Introduction
Esophageal cancer is the sixth most common
malignancy worldwide (1,2), responsible for ~482,300 new cases and
406,800 mortalities in 2008 (3–5). The
highest incidence rates are in South and East Africa and East Asia,
while the lowest rates are in the West and middle of Africa, and
Central America (4–6). Esophageal squamous cell carcinoma
(ESCC) accounts for >90% of cases of esophageal cancer in the
Asia-Pacific region, including China (6–8). ESCC
patients are usually diagnosed at a late stage, resulting in a poor
prognosis (9–11). Efforts to improve the early
detection of ESCC have focused on cytological or endoscopic
screening, as well as the application of genetic and epigenetic
biomarkers (8–12). Although biomarkers exhibit a high
sensitivity, they are unable to conclusively identify which
patients are at a low or high risk for disease recurrence.
Therefore, there is a requirement for novel prognostic markers and
therapeutic targets for ESCC.
MicroRNAs (miRNA) are non-coding RNA molecules
(length, 21–25 nt) that inhibit gene expression at the
transcriptional and post-transcriptional level by binding to the
3′-untranslated region (3′-UTR) of target mRNAs (12–14).
miRNAs bind to partially complementary recognition sequences of
mRNA, subsequently causing mRNA degradation or translation
inhibition and effectively silencing their target genes (15). Bioinformatic studies indicate that a
third of all of the known genes may be regulated by miRNAs. miRNAs
have been reported to participate in various important cellular
processes, such as apoptosis, cell differentiation and
proliferation, tumor suppression, development and metabolism
(14,16,17).
Recent studies have detected a large number of miRNAs by microarray
analysis or other advanced technologies (18–20).
Thus, to elucidate the underlying molecular mechanisms associated
with ESCC cell proliferation, identification of the regulatory
target genes of miRNAs is considered to be critical.
Kirsten rat sarcoma viral oncogene homolog (KRAS),
which promotes cell proliferation, was identified as a potential
target gene of miR-27a and, thus, was the focus of the present
study. The KRAS gene is a member of the mammalian ras gene family
and encodes K-ras, a member of the small guanosine triphosphatase
superfamily. An activating mutation can be caused by a single amino
acid substitution, with the resultant transforming protein
identified as an important factor in various malignancies,
including lung adenocarcinoma, mucinous adenoma, ductal carcinoma
of the pancreas and colorectal carcinoma. The present study
examined the correlation between the expression levels of miR-27a
and KRAS in ESCC patients and revealed the biological function of
miR-27a in ESCC cell lines.
Materials and methods
Cell culture
Human ESCC cell lines (TE-1, TE-10, TE-11 and
ECA-109) and human esophageal epithelial cells (HEEC), Het-1A, were
obtained from the Cell Bank of the China Academy of Sciences
(Shanghai, China). All of the human ESCC cell lines were cultured
in RPMI-1640 medium (Gibco-BRL, Gaithersburg, MD, USA). HEEC cells
were cultured in LHC-9 medium containing 10% fetal bovine serum
(HyClone Laboratories, Inc., Logan, UT, USA). All of the media were
supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin,
and the cells were cultured at 37°C in a 5% CO2
atmosphere.
Construction of recombinant expression
vectors
The predicted binding sites on the 3′-UTR of KRAS
and 741 bp of the contiguous sequences were cloned into a pGL3
Luciferase® Reporter Vector (Promega Corporation,
Madison, WI, USA) and designated as pGL3-Kras-3′-UTR. The mutation
plasmid, pGL3-Kras-3′-UTR/mut was also constructed. The coding
region sequences of KRAS and binding sequences or site mutation
sequences of miR-27a were cloned into the pcDNA3.1(−) plasmid and
termed, pcDNA3.1-Kras and pcDNA3.1-Kras/mut, respectively. The
primer sequences used in the present study are presented in
Table I. To identify miRNAs which
are differentially expressed in ESCC and the corresponding adjacent
healthy tissues, miRNA Solexa analysis was performed. The
expression level of miR-27a was significantly downregulated in ESCC
tissues, thus, the target genes of miR-27a were predicted using
TargetScan software (http://www.targetscan.org/).
 | Table IPrimer sequences for the construction
of the recombinant expression vectors used in the present
study. |
Table I
Primer sequences for the construction
of the recombinant expression vectors used in the present
study.
| Plasmid | Primer sequence | Restriction
enzyme |
|---|
| pGL3-Kras-3′-UTR | Forward:
GAGCAAAGATGGTAAAAAGA | XbaI |
| Reverse:
TAAATATAGCCCCAAAATGG | EcoRV |
|
pGL3-Kras-3′-UTR/mut | Forward:
AACTAGCAATGCGTCTCATAAAGAAACTGAATACCTAAGATTTCTGTC | |
| Reverse:
GACAGAAATCTTAGGTATTCAGTTTCTTTATGAGACGCATTGCTAGTT | |
| pcDNA3.1-Kras | Forward:
ATGACTGAATATAAACTTGTGGTAG | XhoI |
| Reverse:
ACTAGATAAAACACAGAATAGGGAT | EcoRV |
|
pcDNA3.1-Kras/mut | Forward:
AACTAGCAATGCGTCTCATAAAGAAACTGAATACCTAAGATTTCTGTC | |
| Reverse:
GACAGAAATCTTAGGTATTCAGTTTCTTTATGAGACGCATTGCTAGTT | |
| pEGFP-miR-27a | Forward:
AAGTTGCTGTAGCCTCCTTGTCC | XbaI |
| Reverse:
CCCACTCACCCACCTATCTATGC | EcoRI |
Dual-Luciferase® Reporter
assay
The Dual-Luciferase® Reporter assay
system (Promega Corporation) was used to measure the luciferase
activity of cells that had been transfected with 400 ng luciferase
vector pGL3-Kras-3′-UTR or pGL3-Kras-3′-UTR/mut and either miR-27a
mimics or miRNA-negative control (NC). To determine the
transfection efficiency, 20 ng pRL-SV-40 (Promega) was
cotransfected as the control. Reporter assays were performed at 48
h post-transfection using the Dual-Luciferase® Reporter
assay system (Promega Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cell cultures using
TRIzol reagent (Bio Basic Inc., Toronto, ON, Canada) according to
the manufacturer’s instructions. RT was performed using the the
PrimeScriptTM RT reagent Kit (Takara Biotechnology Co.,
Ltd., Dalian, China). A cDNA library of miRNAs was synthesized by
the QuantiMir™ RT kit (Takara Biotechnology Co., Ltd.). U6 small
nuclear RNA and the reference gene, 18S RNA served as the
endogenous controls for miRNA and mRNA, respectively. The target
genes and controls were treated under the same conditions and
analyzed by RT-qPCR using SYBR® Premix Ex Taq™ (Takara
Biotechnology Co., Ltd.) according to the manufacturer’s
instructions.
Western blot analysis
Protein for western blot analysis was precipitated
according to the standard protocol (15). Equal quantities of protein samples
were subjected to SDS-PAGE and transferred to a polyvinylidene
fluoride membrane. The membrane was soaked in Tris-buffered saline
and Tween-20 (TBST) buffer containing 5% low-fat milk for 60 min
with gentle agitation. The membrane was then incubated with
monclonal rabbit anti-human c-Kras (1:1,000) and mouse anti-human
GAPDH (1:1,000) antibodies (Cell Signaling Technologies, Inc.,
Danvers, MA, USA) overnight followed by washing with TBST buffer
and a further incubation with monoclonal rabbit anti-mouse and
mouse anti-rabbit secondary antibodies (1;10,000; Cell Signaling
Technologies, Inc.). Finally, an enhanced chemiluminescence reagent
kit (Thermo Scientific, Waltham, MA, USA) was used to detect of the
protein bands, which were quantified by densitometry (Image Lab™
analysis software; Bio-Rad, Hercules, CA, USA), normalized to GAPDH
and expressed as the fold of the control. The primary antibodies
used were rabbit anti-c-Kras (1:1,000) and mouse anti-GAPDH
(1:1,000). The secondary antibodies were rabbit anti-mouse
(1:10,000) and mouse anti-rabbit (1:10,000). All of the antibodies
were purchased from Cell Signaling Technology, Inc.
Cell proliferation
To investigate the effect of miR-27a on cell
proliferation, a comparison of the growth rates of ESCC cells
transduced with miR-27a or miRNA-NC was performed. Cell growth was
determined using a CellTiter 96® Aqueous One Solution
Cell Proliferation Assay kit (Promega Corporation). A total of
~5,000 cells were seeded in a 96-well plate 48 h post-transfection
and incubated at 37°C for three days. Cell growth was then detected
using a 3-(4,5-dimethylthiazol-2-yl)
5-(3-carboxymethoxyphenyl)2-(4-sulfophenyl)-2H-tetrazolium, inner
salt (MTS) reduction cell proliferation assay kit every 24 h (0,
24, 48 and 72 h). Absorbance at a wavelength of 490 nm was
determined using a microplate reader (OrionL Microplate
Luminometer; Titertek-Berthold, Pforzheim, Germany). To investigate
whether miR-27a suppresses tumor progression in vivo, TE-1
cells were transfected with pEGFP-miR-27a, and G418 was added to
the medium and the cells were cultured for one month. The resultant
TE-1 cells, which stably expressed miR-27a, were subcutaneously
implanted into nude mice to generate tumor xenograft models. Four
days after implantation, all of the animals in the control group
developed palpable tumors, compared with the mice overexpressing
miR-27a, which lacked any detectable tumors.
Subcutaneous tumor assay
A total of five six-week-old BALB/c-A nude mice were
purchased from the animal center of the Cancer Institute of the
Chinese Academy of Medical Science (Beijing, China). All
experimental procedures were conducted according to the
regulations, and the internal biosafety and bioethics guidelines of
Liaocheng Hospital (Liaocheng, China). The TE-1 subcutaneous model
was established as previously described (22). TE-1 cells stably expressing miR-27a
were injected into the mice. Treatment was conducted at four-day
intervals until completion of the experiment. The tumor volume was
measured with a caliper every four days and the following formula
was used: volume (mm3) = (length × width2)/2.
At the end of a 24-day observation period, the mice were
sacrificed, and the tumor tissues were collected for formalin
fixation and preparation of paraffin-embedded sections for
immunohistochemical analysis.
Statistical analysis
Results are expressed as the group means ± standard
error of the mean and were analyzed using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA) using unpaired t-tests
for two-group comparisons and one-way analysis of variance for
three or more group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-27a directly targets the KRAS gene by
interaction with the 3′-UTR
TargetScan (http://www.targetscan.org) and PicTar (http://pictar.mdc-berlin.de) are types of software
broadly used online to predict miRNA targets. The present study
utilized TargetScan and PicTar to predict the target miRNA of KRAS
(Fig. 1A and B) and demonstrated
that miR-27a targets the 3′-UTR of KRAS. To clarify this,
pGL3-Kras-3′-UTR containing the miR-27a binding sequences and
pGL3-Kras-3′-UTR/mut were constructed. Analysis of luciferase
activity demonstrated that the activity of miR-27a mimics that were
cotransfected with pGL3-Kras-3′-UTR was significantly more
inhibited when compared with the miRNA-NC (P<0.01). However, the
activity of miR-27a mimics that were cotransfected with
pGL3-Kras-3′-UTR/mut exhibited no significant difference when
compared with the miRNA-NC (Fig.
1C). Thus, the luciferase activity assay indicated that the
mutated 3′-UTR affected the binding of miR-27a.
Furthermore, to investigate whether miR-27a affects
KRAS expression at the transcriptional and translation levels, two
types of expression plasmid were constructed. The expression
plasmids, pcDNA3.1-Kras and pcDNA3.1-Kras/mut contain the coding
regions and 3′-UTR sequence of KRAS, however, the pcDNA3.1-Kras/mut
contains the mutated miR-27a binding sequences. Western blot
analysis demonstrated that the expression level of miR-27a
cotransfected with pcDNA3.1-Kras was markedly lower when compared
with the miRNA-NC. However, no significant difference was
identified between miR-27a mimics and miRNA-NC cotransfected with
pcDNA3.1-Kras/mut (Fig. 1D).
Finally, the endogenous KRAS was detected by RT-qPCR following
transfection of the miR-27a inhibitor into TE-10 cells or
pEGFP-miR-27a into TE-1 cells. The expression levels of miR-27a and
KRAS demonstrated negative correlation (Fig. 1E and F). These data indicated that
miR-27a directly targets KRAS in ESCC by binding to the 3′-UTR of
the KRAS gene.
Expression level of miR-27a and KRAS in
ESCC cell lines and patient tissue samples
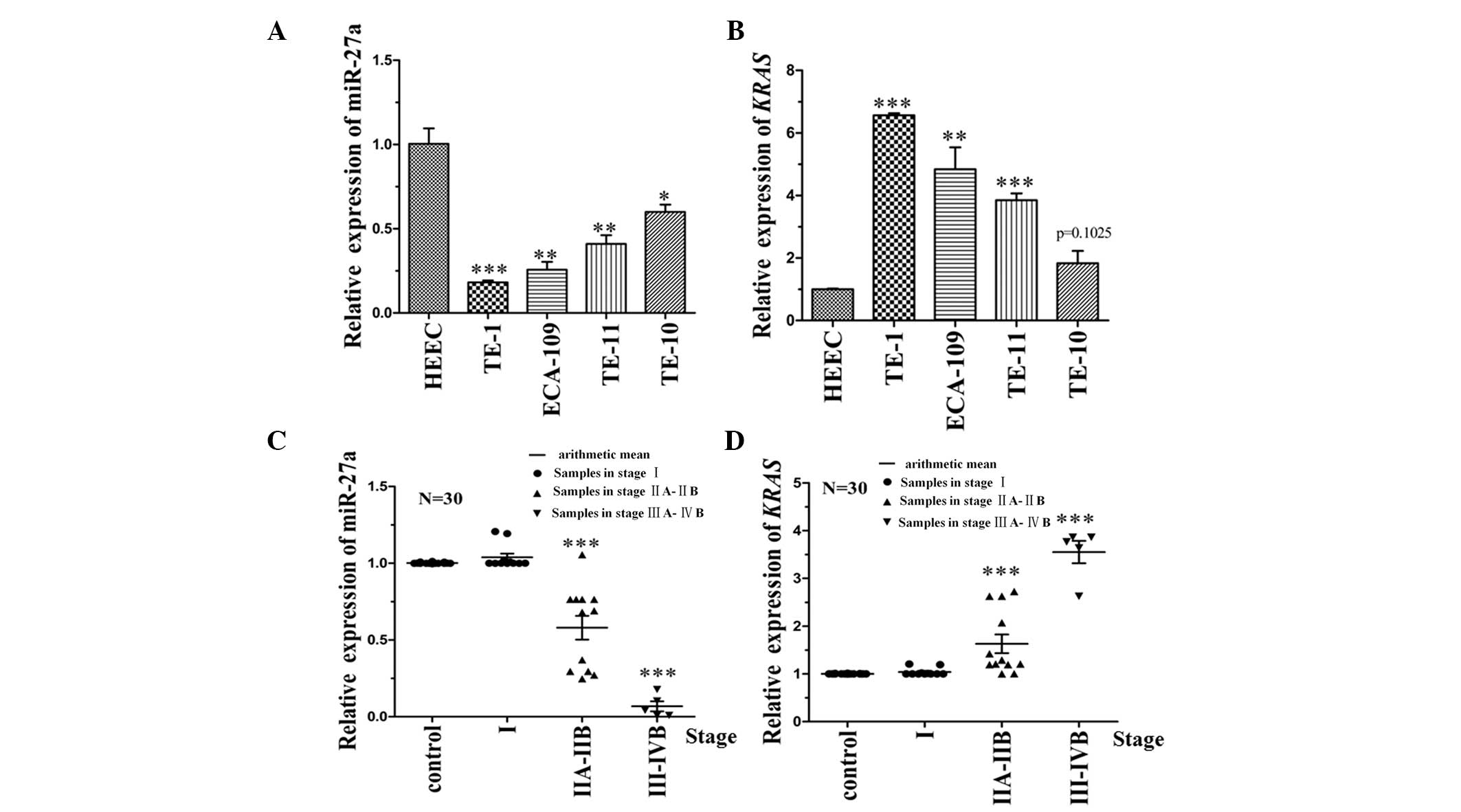
To identify the correlation between miR-27a and KRAS
expression levels in ESCC cells, RT-qPCR analysis was performed on
four different ESCC cell lines (TE-10, TE-11, TE-1 and Eca-109) and
one healthy HEEC line (Het-1A), which served as the control. The
data demonstrated that the expression level of miR-27a was
negatively correlated with KRAS (Fig.
2A and B). The expression level of miR-27a and its target,
KRAS, were also detected in 30 patient tissue samples. The
clinicopathological characteristics of the 30 patients are
indicated in Table II. The
expression level of KRAS in stage III–IVB tumor samples (23) was significantly higher than that in
stage I tissue samples (P<0.001). The corresponding target gene
(miR-27a) was negatively correlated with the KRAS expression level
(Fig. 2C and D). Thus, the results
of the present study indicated that miR-27a affects KRAS expression
levels.
 | Table IIData of the esophageal cancer
patients. |
Table II
Data of the esophageal cancer
patients.
| | | | TNM staging |
|---|
| | | |
|
|---|
| No. | Gender | Age, years | Comprehensive
stage | Tumor | Lymph node | Metastasis |
|---|
| 1 | M | 56 | IA | T1B | N0 | M0 |
| 2 | M | 56 | IA | T1B | N0 | M0 |
| 3 | M | 55 | IB | T2B | N0 | M0 |
| 4 | F | 48 | IIA | T1B | N1 | M0 |
| 5 | M | 86 | IA | T1B | N0 | M0 |
| 6 | F | 57 | IA | T1B | N0 | M0 |
| 7 | M | 62 | IIA | T1B | N1 | M0 |
| 8 | F | 54 | IIA | T1B | N1 | M0 |
| 9 | F | 59 | IIIA | T2A | N2 | M0 |
| 10 | F | 63 | IIB | T2A | N1 | M0 |
| 11 | M | 72 | IB | T2B | N0 | M0 |
| 12 | M | 61 | IIA | T1B | N1 | M0 |
| 13 | M | 78 | IIB | T2A | N1 | M0 |
| 14 | M | 66 | IIA | T2A | N1 | M0 |
| 15 | F | 45 | IB | T2A | N0 | M0 |
| 16 | F | 63 | IIA | T1B | N1 | M0 |
| 17 | F | 52 | IIIA | T3 | N2 | M0 |
| 18 | F | 54 | IB | T2B | N0 | M0 |
| 19 | M | 58 | IIIA | T3 | N2 | M0 |
| 20 | F | 64 | IB | T2A | N0 | M0 |
| 21 | F | 67 | IIA | T2A | N1 | M0 |
| 22 | M | 52 | IIA | T2A | N1 | M0 |
| 23 | M | 80 | IB | T2 | N0 | M0 |
| 24 | M | 56 | IIIB | T4 | N2 | M1 |
| 25 | F | 63 | IIA | T2A | N1 | M0 |
| 26 | F | 54 | IIA | T2A | N1 | M0 |
| 27 | M | 71 | IIA | T2A | N1 | M0 |
| 28 | M | 72 | IV | T1 | N0 | M1 |
| 29 | F | 68 | IA | T1 | N0 | M0 |
| 30 | M | 76 | IIA | T2 | N1 | M0 |
miR-27a inhibits cell proliferation by
reducing the expression level of KRAS in ESCC
To investigate whether miR-27a functions as a tumor
suppressor, an MTS assay was performed to detect cell viability.
ECA-109, TE-11 and TE-1 cell lines transfected with miR-27a were
observed to grow at a reduced rate when compared with those cells
transfected with mRNA-NC (Fig.
3A–C). Thus, the results of the present study indicated ectopic
miR-27a expression may inhibit the proliferation of ESCC cell
lines.
 | Figure 3miR-27a inhibits cell proliferation by
reducing the expression of KRAS in ESCC cell lines and animal
models. (A) TE-11, (B) ECA-109 and (C) TE-1 cells were transfected
with miR-27a or miRNA-NC. Following reseeding, cell numbers were
measured using an MTS assay kit at 0, 24, 48 and 72 h. (D) KRAS
expression was detected by reverse transcription-quantitative
polymerase chain reaction following siK-ras transfection into the
TE-1 cell line or siNC as a control. (E) TE-1 cells were
transfected with siK-ras or siNC, using the same treatment as the
miR-27a mimic transfection. (F) Western blots demonstrating the
expression of K-ras following miR-27a mimic or or miRNA-NC
transfection. (G) Western blots demonstrating the expression of
K-ras following siK-ras or siNC transfection. GAPDH served as the
internal control. (H) Nude mice were photographed 24 days after
injection with TE-1 cells transfected with pEGFP-miR-27 or pEGFP as
a control. (I) Tumor size was measured every four days and tumor
growth curves were generated. Each assay was performed in
triplicate.*P<0.05, **P<0.01;
***P<0.001. miRNA, microRNA; KRAS, Kirsten rat
sarcoma viral oncogene homolog; ESCC, esophagus squamous cell
carcinoma; NC, negative control; siK-ras, small interfering K-ras;
siNC, siK-ras negative control. |
To identify whether the downregulation of KRAS alone
inhibits the proliferation of the TE-1 cell line, a small
interfering (si)K-ras expression vector was constructed. Results
from the present study indicated that the level of KRAS expression
was significantly reduced when compared with the control (Fig. 3D). MTS was subsequently performed to
detect the cell viability following siK-ras expression vector
transfection. The results were consistent with cell viability
following miR-27a mimic transfection, when compared with the
control (Fig. 3E). Furthermore, the
expression level of the endogenous K-ras protein was detected by
western blot analysis, indicating that the expression level of KRAS
was obviously reduced in miR-27a-transfected and siK-ras
vector-transfected ESCC cells (Fig. 3F
and G). Thus, miR-27a promotes cell proliferation by reducing
the expression of KRAS in ESCC cell lines.
Upon termination of the experiment, the tumor volume
demonstrated that the tumor growth rate was substantially lower in
mice implanted with the cells overexpressing miR-27a compared with
the control mice (Fig. 3H). On the
24th day after implantation, the mean tumor volume of the miR-27a
overexpression group (270 mm3) was significantly smaller
than that of the control group (710 mm3) (Fig. 3I; P<0.001).
Discussion
miRNAs are key in the regulation of cell
proliferation, apoptosis and other important cellular processes.
The role of miRNA in each specific cell line is dependent on the
specific target gene of the miRNA (15,24–26).
Thus, a single miRNA may exhibit an opposite role in a different
cell line. Therefore, identifying the target gene of miRNA is
considered to be critical. miR-27a was identified to be
downregulated in acute leukemia cell lines and primary samples when
compared with hematopoietic stem-progenitor cells (HSPCs), which
indicates that miR-27a may exert a tumor suppressor-like action in
acute leukemia, possibly via the regulation of apoptosis (27–30).
However, miR-27a expression was upregulated during C2C12 myoblast
proliferation, indicating that miR-27a may promote myoblast
proliferation by targeting myostatin (31–36).
The present study investigated the variation in miRNA expression
levels in ESCC. The expression of miR-27a was significantly
downregulated when compared with a healthy animal model and human
esophageal tissues, which indicated that miR-27a may function as a
tumor suppressor in ESCC. Furthermore, an MTS assay identified the
role of miR-27a in ESCC.
miR-27a may target a number of genes in ESCC.
TargetScan software was used to predict the target gene of miR-27a.
The KRAS gene was selected as the potential target for further
investigation. Previously, KRAS has been identified as a possible
target for cancer therapeutics, due to its activation driving a
number of traits associated with tumor cells, in particular cell
growth and proliferation. KRAS is a target of miR-27a in tumors,
particularly in ESCC, and downregulation of the KRAS oncogene may
provide a novel treatment strategy for cancer patients by
attenuating tumor growth. The Dual-Luciferase® Reporter
assay indicated that the target gene (KRAS) may be directly
targeted by miR-27a and western blot analysis consistently
indicated that the endogenous K-ras protein is inhibited by
miR-27a. Furthermore, the proliferation of the TE-1 cell line was
significantly inhibited upon siK-ras and miR-27a transfection.
In conclusion, the present study identified that
miR-27a functions as a tumor suppressor in ESCC, by direct
targeting of the KRAS gene.
Acknowledgements
The authors would like to thank Miss Yuzhi Jiang for
RT-qPCR assistance and Miss Yuting Duan for assisting in the
preparation of revisions to this article. The authors would also
like to thank the Liaocheng Hospital Targeted Investment in
Excellence Award and the Comparative Pathology and Mouse
Phenotyping Shared Resource for research support.
References
|
1
|
Lin SW, Abnet CC, Freedman ND, et al:
Measuring telomere length for the early detection of precursor
lesions of esophageal squamous cell carcinoma. BMC Cancer.
13:5782013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu C, Li M, Hu C and Duan H: Prognostic
role of microRNA polymorphisms in patients with advanced esophageal
squamous cell carcinoma receiving platinum-based chemotherapy.
Cancer Chemother Pharmacol. 73:335–341. 2014. View Article : Google Scholar
|
|
3
|
Ku GY and Ilson DH: Adjuvant
(postoperative) therapy for esophageal cancer. Thorac Surg Clin.
23:525–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu L, Zuo LF and Guo JW: Reversal of
multidrug resistance by the anti-malaria drug artesunate in the
esophageal cancer Eca109/ABCG2 cell line. Oncol Lett. 6:1475–1481.
2013.PubMed/NCBI
|
|
5
|
Liu R, Yang M, Meng Y, et al:
Tumor-suppressive function of miR-139–5p in esophageal squamous
cell carcinoma. PLoS One. 8:e770682013. View Article : Google Scholar
|
|
6
|
Marks J, Rice DC and Swisher SG: Salvage
esophagectomy in the management of recurrent or persistent
esophageal carcinoma. Thorac Surg Clin. 23:559–567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Migita K, Sho M, Shimada K, et al:
Significant involvement of herpesvirus entry mediator in human
esophageal squamous cell carcinoma. Cancer. 120:808–817. 2014.
View Article : Google Scholar
|
|
8
|
Moghbeli M, Forghanifard MM, Aarabi A,
Mansourian A and Abbaszadegan MR: Clinicopathological sex-related
relevance of Musashi1 mRNA expression in esophageal squamous cell
carcinoma patients. Pathol Oncol Res. Oct 28–2013.(Epub ahead of
print).
|
|
9
|
Nurkin SJ, Nava HR, Yendamuri S, et al:
Outcomes of endoscopic resection for high-grade dysplasia and
esophageal cancer. Surg Endosc. 28:1090–1095. 2014. View Article : Google Scholar
|
|
10
|
Oze I, Matsuo K, Kawakita D, et al: Coffee
and green tea consumption is associated with upper aerodigestive
tract cancer in Japan. Int J Cancer. 135:391–400. 2014. View Article : Google Scholar
|
|
11
|
Paul S and Altorki N: Induction therapy
for esophageal cancer. Thorac Surg Clin. 23:499–507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murata K, Ito H, Yoshitomi H, et al:
Inhibition of miR-92a enhances fracture healing via promoting
angiogenesis in a model of stabilized fracture in young mice. J
Bone Miner Res. 29:316–326. 2014. View Article : Google Scholar
|
|
13
|
Menigatti M, Staiano T, Manser CN, et al:
Epigenetic silencing of monoallelically methylated miRNA loci in
precancerous colorectal lesions. Oncogenesis. 2:e562013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo L, Zhao Y, Zhang H, Yang S and Chen F:
Close association between paralogous multiple isomiRs and
paralogous/orthologues miRNA sequences implicates dominant sequence
selection across various animal species. Gene. 527:624–629. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Min L, Zhang X, et al: Decreased
miRNA-148a is associated with lymph node metastasis and poor
clinical outcomes and functions as a suppressor of tumor metastasis
in non-small cell lung cancer. Oncol Rep. 30:1832–1840.
2013.PubMed/NCBI
|
|
16
|
Edelstein LC, McKenzie SE, Shaw C, et al:
MicroRNAs in platelet production and activation. J Thromb Haemost.
11(Suppl 1): 340–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu J, Hu W, Wu J, et al: miRNA genes of an
invasive vector mosquito, Aedes albopictus. PLoS One. 8:e676382013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anwar SL, Albat C, Krech T, et al:
Concordant hypermethylation of intergenic microRNA genes in human
hepatocellular carcinoma as new diagnostic and prognostic marker.
Int J Cancer. 133:660–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Biscontin A, Casara S, Cagnin S, et al:
New miRNA labeling method for bead-based quantification. BMC Mol
Biol. 11:442010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Huang Z, Chen D, Yang T and Liu G:
MicroRNA-27a is induced by leucine and contributes to
leucine-induced proliferation promotion in C2C12 cells. Int J Mol
Sci. 14:14076–14084. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
No authors listed. Update of the NCCN
guidelines for treatment of breast cancer. Oncology (Williston
Park). 11:199–220. 1997.
|
|
22
|
Zhang J, Lu Y, Yue X, et al: MiR-124
suppresses growth of human colorectal cancer by inhibiting STAT3.
PLoS One. 8:e703002013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riedl CC, Slobod E, Jochelson M, et al:
Retrospective Analysis of 18F-FDG PET/CT for Staging Asymptomatic
Breast Cancer Patients Younger Than 40 Years. J Nucl Med.
55:1578–1583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaspi H, Chapnik E, Levy M, et al: Brief
report: miR-290–295 regulate embryonic stem cell differentiation
propensities by repressing Pax6. Stem Cells. 31:2266–2272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao WT, Li TT, Wang ZG, et al:
microRNA-224 promotes cell proliferation and tumor growth in human
colorectal cancer by repressing PHLPP1 and PHLPP2. Clin Cancer Res.
19:4662–4672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu J and Bushel PR: Dynamic expression of
3′ UTRs revealed by Poisson hidden Markov modeling of RNA-Seq:
implications in gene expression profiling. Gene. 527:616–623. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pincini A, Tornillo G, Orso F, et al:
Identification of p130Cas/ErbB2-dependent invasive signatures in
transformed mammary epithelial cells. Cell Cycle. 12:2409–2422.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Q, He CY, Liu JW and Yuan Y:
Pre-miR-27a rs895819A/G polymorphisms in cancer: a meta-analysis.
PLoS One. 8:e652082013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Wang ZH, Wu YY, et al: Melatonin
attenuates scopolamine-induced memory/synaptic disorder by rescuing
EPACs/miR-124/Egr1 pathway. Mol Neurobiol. 47:373–381. 2013.
View Article : Google Scholar
|
|
30
|
Lin XZ, Luo J, Zhang LP, et al: MiR-27a
suppresses triglyceride accumulation and affects gene mRNA
expression associated with fat metabolism in dairy goat mammary
gland epithelial cells. Gene. 521:15–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Hu X, Dai Y, et al: MicroRNA-27a
activity is not suppressed in porcine oocytes. Front Biosci (Elite
Ed). 4:2679–2685. 2012. View
Article : Google Scholar
|
|
32
|
Fletcher CE, Dart DA, Sita-Lumsden A, et
al: Androgen-regulated processing of the oncomir miR-27a, which
targets Prohibitin in prostate cancer. Hum Mol Genet. 21:3112–3127.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoon KA, Yoon H, Park S, et al: The
prognostic impact of microRNA sequence polymorphisms on the
recurrence of patients with completely resected non-small cell lung
cancer. J Thorac Cardiovasc Surg. 144:794–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hezova R, Kovarikova A, Bienertova-Vasku
J, et al: Evaluation of SNPs in miR-196-a2, miR-27a and miR-146a as
risk factors of colorectal cancer. World J Gastroenterol.
18:2827–2831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Z, Chen X, Yu B, He J and Chen D:
MicroRNA-27a promotes myoblast proliferation by targeting
myostatin. Biochem Biophys Res Commun. 423:265–269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lü MH, Li CZ, Hu CJ, et al: microRNA-27b
suppresses mouse MSC migration to the liver by targeting SDF-1α in
vitro. Biochem Biophys Res Commun. 421:389–395. 2012. View Article : Google Scholar
|