Introduction
Hepatocellular carcinoma (HCC) is the fifth most
prevalent form of cancer and the third leading cause of
cancer-associated mortality worldwide (1). Tumor cells escape from immune-cell
recognition and antitumor immune responses using numerous
strategies (2). Alterations in
human leukocyte antigen (HLA) expression, including HLA total loss,
HLA haplotype loss, HLA-specific locus downregulation, HLA allelic
losses and a combination of these phenotypes, are mechanisms widely
used by tumor cells, and are critical in the development and
progression of various types of malignancy (3). The non-classical HLA-class I
molecules, including HLA-E, HLA-G and HLA-F, function as potential
immunosuppressive molecules, directly or indirectly interacting
with various types of immune cell, including natural killer (NK)
cells, T cells, monocytes, macrophages and dendritic cells, to
achieve immunosuppression (4,5). A
number of studies have revealed alterations in HLA-G and HLA-E
expression in >30 types of malignant tumor, including ovarian
cancer, breast cancer, colon cancer, pituitary tumors and leukemia,
and these changes were associated with poor patient survival
(6). Recently, HLA-F has been
widely investigated. Lepin et al (7) demonstrated that HLA-F/β2-tetramers
bind to the immune inhibitory receptor immunoglobulin-like
transcripts (ILT)-2 and ILT-4, indicating a potential role for
HLA-F in the regulation of immune cell functions. A study by Zhang
et al (8) indicated that the
HLA-F*01:04 allele is associated with the risk of HCC
pathogenesis. Furthermore, Noguchi et al (9) reported that anti-HLA-F IgG antibodies
were present in sera derived from HCC patients.
However, thus far, no studies have been conducted
with regard to the clinical relevance of HLA-F expression in HCC.
In the present study, HLA-F expression in HCC was analyzed by
immunohistochemistry, and its correlation with clinicopathological
parameters and patient outcome were evaluated.
Patients and methods
Patients and specimens
A total of 90 primary tumor lesions and 55
case-matched adjacent normal liver tissue samples were
consecutively collected from HCC patients undergoing curative
resection at Taizhou Hospital of Zhejiang Province, Wenzhou Medical
University (Linhai, China) between September 12, 2005 and October
12, 2011. A total of 78 male and 12 female patients with a median
age of 53 years (range: 12 years to 74 years) were enrolled on this
study. None of the patients had received preoperative radiotherapy,
chemotherapy or any other medical intervention. The
clinicopathological findings were determined according to the World
Health Organization criteria (10)
and the seventh edition of the tumor-node metastasis (TNM)
classification of the American joint committee on cancer (11). The patient data collected included
information regarding age, gender, tumor diameter, lymphatic or
venous invasion, clinical tumor stage, date of initial diagnosis,
and the date of fatality from HCC or the date of the last
follow-up. Among the patients, 62.2% (56/90) were diagnosed with
TNM stage I, 7.8% (7/90) were TNM stage II, 30.0% (27/90) were TNM
stage III and no case was stage IV. Of the 90 cases, 56 were
suitable for follow-up. The follow-up period was 60 months or until
the patient succumbed to the disease. The average follow-up for all
patients was 33.6 months (range, 8–60 months) and during the entire
period, 30 cancer-associated fatalities (53.6%) were recorded. The
study was performed after the Ethics Review Board of Taizhou
Hopsital of Zheijiang Province approved the study procedure to
investigate the molecular markers associated with HCC pathogenesis
and informed consent was obtained from all patients.
Immunohistochemistry and staining
evaluation
Immunohistochemistry was performed according to
standard methods as previously described (12). The 14670-1-AP rabbit polyclonal
anti-human HLA-F antibody (1:300; Proteintec Group, Chicago, IL,
USA) was used to probe for the expression of HLA-F overnight at
4°C. Goat polyclonal polyperoxidase anti-mouse and anti-rabbit IgG
(Dako, Glostrup, Denmark) secondary antibody was then applied for
30 min at 37°C. Diaminobenzidine solution was used as a chromogen.
Finally, sections were counterstained with hematoxylin and mounted
with glycerol gelatin (Zhongshan Biological Technology Co., Ltd.,
Beijing, China). The extent of HLA-F staining in the HCC tissues
was determined by three independent pathologists, who were blinded
to the clinical data and the disease outcome. The percentage of
HLA-F-positive tumor cells was assessed by each observer and the
average of the scores was recorded as the final result. A lesion
was scored as positive when the percentage of HLA-F-positive tumor
cells in the entire lesion was >5% and negative when the
percentage was ≤5%. Both membrane and cytoplasmic expression of
HLA-F were interpreted as positive. The percentage of positive
cells was assigned a value as determined by the presence or absence
of HLA-F staining, regardless of the staining intensity.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Chicago, IL, USA). Correlations between HLA-F
expression and clinical parameters were calculated using the
Pearson χ2 test. The overall patient survival time was
calculated as the time period between the date of diagnosis and the
date of last follow up (censored) or date of patient mortality
(event). The survival probabilities were determined using the
Kaplan-Meier method and statistical significance was calculated
using the log-rank test. The correlations between survival time and
multiple clinicopathological variables in univariate and
multivariate analysis were calculated using Cox regression
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
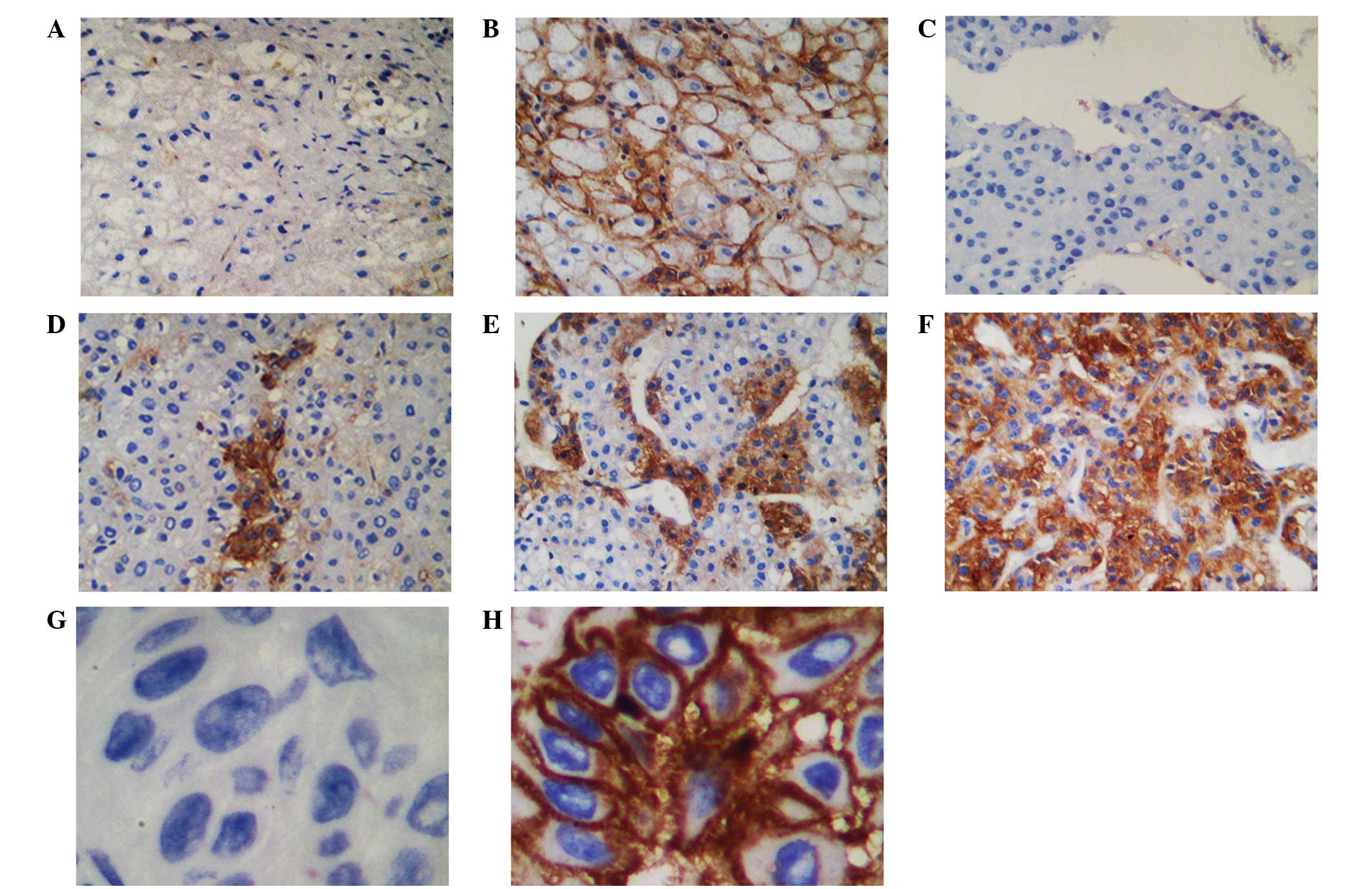
HLA-F expression in primary HCC lesions
and normal liver tissues
The membrane and cytoplasmic expression of HLA-F in
the specimens was determined through immunohistochemical staining.
The intensity of staining varied among tumors and among tumor areas
within the same specimen. Heterogeneous staining was observed in
all HLA-F-positive HCC lesions. Lesions from skin cancer patients
treated at Taizhou Hopsital of Zhejiang Province served as internal
positive and negative (with isotype IgG1) controls for HLA-F
expression. HLA-F expression was observed in 47.8% (43/90) of the
HCC lesions and in 10.9% (6/55) of the normal liver tissues
(Fig. 1; χ2=20.741,
P<0.05).
HLA-F expression in HCC lesions relative
to clinicopathological parameters
The data revealed that venous or lymphatic invasion
(χ2=5.388, P=0.020), and patient gender
(χ2=5.371, P=0.020) were significantly associated with
positive HLA-F expression. However, no significant differences in
HLA-F expression were observed between other clinical parameters,
such as patient age (χ2=0.156, P=0.693), tumor diameter
(χ2=0.002, P=0.962) and TNM stage (χ2=0.584,
P=0.445) (Table I).
 | Table IAssociation of HCC lesion HLA-F
expression with patient clinicopathological parametersa. |
Table I
Association of HCC lesion HLA-F
expression with patient clinicopathological parametersa.
| | HLA-F expression |
|---|
| |
|
|---|
| Variable | No. of cases | Negative (%) | Positive (%) | χ2 | P-value |
|---|
| Total | 90 | 47 (52.2) | 43 (47.8) | | |
| Gender | | | | 5.371 | 0.020 |
| Male | 78 | 37 (47.4) | 41 (52.6) | | |
| Female | 12 | 10 (83.3) | 2 (16.7) | | |
| Age (years) | | | | 0.156 | 0.693 |
| ≤53 | 48 | 26 (54.2) | 22 (45.8) | | |
| >53 | 42 | 21 | 21 | | |
| T factor (cm) | | | | 0.002 | 0.962 |
| ≤5 | 50 | 26 (52.0) | 24 (48.0) | | |
| >5 | 40 | 21 (52.5) | 19 (47.5) | | |
| V/Ly factor | | | | 5.388 | 0.020 |
| Yes | 72 | 42 (58.3) | 30 (41.7) | | |
| No | 18 | 5 (27.7) | 13 (72.3) | | |
| TNM stage | | | | 0.584 | 0.445 |
| I | 56 | 31 (55.4) | 25 (44.6) | | |
| II/III | 34 | 16 (47.1) | 8 (52.9) | | |
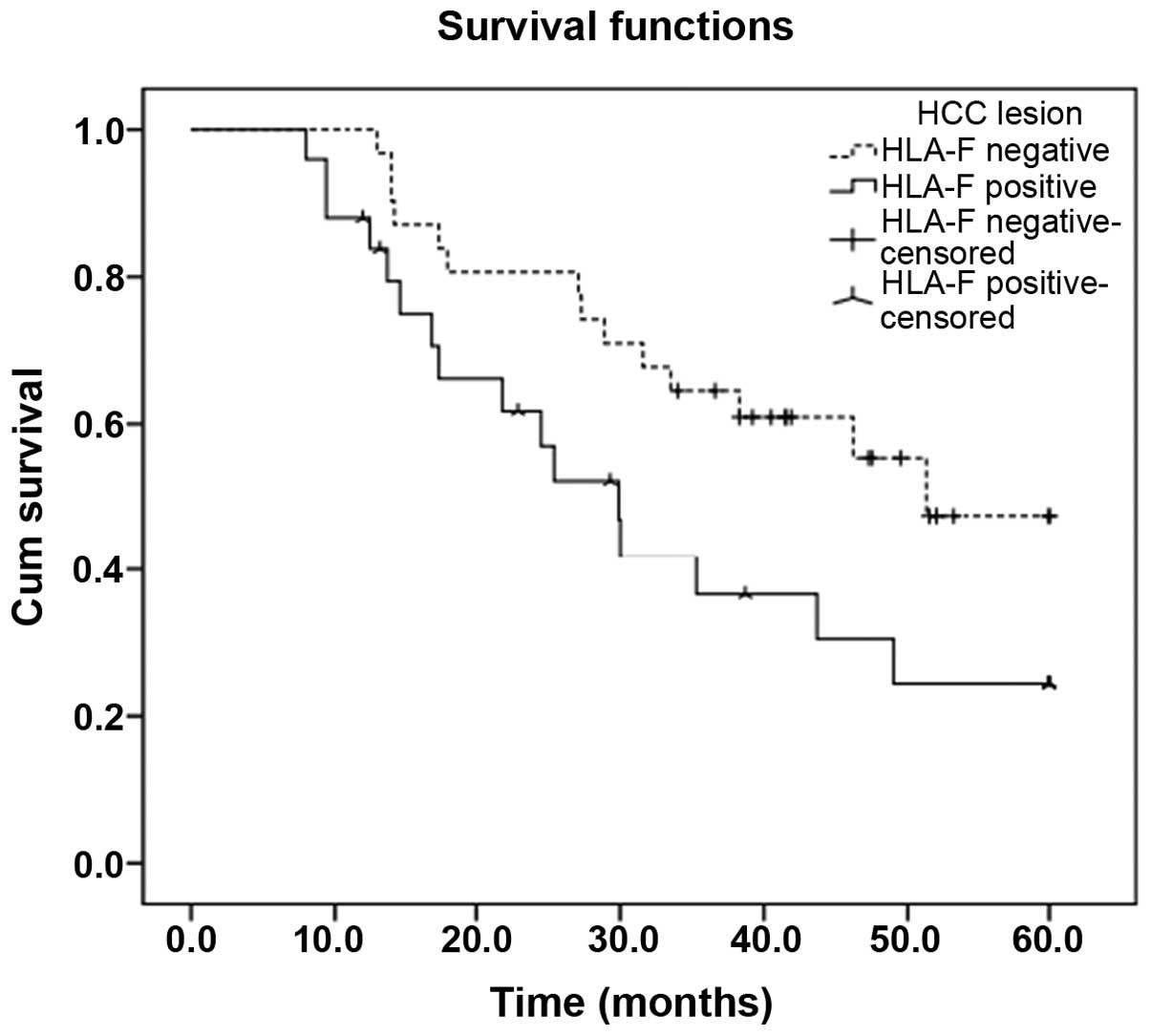
HLA-F expression is associated with
survival times in HCC patients
Patient survival times were defined as the duration
from the date of diagnosis to the date of death. Patients with
HLA-F-positive primary tumors exhibited significantly shorter
survival times than patients with HLA-F-negative tumors
(χ2=4.210, P=0.04; Fig.
2). The mean survival time of the HLA-F-positive HCC patients
was 33.0 months [95% confidence interval (CI), 25.1–40.8 months],
which was significantly shorter than that of the HLA-F-negative HCC
patients [44.2 months (95% CI, 37.7–50.7 months)] (P=0.040;
Fig. 2). In addition, Cox
proportional-hazards model analysis was performed to assess the
prognostic parameters in patients with HCC. In the univariate
analysis, HLA-F-positive expression (HR, 3.061; P=0.012) and TNM
stages II/III (HR, 1.632; P=0.033) had significantly higher hazard
ratios than HLF-A-negative expression and TNM stage I,
respectively, indicating a poor prognosis. Furthermore,
multivariate analysis revealed that positive HLA-F expression was
an independent prognostic factor (HR, 2.149; P=0.039; Table II).
 | Table IICox proportional-hazards model
analysis of variables affecting survival rates in HCC
patientsa. |
Table II
Cox proportional-hazards model
analysis of variables affecting survival rates in HCC
patientsa.
| | Overall survival
rate |
|---|
| |
|
|---|
| | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|---|
| Variable | Category | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Gender | Male (vs.
female) | 2.024
(0.576–7.108) | 0.271 | | |
| Age (years) | >53 (vs. ≤53) | 0.504
(0.207–1.225) | 0.130 | | |
| T factor (cm) | >5 (vs. ≤5) | 1.871
(0.893–3.919) | 0.097 | | |
| V/Ly factor | Yes (vs. no) | 0.744
(0.247–2.246) | 0.600 | | |
| TNM stage | Stage II/III (vs.
I) | 1.632
(1.041–2.560) | 0.033 | 1.558
(1.038–2.338) | 0.032 |
| Lesion HLA-F | Positive (vs.
negative) | 3.061
(1.285–7.295) | 0.012 | 2.149
(1.040–4.441) | 0.039 |
Discussion
In the present study, HLA-F was observed to be more
frequently expressed in HCC lesions than in normal adjacent tissue
sections. Notably, HLA-F expression was significantly correlated
with the degree of lymphatic or venous invasion. In addition, HLA-F
expression was an independent prognostic factor for HCC
patients.
HLA-F expression is exhibited in different manners
in various types of tumor. In non-small-cell lung cancer (NSCLC)
patients (12), HLA-F expression
was detected in 24.1% (20/83) of NSCLC primary lesions but not in
any of the adjacent normal lung tissues. Furthermore, HLA-F
expression was not significantly associated with certain clinical
parameters, such as patient age, gender, tumor histological type,
tumor diameter, grade of tumor differentiation or TNM stage.
However, patients with HLA-F-positive tumors had a significantly
poorer prognosis than those who were HLA-F-negative; thus, HLA-F
expression status was an independent prognostic factor for NSCLC
patients. In esophageal squamous cell carcinoma (ESCC) patients
(13), positive HLA-F expression
was observed not only in tumor lesions (58.1%) but also in the
corresponding adjacent normal esophageal tissues (54.8%). ESCC
patients with HLA-F-positive tumors also had worse survival times
than patients with HLA-F-negative tumors. Zhang et al
(14) analyzed HLA-F expression in
277 primary gastric cancer (GC) lesions and HLA-F expression was
observed in 71.1% (197/277) of the patients. The authors found that
lesion HLA-F expression was not associated with clinical
parameters, such as gender, age or disease TNM stage and, unlike in
NSCLC and ESCC patients, HLA-F expression was not associated with
GC patient prognosis, and therefore may exert a cancer-type
dependent effects.
In the present study, the HLA-F expression in the
HCC lesions was significantly correlated with the lymphatic or
venous invasion. Ishigami et al (15) also demonstrated that HLA-F
expression in GC lesions was significantly associated with
lymphatic and venous invasion, as well as depth of invasion and
nodal involvement. Thus, HLA-F expression may be associated with
aggressive tumor behavior, and the promotion of tumor cell invasion
and metastasis. However, further studies are required to
investigate this hypothesis.
HLA-F, which is encoded by a gene located on the
short arm of human chromosome 6, was first identified by Geraghty
in 1990. The HLA-F protein is 5.4 kb in length and is highly
homologous with other types of HLA-I molecule (16,17).
HLA-F is shorter than a typical HLA class I protein due to the
exclusion of exon 7 from the mature HLA-F mRNA, resulting in a
protein with a shortened cytoplasmic domain (16). HLA-F was found to be entirely
dependent on its cytoplasmic tail for export from the endoplasmic
reticulum, with the assistance of the C-terminal valine residue and
the RxR motif (18). However,
whether HLA-F is expressed on the cell surface remains
controversial. Early studies indicated that HLA-F exhibits
predominantly intracellular expression, although this was only
observed in the cytoplasm of peripheral blood B cells, B cell
lines, tissues containing B cells, and various other types of
tissues and cell lines, such as bladder and skin cell lines
(7,19). Later studies observed that HLA-F
protein may be expressed at the surface of certain cell types; for
example, EBV-transformed lymphoblastoid cell lines or particular
monocyte cell lines, extravillous trophoblast cells invading the
decidua in term placental tissues, and activated B cells, T cells,
NK cells and monocytes (20–22).
The present study revealed both membrane and cytoplasmic expression
of HLA-F.
Studies concerning the function of HLA-F have rarely
been reported. A previous study (7)
demonstrated that HLA-F tetramers were capable of interacting with
the inhibitory receptors ILT-2 and ILT-4, indicating a mechanism by
which HLA-F exerts an immune tolerance function. Goodridge et
al (23) revealed that HLA-F
and the open conformers of major histocompatibility complex I
(MHC-I) expressed on activated cells cooperate in a MHC-I antigen
cross-presentation pathway for the presentation of exogenous
proteins by MHC-I, which may significantly contribute to the
regulation of immune system functions and immune defense. HLA-F has
also been shown to be involved in maternal-fetal tolerance. Shobu
et al (24) indicated that
HLA-F may function together with HLA-G/E to prepare an environment
to support fetal growth. We hypothesize that HLA-F expression may
also enable tumor cells to escape from recognition by the host
immune system.
In conclusion, in the present study, positive HLA-F
expression was associated with poor survival in HCC patients, and
may be correlated with invasion and metastasis of tumor cells. This
finding provides a novel area for the analysis of HCC. However, the
biological functions and clinical significance of HLA-F are far
from established, and further investigation into HLA-F expression
is urgently required.
Acknowledgements
This study was supported by the Science Technology
Program of Zhejiang Province on the Scientific Research Project
(grant no. 2009C33096) and the Zhejiang Provincial Health
Department Project (grant nos. 2009A220, 2014KYA227 and
2014KYB308).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reiman JM, Kmieciak M, Manjili MH and
Knutson KL: Tumor immunoediting and immunosculpting pathways to
cancer progression. Semin Cancer Biol. 17:275–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smyth MJ, Dunn GP and Schreiber RD: Cancer
immunosurveillance and immunoediting: The roles of immunity in
suppressing tumor development and shaping tumor immunogenicity. Adv
Immunol. 90:1–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carosella ED, HoWangYin KY, Favier B and
LeMaoult J: HLA-G-dependent suppressor cells: Diverse by nature,
function, and significance. Hum Immunol. 69:700–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pietra G, Romagnani C, Manzini C, Moretta
L and Mingari MC: The emerging role of HLA-E-restricted
CD8+ T lymphocytes in the adaptive immune response to
pathogens and tumors. J Biomed Biotechnol. 2010:9070922010.
View Article : Google Scholar
|
|
6
|
Yan WH: HLA-G expression in hematologic
malignancies. Expert Rev Hematol. 3:67–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lepin EJ, Bastin JM, Allan DS, et al:
Functional characterization of HLA-F and binding of HLA-F tetramers
to ILT2 and ILT4 receptors. Eur J Immunol. 30:3552–3561. 2000.
View Article : Google Scholar
|
|
8
|
Zhang J, Pan L, Chen L, Feng X, Zhou L and
Zheng S: Non-classical MHC-Iota genes in chronic hepatitis B and
hepatocellular carcinoma. Immunogenetics. 64:251–258. 2012.
View Article : Google Scholar
|
|
9
|
Noguchi K, Isogai M, Kuwada E, Noguchi A,
Goto S and Egawa K: Detection of anti-HLA-F antibodies in sera from
cancer patients. Anticancer Res. 24:3387–3392. 2004.PubMed/NCBI
|
|
10
|
Ishak KG, Anthony PP and Sobin LH:
Histological Typing of Tumors of the Liver. 2nd edition. Springer;
Berlin: 1994, View Article : Google Scholar
|
|
11
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin A, Zhang X, Ruan YY, Wang Q, Zhou WJ
and Yan WH: HLA-F expression is a prognostic factor in patients
with non-small-cell lung cancer. Lung Cancer. 74:504–509. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Lin A, Zhang JG, et al:
Alteration of HLA-F and HLA I antigen expression in the tumor is
associated with survival in patients with esophageal squamous cell
carcinoma. Int J Cancer. 132:82–89. 2013. View Article : Google Scholar
|
|
14
|
Zhang JG, Zhang X, Lin A and Yan WH:
Lesion HLA-F expression is irrelevant to prognosis for patients
with gastric cancer. Hum Immunol. 74:828–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishigami S, Arigami T, Setoyama T, et al:
Clinical-pathological implication of human leukocyte
antigen-F-positive gastric adenocarcinoma. J Surg Res. 184:802–806.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geraghty DE, Wei XH, Orr HT and Koller BH:
Human leukocyte antigen F (HLA-F). An expressed HLA gene composed
of a class I coding sequence linked to a novel transcribed
repetitive element. J Exp Med. 171:1–18. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geraghty DE: Structure of the HLA class I
region and expression of its resident genes. Curr Opin Immunol.
5:3–7. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boyle LH, Gillingham AK, Munro S and
Trowsdale J: Selective export of HLA-F by its cytoplasmic tail. J
Immunol. 176:6464–6472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wainwright SD, Biro PA and Holmes CH:
HLA-F is a predominantly empty, intracellular, TAP-associated MHC
class Ib protein with a restricted expression pattern. J Immunol.
164:319–328. 2000. View Article : Google Scholar
|
|
20
|
Lee N and Geraghty DE: HLA-F surface
expression on B cell and monocyte cell lines is partially
independent from tapasin and completely independent from TAP. J
Immunol. 171:5264–5271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee N, Ishitani A and Geraghty DE: HLA-F
is a surface marker on activated lymphocytes. Eur J Immunol.
40:2308–2318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishitani A, Sageshima N, Lee N, et al:
Protein expression and peptide binding suggest unique and
interacting functional roles for HLA-E, F, and G in
maternal-placental immune recognition. J Immunol. 171:1376–1384.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goodridge JP, Lee N, Burian A, et al:
HLA-F and MHC-I open conformers cooperate in a MHC-I antigen
cross-presentation pathway. J Immunol. 191:1567–1577. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shobu T, Sageshima N, Tokui H, et al: The
surface expression of HLA-F on decidual trophoblasts increases from
mid to term gestation. J Reprod Immunol. 72:18–32. 2006. View Article : Google Scholar : PubMed/NCBI
|