like growth factor 2 mRNA binding protein 3 expression is
an independent prognostic factor in pediatric pilocytic and
pilomyxoid astrocytoma. J Neuropathol Exp Neurol. 72:442–449. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanoke A, Kanamori M, Kumabe T, et al:
Metachronous, multicentric glioma of pilocytic astrocytoma with
oligodendroglioma-like component and oligodendroglioma through
distinct genetic aberrations. J Neurosurg. 118:854–858. 2013.
View Article : Google Scholar
|
|
11
|
Mascelli S, Raso A, Biassoni R, et al:
Analysis of NADP+-dependent isocitrate dehydrogenase-1/2 gene
mutations in pediatric brain tumors: report of a secondary
anaplastic astrocytoma carrying the IDH1 mutation. J Neurooncol.
109:477–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Looso M, Michel CS, Konzer A, et al:
Spiked-in pulsed in vivo labeling identifies a new member of the
CCN family in regenerating newt hearts. J Proteome Res.
11:4693–4704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minhas U, Martin TA, Ruge F, Harding KG
and Jiang WG: Pattern of expression of CCN family members Cyr61,
CTGF and NOV in human acute and chronic wounds. Exp Ther Med.
2:641–645. 2011.PubMed/NCBI
|
|
15
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lake AC and Castellot JJ Jr: CCN5
modulates the antiproliferative effect of heparin and regulates
cell motility in vascular smooth muscle cells. Cell Commun Signal.
1:52003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schutze N, Noth U, Schneidereit J,
Hendrich C and Jakob F: Differential expression of CCN-family
members in primary human bone marrow-derived mesenchymal stem cells
during osteogenic, chondrogenic and adipogenic differentiation.
Cell Commun Signal. 3:52005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banerjee S, Sengupta K, Saxena NK, Dhar K
and Banerjee SK: Epidermal growth factor induces WISP-2/CCN5
expression in estrogen receptor-alpha-positive breast tumor cells
through multiple molecular cross-talks. Mol Cancer Res. 3:151–162.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhar K, Banerjee S, Dhar G, Sengupta K and
Banerjee SK: Insulin-like growth factor-1 (IGF-1) induces
WISP-2/CCN5 via multiple molecular cross-talks and is essential for
mitogenic switch by IGF-1 axis in estrogen receptor-positive breast
tumor cells. Cancer Res. 67:1520–1526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhar G, Banerjee S, Dhar K, et al: Gain of
oncogenic function of p53 mutants induces invasive phenotypes in
human breast cancer cells by silencing CCN5/WISP-2. Cancer Res.
68:4580–4587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dhar G, Mehta S, Banerjee S, et al: Loss
of WISP-2/CCN5 signaling in human pancreatic cancer: a potential
mechanism for epithelial-mesenchymal-transition. Cancer Lett.
254:63–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ouelaa-Benslama R, De Wever O, Hendrix A,
et al: Identification of a GαGβγ, AKT and PKCα signalome associated
with invasive growth in two genetic models of human breast cancer
cell epithelial-to-mesenchymal transition. Int J Oncol. 41:189–200.
2012.PubMed/NCBI
|
|
23
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Henriksson R, Asklund T and Poulsen HS:
Impact of therapy on quality of life, neurocognitive function and
their correlates in glioblastoma multiforme: a review. J
Neurooncol. 104:639–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Florian IS, Tomuleasa C, Soritau O, et al:
Cancer stem cells and malignant gliomas. From pathophysiology to
targeted molecular therapy. J BUON. 16:16–23. 2011.PubMed/NCBI
|
|
26
|
Pennica D, Swanson TA, Welsh JW, et al:
WISP genes are members of the connective tissue growth factor
family that are up-regulated in wnt-1-transformed cells and
aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA.
95:14717–14722. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bargonetti J and Manfredi JJ: Multiple
roles of the tumor suppressor p53. Curr Opin Oncol. 14:86–91. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hock AK and Vousden KH: Tumor suppression
by p53: fall of the triumvirate? Cell. 149:1183–1185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banerjee S, Dhar G, Haque I, et al:
CCN5/WISP-2 expression in breast adenocarcinoma is associated with
less frequent progression of the disease and suppresses the
invasive phenotypes of tumor cells. Cancer Res. 68:7606–7612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banerjee SK and Banerjee S: CCN5/WISP-2: A
micromanager of breast cancer progression. J Cell Commun Signal.
6:63–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fritah A, Saucier C, De Wever O, et al:
Role of WISP-2/CCN5 in the maintenance of a differentiated and
noninvasive phenotype in human breast cancer cells. Mol Cell Biol.
28:1114–1123. 2008. View Article : Google Scholar :
|
|
32
|
Haque I, Banerjee S, Mehta S, et al:
Cysteine-rich 61-connective tissue growth
factor-nephroblastoma-overexpressed 5 (CCN5)/Wnt-1-induced
signaling protein-2 (WISP-2) regulates microRNA-10b via
hypoxia-inducible factor-1α-TWIST signaling networks in human
breast cancer cells. J Biol Chem. 286:43475–43485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang CH, Fan TC, Yu JC, et al: The
prognostic significance of RUNX2 and miR-10a/10b and their
inter-relationship in breast cancer. J Transl Med. 12:2572014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haque I, Banerjee S, De A, et al:
CCN5/WISP-2 promotes growth arrest of triple-negative breast cancer
cells through accumulation and trafficking of p27 via Skp2 and
FOXO3a regulation. Oncogene. Aug 18–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
35
|
Han X, Yan S, Weijie Z, et al: Critical
role of miR-10b in transforming growth factor-β1-induced
epithelial-mesenchymal transition in breast cancer. Cancer Gene
Ther. 21:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ibrahim SA, Yip GW, Stock C, et al:
Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer
cell motility and invasiveness via a Rho-GTPase- and
E-cadherin-dependent mechanism. Int J Cancer. 131:E884–E896. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferrand N, Stragier E, Redeuilh G and
Sabbah M: Glucocorticoids induce CCN5/WISP-2 expression and
attenuate invasion in oestrogen receptor-negative human breast
cancer cells. Biochem J. 447:71–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma L: Role of miR-10b in breast cancer
metastasis. Breast Cancer Res. 12:2102010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Davies SR, Watkins G, Mansel RE and Jiang
WG: Differential expression and prognostic implications of the CCN
family members WISP-1, WISP-2, and WISP-3 in human breast cancer.
Ann Surg Oncol. 14:1909–1918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Banerjee S, Saxena N, Sengupta K, et al:
WISP-2 gene in human breast cancer: estrogen and progesterone
inducible expression and regulation of tumor cell proliferation.
Neoplasia. 5:63–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inadera H, Hashimoto S, Dong HY, et al:
WISP-2 as a novel estrogen-responsive gene in human breast cancer
cells. Biochem Biophys Res Commun. 275:108–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kouzu Y, Uzawa K, Kato M, et al: WISP-2
expression in human salivary gland tumors. Int J Mol Med.
17:567–573. 2006.PubMed/NCBI
|