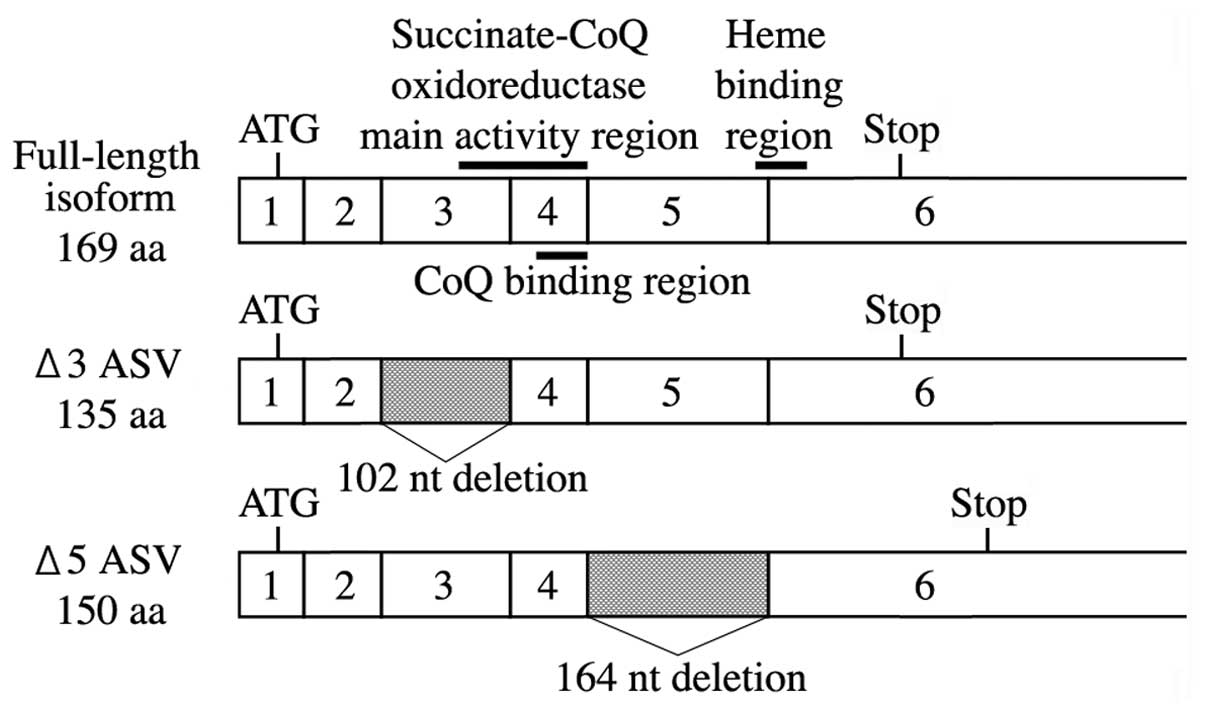
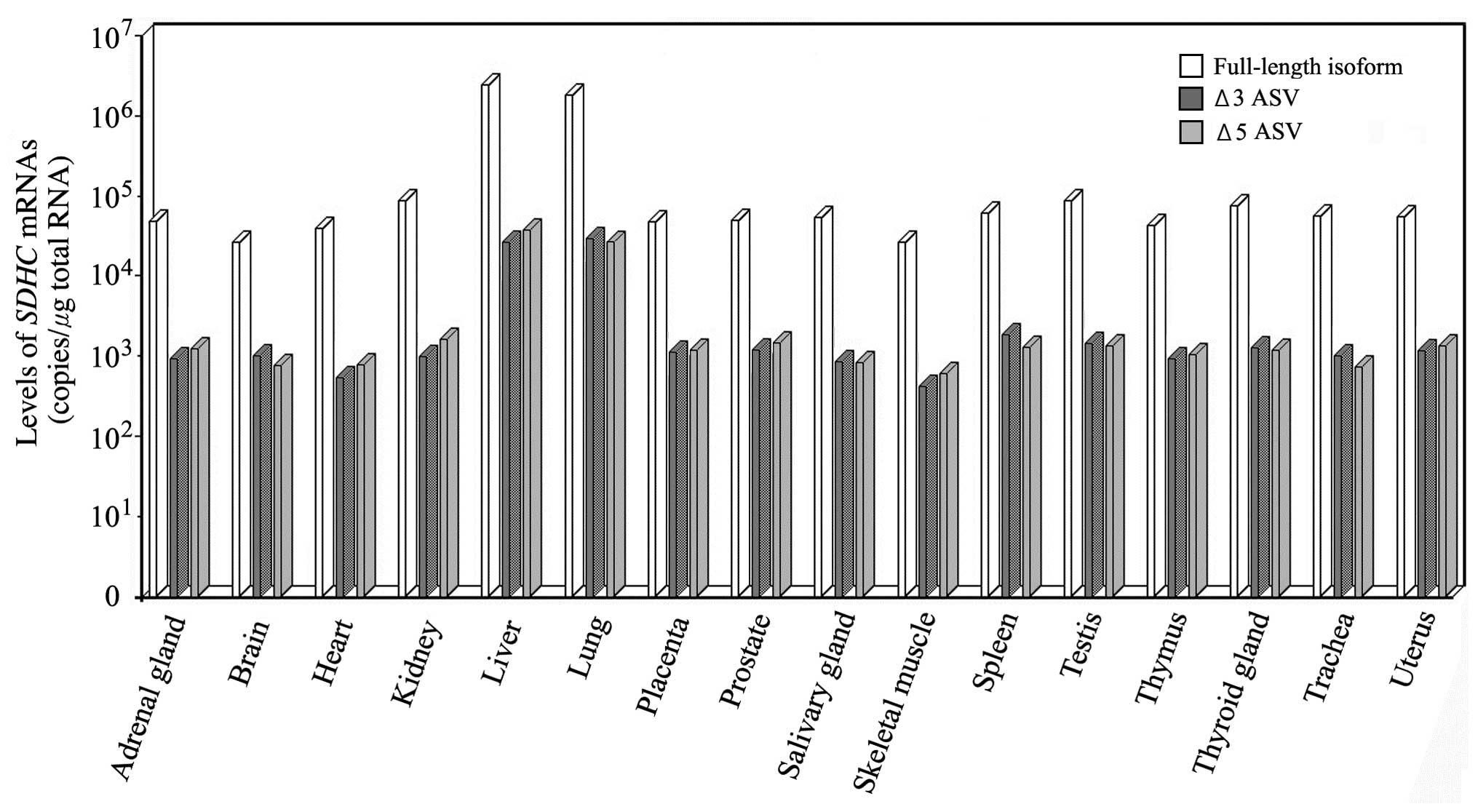
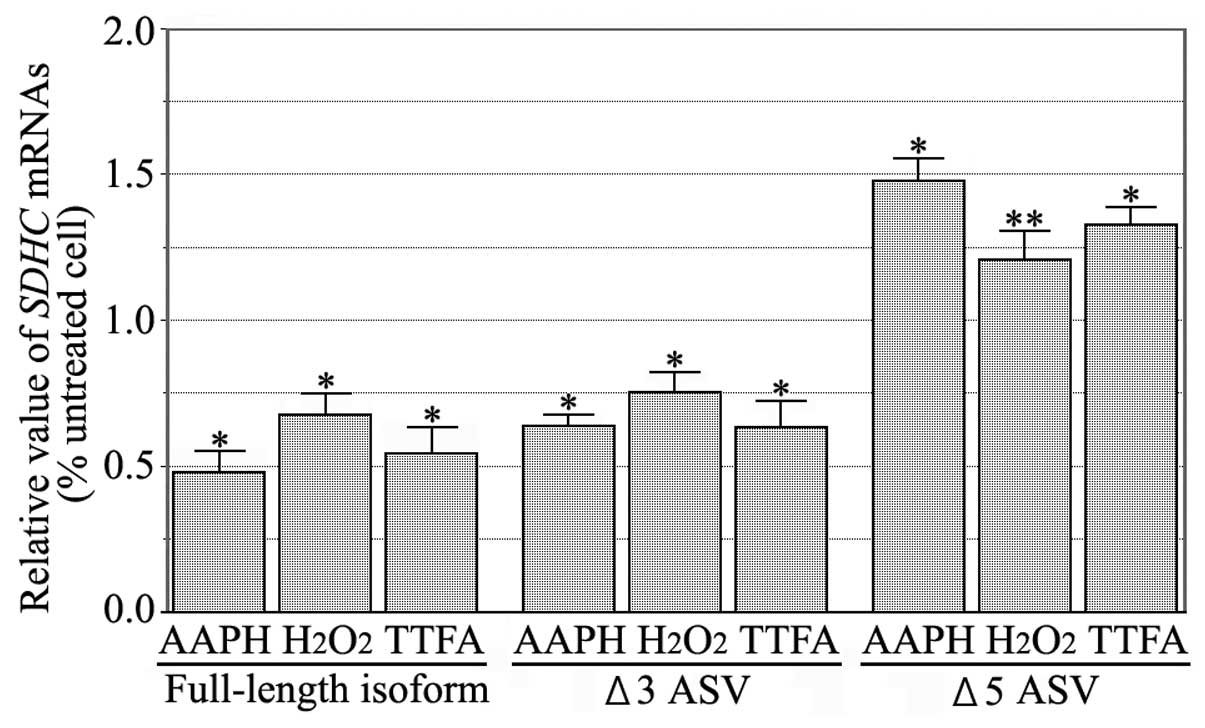
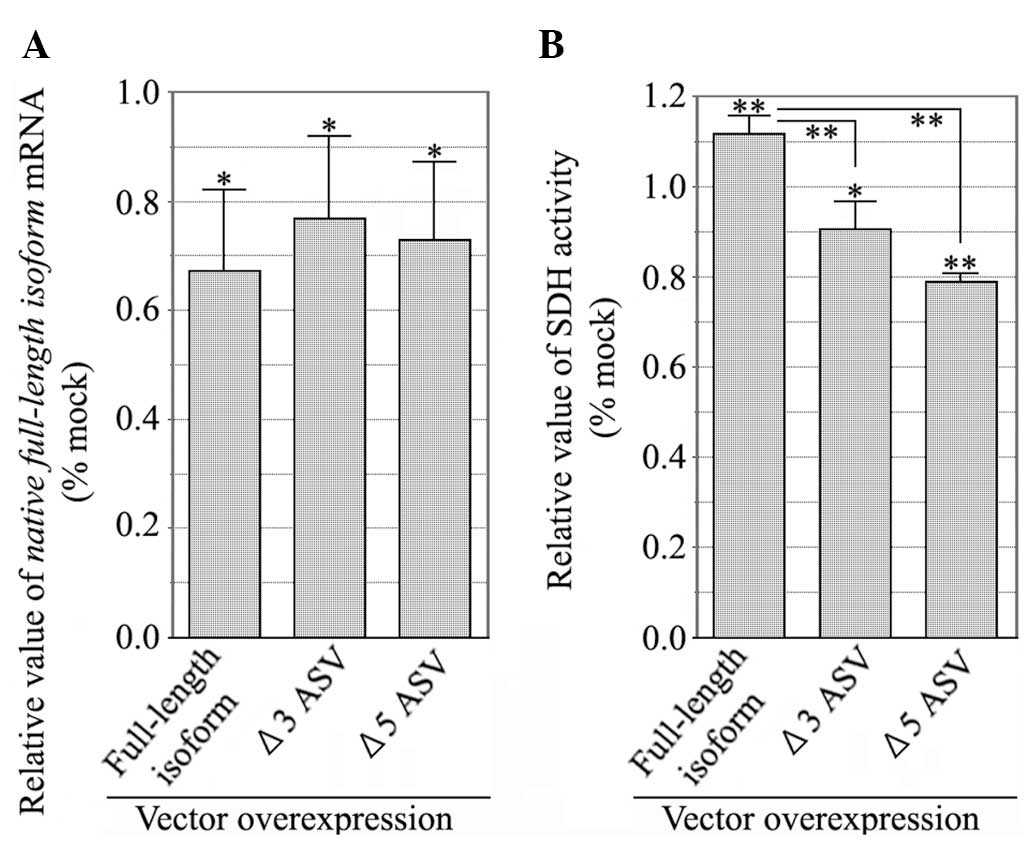
|
1
|
López-Jiménez E, de Campos JM, Kusak EM,
et al: SDHC mutation in an elderly patient without familial
antecedents. Clin Endocrinol (Oxf). 69:906–910. 2008. View Article : Google Scholar
|
|
2
|
Zaunmüller T, Kelly DJ, Glöckner FO and
Unden G: Succinate dehydrogenase functioning by a reverse redox
loop mechanism and fumarate reductase in sulphate-reducing
bacteria. Microbiology. 152:2443–2453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selak MA, Armour SM, MacKenzie ED, et al:
Succinate links TCA cycle dysfunction to oncogenesis by inhibiting
HIF-alpha prolyl hydroxylase. Cancer Cell. 7:77–85. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong LF, Low P, Dyason JC, et al:
Alpha-tocopheryl succinate induces apoptosis by targeting
ubiquinone-binding sites in mitochondrial respiratory complex II.
Oncogene. 27:4324–4335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Nederveen FH, Gaal J, Favier J, et al:
An immunohistochemical procedure to detect patients with
paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or
SDHD gene mutations: a retrospective and prospective analysis.
Lancet Oncol. 10:764–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peczkowska M, Cascon A, Prejbisz A, et al:
Extra-adrenal and adrenal pheochromocytomas associated with a
germline SDHC mutation. Nat Clin Pract Endocrinol Metab. 4:111–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aratake K, Kamachi M, Iwanaga N, et al: A
cross-talk between RNA splicing and signaling pathway alters Fas
gene expression at post-transcriptional level: alternative splicing
of Fas mRNA in the leukemic U937 cells. J Lab Clin Med.
146:184–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niemann S and Müller U: Mutations in SDHC
cause autosomal dominant paraganglioma, type 3. Nat Genet.
26:268–270. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schiavi F, Boedeker CC, Bausch B, et al;
European-American Paraganglioma Study Group. Predictors and
prevalence of paraganglioma syndrome associated with mutations of
the SDHC gene. JAMA. 294:2057–2063. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanke J, Brett D, Zastrow I, et al:
Alternative splicing of human genes more the rule than the
exception? Trends Genet. 15:389–390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Qian C and Francke U: Silent
mutation induces exon skipping of fibrillin-1 gene in Marfan
syndrome. Nat Genet. 16:328–329. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stallings-Mann ML, Ludwiczak RL, Klinger
KW and Rottman F: Alternative splicing of exon 3 of the human
growth hormone receptor is the result of an unusual genetic
polymorphism. Pro Natl Acad Sci USA. 93:12394–12399. 1996.
View Article : Google Scholar
|
|
13
|
Hirawake H, Taniwaki M, Tamura A, et al:
Cytochrome b in human complex II (succinate-ubiquinone
oxidoreductase): cDNA cloning of the components in liver
mitochondria and chromosome assignment of the genes for the large
(SDHC) and small (SDHD) subunits to 1q21 and 11q23. Cytogenet Cell
Genet. 79:132–138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maklashina E, Rajagukguk S, McIntire WS
and Cecchini G: Mutation of the heme axial ligand of Escherichia
coli succinate-quinone reductase: implications for heme ligation in
mitochondrial complex II from yeast. Biochim Biophys Acta.
1797:747–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishii N: Role of oxidative stress from
mitochondria on aging and cancer. Cornea. 26(9 Suppl 1): S3–S9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, McMillan-Ward E, Kong J, Israels
SJ and Gibson SB: Mitochondrial electron-transport-chain inhibitors
of complexes I and II induce autophagic cell death mediated by
reactive oxygen species. J Cell Sci. 120:4155–4166. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pasini B and Stratakis CA: SDH mutations
in tumorigenesis and inherited endocrine tumours: lesson from the
phaeochromocytoma-paraganglioma syndromes. J Intern Med. 266:19–42.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pantaleo MA, Astolfi A, Urbini M, et al:
GIST Study Group: Analysis of all subunits, SDHA, SDHB, SDHC, SDHD,
of the succinate dehydrogenase complex in KIT/PDGFRA wild-type
GIST. Eur J Hum Genet. 22:32–39. 2014. View Article : Google Scholar
|
|
19
|
Miyazawa M, Ishii T, Kirinashizawa M, et
al: Cell growth of the mouse SDHC mutant cells was suppressed by
apoptosis throughout mitochondrial pathway. Biosci Trends. 2:22–30.
2008.PubMed/NCBI
|
|
20
|
Ishii T, Miyazawa M, Onodera A, et al:
Mitochondrial reactive oxygen species generation by the SDHC V69E
mutation causes low birth weight and neonatal growth retardation.
Mitochondrion. 11:155–165. 2011. View Article : Google Scholar
|
|
21
|
Lluis JM, Buricchi F, Chiarugi P, Morales
A and Fernandez-Checa JC: Dual role of mitochondrial reactive
oxygen species in hypoxia signaling: activation of nuclear
factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer
Res. 67:7368–7377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sumimoto H: Structure, regulation and
evolution of Nox-family NADPH oxidases that produce reactive oxygen
species. FEBS J. 275:3249–3277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tietze F: Enzymic method for quantitative
determination of nanogram amounts of total and oxidized
glutathione: applications to mammalian blood and other tissues.
Anal Biochem. 27:502–522. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yankovskaya V, Sablin SO, Ramsay RR, et
al: Inhibitor probes of the quinone binding sites of mammalian
complex II and Escherichia coli fumarate reductase. J Biol Chem.
271:21020–21024. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dröse S and Brandt U: The mechanism of
mitochondrial superoxide production by the cytochrome bc1 complex.
J Biol Chem. 283:21649–21654. 2008. View Article : Google Scholar : PubMed/NCBI
|