Introduction
Statistics indicate that the incidence of cancer in
China is increasing each year (1).
Moreover, the clinical diagnosis of many Chinese patients occurs
when these individuals have late-stage cancer and therefore no
longer have the opportunity to receive radical surgical treatment.
Chemotherapy has become an important method of cancer treatment,
however, most patients undergo either relapse or metastasis
following first-line chemotherapy, requiring second-line and
subsequent treatments.
Cisplatin (DDP)-based chemotherapy is significant in
cancer treatment; however, due to its toxicity, particularly
nephro- and neuro-toxicity, DDP-based chemotherapy has limited
applications (2,3). Therefore, researchers have pursued a
new platinum compound. Lobaplatin (LBP), which is a class III
platinum anticancer drug developed by the German firm ASTA Medica
(Degussa), is primarily used for the treatment of advanced breast
cancer, small cell lung cancer (SCLC), and chronic myeloid
leukaemia (4). Research has
demonstrated that LBP has various advantageous properties,
including strong anticancer activity, no significant nephrotoxicity
or neurotoxicity, no requirement for hydration/liquid infusion
(5), a much lower incidence of drug
resistance than DDP, and no cross-resistance with DDP (6,7). The
mechanism of action of LBP is similar to that of other platinum
drugs; in particular, LBP induces the formation of inter-strand
Pt-GG and Pt-AG crosslinks, blocking DNA replication and
transcription and thereby inhibiting gene expression in tumour
cells (6). LBP’s pharmacokinetic
characteristics include the rapid onset of clinical effects, the
persistence of these effects for a long duration, high tumour
tissue concentrations, and low plasma concentrations (8). Thus, the drug demonstrates good
selectivity after entering the body. LBP exhibits superior
pharmacokinetic parameters in Chinese populations compared with
Western populations (8,9). A number of studies have revealed that
LBP has broad-spectrum anticancer activity, including efficacy
against lung cancer, breast cancer, colorectal cancer, testicular
cancer, and lymphoma (2,10,11).
Docetaxel (TXT) is a semisynthetic compound in the
taxane class of anti-cancer drugs. It binds to free tubulin,
promotes the assembly of tubulin into stable microtubules, and
inhibits microtubule depolymerisation (12). These effects significantly decrease
the quantities of free tubulin and thereby inhibit cell mitosis and
proliferation. Single-agent chemotherapy with TXT is an important
treatment approach for a variety of tumours (13–16).
Studies have demonstrated that the administration of LBP in
combination with TXT can produce certain therapeutic effects in
patients with tumour progression after chemotherapy and that in
this combination, LBP and TXT produce synergistic effects (11). However, the optimal LBP dose in this
combination regimen has not been established based on the findings
from phase I/II clinical trials. In particular, although
international studies have established a recommended LBP dose of 50
mg/m2 for single-agent chemotherapy (17–20),
no phase I studies on the appropriate LBP dose in the
aforementioned combination regimen for second-line or third-line
chemotherapy have been reported. In Europe and the USA, the
recommended dose of TXT for second-line therapy is 75–100
mg/m2 (21), however,
Asian studies have suggested that a TXT dose of 60 mg/m2
is more suitable for East Asian populations (22–24).
Our previous studies have demonstrated that Eastern and Western
populations have different tolerances for the same doses of
chemotherapy (cisplatin with 5-fluorouracil, and capecitabine with
docetaxel), with tolerated doses in the combination regimens for
Eastern populations that are equivalent to 70% to 80% of the
corresponding doses for Western populations (25,26).
Therefore, it is unclear whether chemotherapy doses determined
based on studies of Western populations can be applied to Chinese
patients. To further investigate the appropriate LBP dose in the
aforementioned combination regimen, we conducted a dose escalation
trial for LBP in this regimen; this study reports the results of
this trial.
Materials and methods
Eligibility
The patients who participated in this study were
pathologically or cytologically confirmed to have advanced solid
tumours that had progressed after at least first-line chemotherapy.
These patients had at least one evaluable lesion (27,28)
and were in clinical stages III or IV. The following inclusion
criteria were utilised: 18–75 years of age; Karnofsky Performance
Status (KPS) score ≥60, with expected survival of over three
months; a complete recovery to normal from the toxicity of prior
treatment, with ≥four weeks since any previous treatment; marrow
conditions that included a white blood cell (WBC) count,
≥4.0×109/l, neutrophils, ≥1.5×109/l,
platelets (PLT), ≥100×109/l, and haemoglobin, ≥100 g/l;
adequate hepatic and renal function (serum creatinine, aspartate
aminotransferase, alanine aminotransferase and total serum
bilirubin ≤upper limits of normal); normal cardiopulmonary
function, with no obvious infection, gastrointestinal bleeding, or
other serious visceral diseases; no prior treatment with LBP; no
treatment with TXT during the previous six months; favourable
compliance to the chemotherapy regimen during the study period; and
the provision of written informed consent.
Exclusion criteria
Pregnant or lactating women; patients with no
self-awareness, uncontrollable central nervous system metastases,
uncontrollable seizures, or mental illnesses impairing
self-awareness or judgment; patients who had been treated with
chemotherapy drugs other than LBP and TXT or with radiation therapy
within the prior four weeks; patients with organ transplants; and
patients with the long-term use of immunosuppressive agents and
corticosteroids.
Pretreatment evaluation
Within one week prior to the start of treatment, the
researchers obtained the subjects’ medical histories and KPS scores
as well as completing a physical examination, a routine blood
examination, tests of liver and renal function, chest and abdominal
computed tomography (CT) imaging, and an electrocardiogram for each
study subject.
Trial Design
The trial was an open-label, non-randomised dose
escalation study. Each group consisted of at least three patients.
The primary endpoint of this study was to determine the maximum
tolerated dose (MTD) of LBP in a LBP and TXT combination regimen
for the treatment of solid tumours with progression following
chemotherapy. The secondary endpoint of the study was to evaluate
the safety, toxicity, and time to progression (TTP) of this LBP and
TXT combination regimen.
Ethics
The study was approved by the Ethics Committee,
North China Petroleum Bureau General Hospital of Hebei Medical
University, Renqiu, China. It was performed in accordance with the
ethics standards of human experimentation and with the Helsinki
Declaration of 1975, as revised in 2000. All patients provided
written informed consent.
Chemotherapy
A fixed dose of 60 mg/m2 TXT was diluted
in 250 ml of 5% glucose and then intravenously injected on day one
(d1) (22–24). LBP was dissolved in 5 ml of
injectable sterile water, diluted in 250 ml of 5% glucose, and then
intravenously injected in a 2 h treatment on day two (d2). A
prophylactic anti-allergy treatment of 8 mg of dexamethasone was
administered twice per day on the three consecutive days of d-1,
d1, and d2. This treatment cycle was repeated every 21 days for a
minimum of two cycles. The treatment was continued until disease
progression or unacceptable toxicity was observed, up to a maximum
of six cycles of chemotherapy.
During therapy, all patients were given
5-hydroxytryptamine (HT3) receptor antagonists as an anti-emetic
prophylactic treatment. To ensure the continuity of chemotherapy,
at WBC <4.0×109/l and/or absolute neutrophil count
(ANC) <2.0×109/l, supportive treatment using
recombinant human granulocyte colony-stimulating factor was
administered, and when PLT <75×109/l, interleukin-11
treatment was administered. If clinically indicated, additional
supportive care was allowed.
Dose escalation and the determination of
dose-limiting toxicity (DLT)
The evaluation of adverse events was based on the
Common Terminology Criteria for Adverse Events, v3.0 (29). DLT was defined to be the occurrence
of one or more of the following events after the first day of
chemotherapy and prior to the third cycle of chemotherapy: i)
Haematological toxicity in the form of grade IV neutropenia, grade
III febrile neutropenia, grade III or grade IV thrombocytopenia, or
grade III or grade IV anaemia; ii) grade III–IV non-haematological
toxicity (with the exception of alopecia, nausea, vomiting, and
fatigue); and iii) any grade V responses.
A modified Fibonacci method (30) was used, with an initial LBP dose of
30 mg/m2 and a subsequent increase of 5 mg/m2
from one group to the next. The patients received the treatment
specified by the study protocol in accordance with their order of
enrolment, and the study gradually progressed from enrolling
subjects in a low-dose group to enrolling subjects in a high-dose
group. At least three patients were enrolled in each group. If DLT
did not occur in the three cases in a dose group, the subsequent
dose group was initiated. However, repeated administration to the
same patient was not allowed. If one case of DLT occurred within a
dose group, three additional patients were enrolled into the dose
group in question. Enrolment into the subsequent dose group could
only commence if none of these additional three patients
experienced DLT. If one or more cases of DLT occurred among these
additional three patients, the trial was terminated. The dose used
in the final group was regarded as the dose that produced DLT, and
the dose immediately below the dose that produced DLT was regarded
as the MDT.
Evaluation Standards
We used RECIST (Response Evaluation Criteria in
Solid Tumours) 1.1 to evaluate short-term efficacy (31). The time point at which the efficacy
evaluation was performed was one week prior to the third
chemotherapy cycle. The following classifications were used for
this evaluation: complete remission (CR), partial response (PR),
stable disease (SD), and progressive disease (PD). The response
rate (RR) was defined to be CR + PR, and the disease control rate
(DCR) was defined to be CR + PR + SD. The main image-based evidence
used for these evaluations consisted of the CT/magnetic resonance
imaging (MRI) results. It was thought that with the exception of
cases involving PD, no evaluations of efficacy could be performed
after only one cycle of chemotherapy. Each patient received at
least two cycles of chemotherapy. The chemotherapy cycle was
repeated every 21 days, and treatment was continued until the
occurrence of disease progression or unacceptable toxicity.
Subsequent treatment
Based on the judgements of the research team,
patients with disease progression received either third-line
treatment or the best available supportive care.
Follow-up
Following the completion of the treatment, the
patients underwent follow-up studies once per month for the first
six months and once every three months subsequently. Each follow-up
exam included the acquisition of a medical history, a physical
examination, a routine blood examination, comprehensive biochemical
tests, and a chest CT; in addition, an abdominal CT was performed
once every three months. All patients were followed up by
outpatient examinations and telephone, and follow-up continued
until mortality occurred.
Statistical analysis
Data analysis was performed using SPSS 18.0, and the
Kaplan-Meier method was used to calculate patients’ TTP.
Results
Patient characteristics
Between May 2012 and November 2013, 17 patients with
malignant solid tumours were enrolled in this study. These patients
included nine males and eight females, and their ages ranged from
45–76 years (with a median age of 62 years). The median KPS score
of the study participants was 80 points (with a range of 60–90).
Patients’ body surface areas ranged from 1.41–1.94 m2
(median, 1.66 m2). There were 11 cases of non-small cell
lung cancer (NSCLC), two cases of SCLC, two cases of breast cancer,
one case of gastric cancer, and one case of endometrial carcinoma.
There was one stage IIIA patient, two stage IIIB patients, and 14
stage IV patients. A total of 14 patients among the entire group of
study subjects were evaluable for efficacy; all 17 patients were
evaluable for toxicity (Table I).
Until 5th January 2014, no cases had been lost to follow-up, and
the follow-up rate was 100%.
 | Table IPatient characteristics |
Table I
Patient characteristics
| Characteristic | Patients, n |
|---|
| Gender |
| Male | 9 |
| Female | 8 |
| Age, year |
| Range | 45–76 |
| Median | 62 |
| Stage |
| IIIA | 1 |
| IIIB | 2 |
| IV | 14 |
| KPS |
| Range | 70–90 |
| Median | 90 |
Compliance
A total of 17 patients completed a total of 58
cycles of chemotherapy. The median number of chemotherapy cycles
completed by a patient was four (range, 1–6 cycles). Two patients
completed one chemotherapy cycle, six patients completed two
chemotherapy cycles, five patients completed four chemotherapy
cycles, and four patients completed six chemotherapy cycles.
Haematological toxicity
Table II describes
the haematological toxicities associated with the tested
treatments. The incidence of leukopenia was 82.35% (11 cases), the
incidence of grade III leukopenia was 29.41% (five cases), and
there were no cases of grade IV leukopenia. The incidence of
neutropenia was 58.82% (ten cases), the incidence of grade III
neutropenia was 29.41% (five cases), and the incidence of grade IV
neutropenia was 11.76% (two cases). The incidence of anaemia was
58.82% (ten cases), with no cases of grade III or grade IV anaemia.
The incidence of thrombocytopenia was 46.2% (six cases), the
incidence of grade III thrombocytopenia was 5.88% (one case in the
40 mg/m2 group), and there were no cases of grade IV
thrombocytopenia.
 | Table IIHaematological toxicities. |
Table II
Haematological toxicities.
| 30
mg/m2 | 35
mg/m2 | 40
mg/m2 |
|---|
|
|
|
|
|---|
| Cases, n | % | Cases, n | % | Cases, n | % |
|---|
| Leukopenia |
| 0 | 1 | 33.3 | 1 | 10.0 | 1 | 25.0 |
| I–II | 2 | 66.6 | 6 | 60.0 | 1 | 25.0 |
| III | 0 | 0 | 3 | 30.0 | 2 | 50.0 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia |
| 0 | 3 | 100 | 3 | 30.0 | 1 | 25.0 |
| I–II | 0 | 0 | 2 | 20.0 | 1 | 25.0 |
| III | 0 | 0 | 4 | 40.0 | 1 | 25.0 |
| IV | 0 | 0 | 1 | 10.0 | 1 | 25.0 |
| Anemia |
| 0 | 2 | 66.6 | 4 | 40.0 | 1 | 25.0 |
| I–II | 1 | 33.3 | 6 | 60.0 | 3 | 75.0 |
| III | 0 | 0 | 0 | 0 | 0 | 0 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 |
|
Thrombocytopenia |
| 0 | 2 | 66.6 | 7 | 70.0 | 2 | 50.0 |
| I–II | 1 | 33.3 | 3 | 30.0 | 1 | 25.0 |
| III | 0 | 0 | 0 | 0 | 1 | 25.0 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 |
Non-haematological toxicity
The subjects experienced only mild
non-haematological toxicities of grades I–II. There were no
treatment-related deaths. There was one case of diarrhoea (5.88%),
one case of phlebitis (5.88%), five cases of fatigue (29.41%),
three cases of nausea (17.65%), and no cases of vomiting. All the
patients improved after receiving symptomatic treatment. The
adverse events are detailed in Table
III.
 | Table IIINon-haematological toxicities. |
Table III
Non-haematological toxicities.
| 30
mg/m2 | 35
mg/m2 | 40
mg/m2 |
|---|
|
|
|
|
|---|
| Cases, n | % | Cases, n | % | Cases, n | % |
|---|
| Diarrhea |
| 0 | 3 | 100 | 9 | 90.0 | 4 | 100 |
| I–II | 0 | 0 | 1 | 10.0 | 0 | 0 |
| III–IV | 0 | 0 | 0 | 0 | 0 | 0 |
| Phlebitis |
| 0 | 3 | 100 | 9 | 90.0 | 4 | 100 |
| I–II | 0 | 0 | 1 | 10.0 | 0 | 0 |
| III–IV | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue |
| 0 | 3 | 100 | 6 | 60.0 | 3 | 75.0 |
| I–II | 0 | 0 | 4 | 40.0 | 1 | 25.0 |
| III–IV | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea |
| 0 | 3 | 100 | 9 | 90.0 | 2 | 50.0 |
| I–II | 0 | 0 | 1 | 10.0 | 2 | 50.0 |
| III–IV | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting |
| 0 | 3 | 100 | 10 | 100 | 4 | 100 |
| I–II | 0 | 0 | 0 | 0 | 0 | 0 |
| III–IV | 0 | 0 | 0 | 0 | 0 | 0 |
Determination of MTD
DLT did not occur in the chemotherapy group that
received the initial LBP dose of 30 mg/m2, which was
administered to the first three enrolled patients. In accordance
with the dose escalation method, three patients were then enrolled
into the next highest dose group, the 35 mg/m2 LBP
group; as previously, DLT did not occur. Subsequently, four
patients were enrolled into the next dose group (40
mg/m2 LBP group); the first of these patients withdrew
from the trial on the seventh day after the first cycle of
chemotherapy due to associated pancreatitis. We hypothesise that
this occurrence of pancreatitis, which caused the patient to
succumb to the disease seven days following the onset, was not
directly caused by the chemotherapy. This is due to the fact that a
search of the literature did not identify any studies which
reported that lobaplatin or docetaxel could induce pancreatitis,
and pancreatitis was not mentioned as a side effect in the
instructions for the two drugs. DLT occurred in the second patient.
This patient experienced grade III thrombocytopenia with a PLT of
26×109/L on the 11th day after one cycle of
chemotherapy and succumbed to the disease on the day 12 following
chemotherapy due to massive haemoptysis. This patient, who received
only a single cycle of chemotherapy, exhibited central lung cancer
with T4 lesions. On the third day following chemotherapy, pain and
numbness in the patient’s left arm were significantly reduced, and
swelling on the left side of the patient’s face had subsided
significantly, suggesting that the chemotherapy was effective.
Massive haemoptysis may have occurred due to damage to major blood
vessels caused by rapid tumour regression (32); therefore, this patient’s outcome was
not considered to be a case of chemotherapy-related death. The
third patient in the 40 mg/m2 LBP group experienced
grade IV neutropenia (a case of DLT), and the fourth patient in
this group experienced grade III febrile neutropenia (a case of
DLT). Therefore, at the third dose level, two patients each
completed two cycles of chemotherapy, and experienced DLT (in the
form of neutropenia), and one patient completed one cycle of
chemotherapy, but also experienced DLT (in the form of grade III
thrombocytopenia). We determined that the 40 mg/m2 level
of LBP was the dose that produced DLT, and dose escalation was
therefore terminated. To further evaluate the adverse events
associated with 35 mg/m2 LBP, three additional patients
were enrolled into the LBP 35 mg/m2 group. Among these
patients, one case of DLT (in the form of grade IV neutropenia) was
observed. Subsequently, an additional four patients were enrolled
into this group, however, no other cases of DLT were observed.
Therefore, in the 35 mg/m2 group, there were a total of
10 patients, and one case of DLT was observed. The patient who
experienced DLT exhibited lung cancer with multiple bone
metastases. This patient’s poor bone marrow function may have
contributed to the onset of grade IV neutropenia. The patient’s
neutrophil levels returned to normal after seven days, and no
febrile neutropenia was observed. The aforementioned observations
suggested that the 35 mg/m2 dose level was well
tolerated; therefore, we do not consider this dose to be regularly
associated with DLT. In summary, this study had three dose levels,
as indicated in Table IV.
Initially, three patients were enrolled in the dose group I, which
consisted of 30 mg/m2 LBP on d2 and 60 mg/m2
TXT on d1, and DLT did not occur. In total, 10 patients were
enrolled in dose group II, which consisted of 35 mg/m2
LBP on d2 and 60 mg/m2 TXT on d1, and only one patient
experienced DLT. In contrast, four patients were enrolled in dose
group III, which consisted of 40 mg/m2 LBP on d2 and 60
mg/m2 TXT on d1, however, one patient withdrew from the
group, and the other three patients experienced DLT. These results
suggested that in the tested regimen, the dose level of 40
mg/m2 LBP was overly strong and was not tolerated by the
patients. In accordance with our experimental design, we determined
that the MTD was the highest tested dose less than the dose that
produced DLT; therefore, the MTD for the tested regimen was 35
mg/m2 LBP on d2 and 60 mg/m2 TXT on d1 of a
treatment cycle that repeated every 21 days.
 | Table IVDose escalation level. |
Table IV
Dose escalation level.
| Levels | Patients | Lobaplatin,
mg/m2 | Docetaxel,
mg/m2 |
|---|
| I | 3 | 30 | 60 |
| II | 10 | 35 | 60 |
| III | 4 | 40 | 60 |
Short-term efficacy
Among the 17 examined patients, 14 patients were
evaluable. Among these 14 patients, there were no cases of CR, one
case of PR, 10 cases of SD, and three cases of PD. Thus, the RR was
7.1% (1/14), and the DCR was 78.6% (11/14). Among the 11 examined
NSCLC patients, nine patients were evaluable. These patients
included one case of PR, six cases of SD, and two cases of PD.
Thus, among the NSCLC patients, the RR was 11.1% (1/9), and the DCR
was 77.8 % (7/9).
Survival analysis
Although we conducted a phase I study, the follow-up
time was not extensive, and the overall survival data are not yet
available. However, we have reported the preliminary survival
information in the current study. Fig.
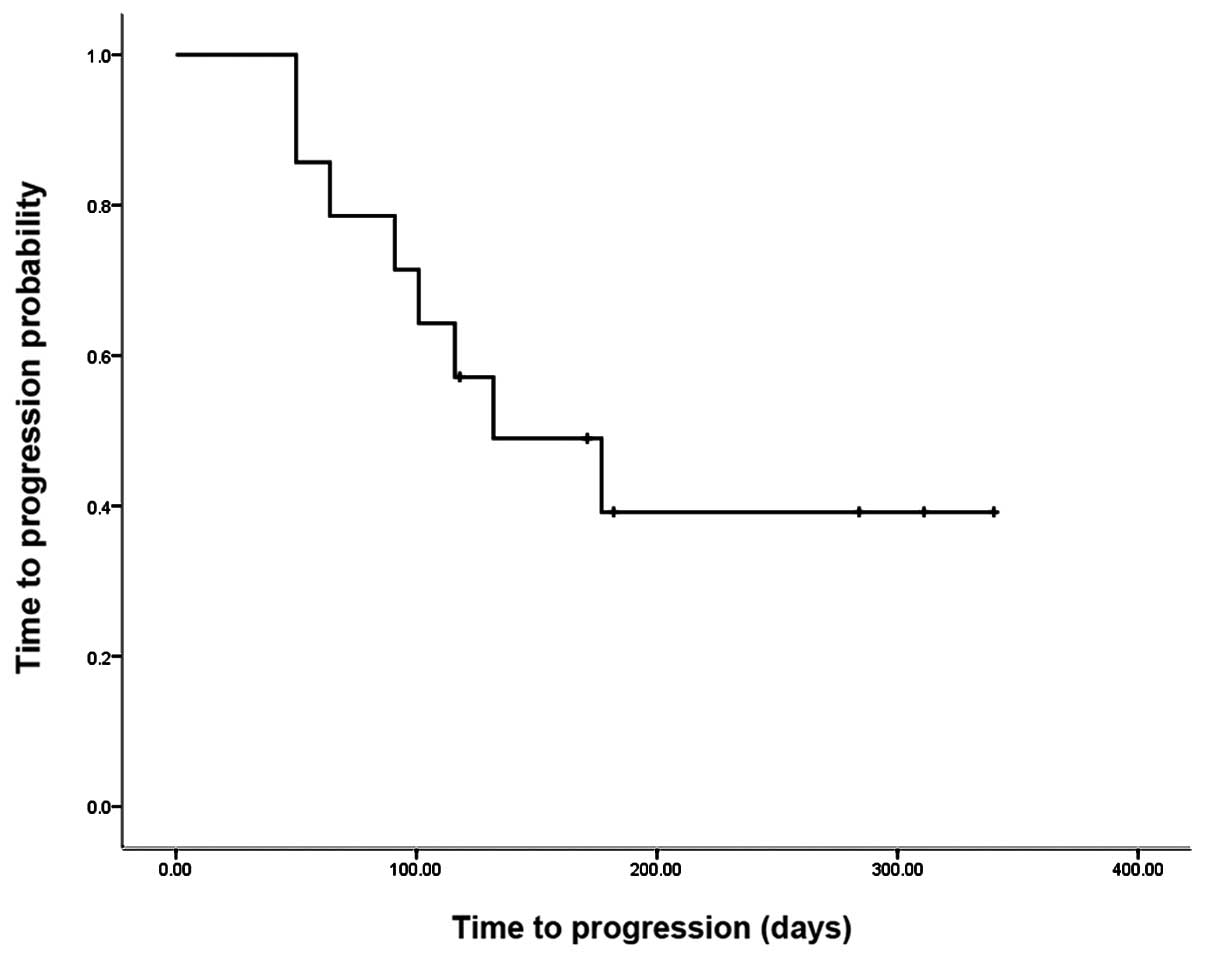
1 indicates that the median TTP among all patients was 132
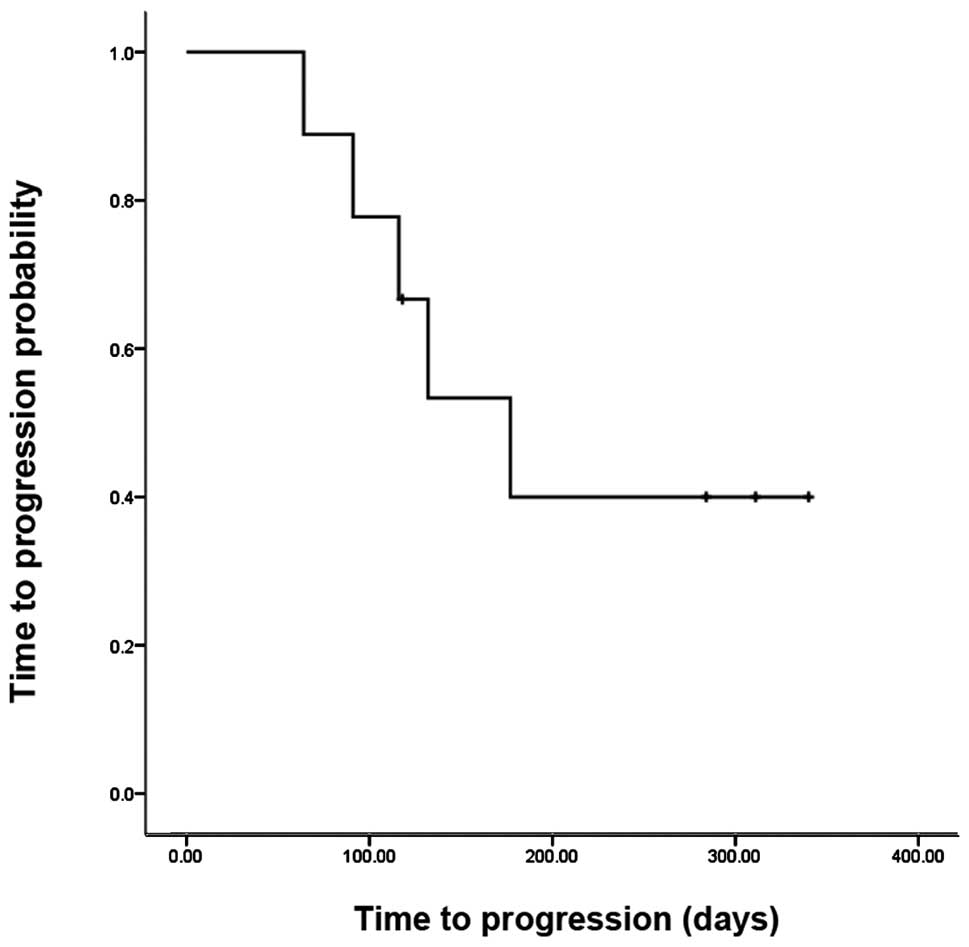
days, and the six month TTP rate was 39.2%. Fig. 2 indicates that among the NSCLC
patients, the median TTP was 177 days (95% confidence interval:
100–255 days), and the 6-month TTP rate was 40%.
Discussion
The current phase I dose escalation clinical trial
demonstrated that the LBP and TXT combination regimen for the
treatment of human solid tumours with progression following
chemotherapy is safe, is associated with a low incidence of serious
adverse effects, and exhibits short-term efficacy.
The wide application of DDP, carboplatin, and other
platinum-based drugs in clinical antitumour therapies has led to
the accumulation of overwhelming evidence indicating that platinum
compounds have good therapeutic effects in the treatment of
different types of cancer and that these compounds are currently
among the most effective anticancer drugs in clinical use. However,
as these drugs not only have significant renal toxicity and
neurotoxicity, but also may cause auditory nerve damage as well as
severe nausea and vomiting, the clinical applications of
platinum-based drugs are subject to certain restrictions.
Furthermore, the general physical condition and treatment tolerance
are often poorer for patients receiving second-line therapy than
for patients receiving first-line treatment. Therefore, current
second-line treatment regimens frequently utilise drugs with lower
toxicity and improved safety compared with platinum-based
compounds.
DDP and the third-generation platinum compound, LBP
have similar mechanisms of action. LBP has anti-cancer activity
against a variety of tumours, including tumours resistant to DDP;
LBP also produces only mild adverse gastrointestinal reactions,
exhibits good water solubility, and has no significant
nephrotoxicity or neurotoxicity (33,34).
LBP was researched and developed by the German company ASTA Medica
(Degussa) (5). In 2002, the Chinese
firm Hainan Chang’an International Pharmaceutical Co., Ltd.
purchased the LBP patent and exclusive LBP production and marketing
rights. In 2005, the State Food and Drug Administration (SFDA) of
China approved LBP and subsequently LBP entered the Chinese market
as a new Class I drug that was predominantly utilised for the
treatment of advanced breast cancer, SCLC, and chronic myelogenous
leukaemia. In China, LBP is generally administered at doses of
30–50 mg/m2, with a 50 mg/m2 dose used for
single-agent chemotherapy with LBP and a 30 mg/m2 dose
used for combination therapies; LBP treatment is typically repeated
every three to four weeks (5).
However, the aforementioned doses have not been determined through
rigorous phase I/II clinical trials. In Germany, phase I/II
clinical trials of LBP were conducted on Western subjects; the
findings from these trials were used to establish a recommended
dose of 50 mg/m2 for single-agent LBP therapy (17). However, considering the different
tolerances for the same doses of chemotherapy between Eastern and
Western populations, it is unclear whether dose recommendations
based on studies of Western subjects are applicable to Eastern
patients. Furthermore, no prior phase I/II studies of LBP doses in
combination regimens were identified. Certain foundational studies
have suggested that LBP and TXT exhibit synergistic antitumour
effects when used in combination (11) and that TXT is a broad-spectrum
antitumour drug that can effectively treat a variety of malignant
solid tumours (35). In addition,
studies from East Asia have demonstrated that the administration of
60 mg/m2 of TXT to Eastern patients is as effective as
and less toxic than the administration of 75–100 mg/m2
of TXT to Western patients (22–24).
This phenomenon may be associated with to a lack of CYP3A
(cytochrome P450, family 3, subfamily A) isoenzymes among Asian
populations, given that these enzymes are involved in the
metabolism of TXT to less active metabolites (22). Our previous studies have also found
that the MTD for Chinese patients in a DDP and 5-fluorouracil
combination regimen was equivalent to 70% of the corresponding MTD
for Western patients (25). The
aforementioned studies based on Asian populations have suggested
that Eastern populations might have lower tolerances for doses of
chemotherapy drugs than Western populations. Due to this, a phase I
trial was conducted to identify MTDs in Eastern subjects; this
clinical trial investigated LBP in combination with TXT to
determine the MTD of LBP in this combination regimen.
Among the four cases of DLT in the current study,
three cases of neutropenia were observed; by contrast, other
studies have reported DLT occurring in the form of severe
thrombocytopenia (17). In multiple
clinical trials, the incidence of grade III–IV thrombocytopenia in
LBP monotherapy (at 50 mg/m2) has ranged from
26.0%–72.7% (3,18,19).
In studies of combination chemotherapy regimens involving LBP,
typically at a dose of 30 mg/m2, the incidence of grade
III–IV thrombocytopenia has ranged from 5.0%–23.8% (12,36);
therefore, this incidence is markedly lower in combination
chemotherapy compared with single-agent chemotherapy. Therefore, we
considered the possibility that in the aforementioned studies, the
incidence of chemotherapy-induced thrombocytopenia primarily
correlated with the LBP dose. The 30 mg/m2 LBP, 35
mg/m2 LBP, and 40 mg/m2 LBP groups were
established. The incidence of grade III–IV thrombocytopenia in the
current study was 5.9% (1/17). The analysed LBP doses (30–40
mg/m2) were lower than the LBP dose used for
single-agent chemotherapy (50 mg/m2), and the observed
incidence of grade III–IV thrombocytopenia (5.9%) was also lower in
our study compared with previous studies of LBP monotherapy
(26.0%–72.7%) (3,18,19).
In the current study, the observed case of grade III
thrombocytopenia occurred in the 40 mg/m2 group, further
indicating that the incidence of thrombocytopenia is associated
with the LBP dose. In this study, the majority of observed
toxicities were mild to moderate, and symptomatic treatment enabled
a return to normal following the adverse events. Therefore,
patients exhibited a favourable tolerance for the tested
regimen.
In the current study, the RR was 7.1% (1/14), and
the DCR was 78.6% (11/14). Among NSCLC patients, the RR was 11.1%
(1/9), and the DCR was 77.8 % (7/9). However, He et al
(37) reported that, for the
second-line treatment of NSCLC with 30 mg/m2 LBP in
combination with 75 mg/m2 TXT, an RR of 26.7% (4/15) and
a DCR of 73.3% (11/15) were observed. Zhang et al (12) reported that, among patients with
anthracycline-resistant advanced breast cancer who were treated
with 30 mg/m2 LBP in combination with 75
mg/m2 TXT, an RR of 54.8% (23/42) and a DCR of 80.9%
(34/42) were observed. The current study reported a lower RR than
those reported previously (12,37).
The following reasons may contribute to explaining this difference.
Firstly, significantly higher treatment efficacy has been observed
for the second-line treatment of breast cancer compared with that
for the second-line treatment of NSCLC. The patients enrolled in
the current study predominantly suffered from NSCLC, which was
involved in 64.7% (11/17) of the cases that were examined. It was
found that the efficacy of second-line chemotherapy for NSCLC was
lower than that for breast cancer. However, Zhang et al
(12) recruited patients with
breast cancer and in the present study the majority of patients
exhibited NSCLC. Thus, RR in the current study may have been lower
than that reported by Zhang et al (12) due to patient differences. Secondly,
this study included several patients who were receiving third-line
NSCLC treatments. These patients accounted for 27% (3/11) of the
examined cases of NSCLC. By contrast, the NSCLC study by He et
al (37) involved only
second-line treatment groups. The DCR calculated in the current
study was consistent with the DCRs calculated in the aforementioned
reports by Zhang et al (12)
and He et al (37).
In the current study, the median TTP among NSCLC
patients was 177 days (95% confidence interval: 74–163 days). In a
review of second-line treatment for advanced NSCLC, Weiss et
al (38) determined that the
median TTP for second-line NSCLC treatment using cytotoxic
chemotherapy agents ranged from 55 to 87 days and that the median
TTP for second-line NSCLC treatment using epidermal growth factor
receptor tyrosine kinase inhibitors varied from 48 to 108 days. The
TTP in the current study was highly comparable with the findings by
Weiss et al (38) in the
review of second-line treatment for NSCLC; furthermore, 27% (3/11)
of NSCLC patients in the current study were third-line patients.
Therefore, with respect to both disease control rate and median
TTP, these findings are encouraging.
In conclusion, the MTD for the examined LBP
combination regimen was 35 mg/m2 LBP on d2 and 60
mg/m2 TXT on d1 of a treatment cycle that was repeated
every 21 days. We are utilising these dosages in a prospective
phase II study to further evaluate the efficacy and safety of the
proposed MTD.
References
|
1
|
Chen WQ, Zheng RS, Zeng HM, Zhang SW, Li
N, Zou XN and He J: Trend analysis and prediction of cancer
incidence in China. Zhonghua Yu Fang Yi Xue Za Zhi. 46:581–586.
2012.(In Chinese). PubMed/NCBI
|
|
2
|
Harstrick A, Bokemeyer C, Scharnofkse M,
Hapke G, Reile D and Schmoll HJ: Preclinical activity of a new
platinum analogue, lobaplatin, in cisplatin-sensitive and
-resistant human testicular, ovarian, and gastric carcinoma cell
lines. Cancer Chemother Pharmacol. 33:43–47. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gietema JA, Guchelaar HJ, de Vries EG,
Aulenbacher P, Sleijfer DT and Mulder NH: A Phase I study of
lobaplatin (D-19466) administered by 72 h continuous infusion.
Anticancer Drugs. 4:51–55. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai SQ and Xu M:
Antineoplastic-lobaplatin. Shi Jie Lin Chuang Yao Wu.
26:3152005.(In Chinese).
|
|
5
|
Yang LQ and Qin SK: Progression of
lobaplatin as the third generation platinum drug. Lin Chuang Zhong
Liu Xue Za Zhi. 14:1134–1139. 2009.(In Chinese).
|
|
6
|
Mckeage MJ: Lobaplatin: a new antitumor
platinum drug. Expert Opin Investig Drugs. 10:119–128. 2001.
View Article : Google Scholar
|
|
7
|
Saris CP, van De Vaart PJ, Rietbroek RC
and Blommaert FA: In Vitro formation of DNA adducts by cisplatin,
lobaplatin and oxaliplatin in calf thymus DNA in solution and in
cultured human cells. Carcinogenesis. 17:2763–2769. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi J, Yuan ZF, Liu WN, et al: Study on
Lobaplatin Pharmacokinetic in Human Plasma and Tumor Tissue of
Malignant Patients. Zhongguo Yaoxue Zazhi. 24:1888–1891. 2007.(In
Chinese).
|
|
9
|
Welink J, Pechstein B and van der Vijgh
WJ: Determination of the two diastereoisomers of lobaplatin
(D-19466) in plasma ultrafiltrate of cancer patients with a normal
or an impaired kidney or liver function by high-performance liquid
chromatography with ultraviolet detection. J Chromatogr B Biomed
Appl. 675:107–111. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng QQ, Huang XE, Ye LH, Lu YY, Liang Y
and Xiang J: Phase II trial of Loubo® (Lobaplatin) and pemetrexed
for patients with metastatic breast cancer not responding to
anthracycline or taxanes. Asian Pac J Cancer Prev. 14:413–417.
2013. View Article : Google Scholar
|
|
11
|
Xie CY, Xu YP, Jin W and Lou LG: Antitumor
activity of lobaplatin alone or in combination with antitubulin
agents in non-small-cell lung cancer. Anticancer Drugs. 23:698–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Liu YG and Sun H: Clinical
observation of docetaxel plus lobaplatin in treating anthracyclines
resistant advanced breast cancer. Xian Dai Zhong Liu Xue Za Zhi.
3:473–474. 2011.(In Chinese).
|
|
13
|
Chouaid C, Atsou K, Hejblum G and
Vergnenegre A: Economics of treatments for non-small cell lung
cancer. Pharmacoeconomics. 27:113–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dancey J, Shepherd FA, Gralla RJ and Kim
YS: Quality of life assessment of second-line docetaxel versus best
supportive care in patients with non-small-cell lung cancer
previously treated with platinum-based chemotherapy: results of a
prospective, randomized phase III trial. Lung Cancer. 43:183–194.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davies AM, Lara PN Jr, Mack PC and Gandara
DR: Docetaxel in non-small cell lung cancer: a review. Expert Opin
Pharmacother. 4:553–565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nabholtz JM and Crown J: Phase III studies
of single-agent docetaxel in patients with metastatic breast cancer
who have progressed despite previous chemotherapy regimens:
preliminary results. Semin Oncol. 25:4–9. 1998.PubMed/NCBI
|
|
17
|
Fiebig HH, Henß H, Mross K, et al: Phase I
clinical trial of lobaplatin (D-19466) after intravenous bolus
injection. Onkologie. 17:142–148. 1994. View Article : Google Scholar
|
|
18
|
Manegold C, Drings P, Gatzemeier U, Pawel
Jv, Fiebig HH, Queißer W and Edler L: Lobaplatin (D-19466) in
Patients with Advanced Non-Small-Cell Lung Cancer: A Trial of the
Association for Medical Oncology (AIO) Phase II Study Group.
Onkologie. 19:248–251. 1996. View Article : Google Scholar
|
|
19
|
Degardin M, Armand JP, Chevallier B,
Cappelaere P, Lentz MA, David M and Roché H: A clinical screening
cooperative group phase II evaluation of lobaplatin (ASTA D-19466)
in advanced head and neck cancer. Invest New Drugs. 13:253–255.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kavanagh JJ, Edwards CL, Freedman RS, et
al: A trial of lobaplatin (D-19466) in platinum-resistant ovarian
cancer. Gynecol Oncol. 58:106–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huisman C, Smit EF, Giaccone G and Postmus
PE: Second-line chemotherapy in relapsing or refractory
non-small-cell lung cancer: a review. J Clin Oncol. 18:3722–3730.
2000.PubMed/NCBI
|
|
22
|
Lai CL, Tsai CM, Chiu CH, Wang GS, Su WJ,
Chen YM and Perng RP: Phase II randomized trial of tri-weekly
versus days 1 and 8 weekly docetaxel as a second-line treatment of
advanced non-small cell lung cancer. Jpn J Clin Oncol. 35:700–706.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kunitoh H, Watanabe K, Onoshi T, Furuse K,
Niitani H and Taguchi T: Phase II trial of docetaxel in previously
untreated advanced non-small-cell lung cancer: a Japanese
cooperativestudy. J Clin Oncol. 14:1649–1655. 1996.PubMed/NCBI
|
|
24
|
Nakamura Y, Kunitoh H, Kubota K, Sekine I,
Yamamoto N, Tamura T, et al: Retrospective analysis of safety and
efficacy of low-dose docetaxel 60 mg/m2 in advanced non-small cell
lung cancer patients previously treated with platinum-based
chemotherapy. Am J Clin Oncol. 26:459–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin Q, Gao XS, Qiao XY, et al: Phase I
trial of escalating-dose cisplatin with 5-fluorouracil and
concurrent radiotherapy in Chinese patients with esophageal cancer.
Acta Med Okayama. 62:37–44. 2008.PubMed/NCBI
|
|
26
|
Lin Q, Liu YE, Chang CL, et al: Phase I
trial of dose escalation of capecitabine combined with fixed
docetaxel in previously treated patients with non-small cell lung
cancer. Chinese-German Journal of Clinical Oncology. 11:6–10. 2012.
View Article : Google Scholar
|
|
27
|
Ardizzoni A, Tiseo M, Boni L, et al:
Pemetrexed versus pemetrexed and carboplatin as second-line
chemotherapy in advanced non-small-cell lung cancer: results of the
GOIRC 02-2006 randomized phase II study and pooled analysis with
the NVALT7 trial. J Clin Oncol. 30:4501–4507. PubMed/NCBI
|
|
28
|
Kim HJ, Yun J, Kim HJ, et al: Phase II
study of palliative S-1 in combination with cisplatin as
second-line chemotherapy for gemcitabine-refractory pancreatic
cancer patients. Oncol Lett. 3:1314–1318. PubMed/NCBI
|
|
29
|
DCTD, NCI, NIH and DHHS. Cancer Therapy
Evaluation Program, Common Toxicity Criteria for Adverse Events,
Version 3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Accessed October 30, 2014
|
|
30
|
Ratain MJ, Mick R, Schilsky RL and Siegler
M: Statistical and ethical issues in the design and conduct of
phase I and II clinical trials of new anticancer agents. J Natl
Cancer Inst. 85:1637–1643. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
32
|
Cho KH, Ahn SJ, Pyo HR, et al: A Phase II
study of synchronous three-dimensional conformal boost to the gross
tumor volume for patients with unresectable Stage III
non-small-cell lung cancer: results of Korean Radiation Oncology
Group 0301 study. Int J Radiat Oncol Biol Phys. 74:1397–1404. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ali I, Wani WA, Saleem K and Haque A:
Platinum compounds: a hope for future cancer chemotherapy.
Anticancer Agents in Med Chem. 13:296–306. 2013. View Article : Google Scholar
|
|
34
|
Wheate NJ, Walker S, Craig GE and Oun R:
The status of platinum anticancer drugs in the clinic and in
clinical trials. Dalton Trans. 39:8113–8127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cortes JE and Pazdur R: Docetaxel. J Clin
Oncol. 13:2643–2655. 1995.PubMed/NCBI
|
|
36
|
Yang LQ, Shi Y, Qin SK, et al: Clinical
study of Lobaplatin combined with Navelbine for advanced non-small
cell lung cancer. Lin Chuang Zhong Liu Xue Za Zhi. 12:890–894.
2006.(In Chinese).
|
|
37
|
He AB, Luo YX, Wang Q, Tong WX and Liu AH:
The efficacy of lobaplatin combined with docetaxel versus docetaxel
alone as the second-line treatment for advanced non-small cell lung
cancer. Lin Chuang Zhong Liu Xue Za Zhi. 10:923–926. 2012.(In
Chinese).
|
|
38
|
Weiss JM and Stinchcombe TE: Second-Line
Therapy for Advanced NSCLC. Oncologist. 18:947–953. 2013.
View Article : Google Scholar : PubMed/NCBI
|