Introduction
Resistance to thyroid hormone (RTH), also known as
Refetoff syndrome, is a rare syndrome that manifests as reduced
end-organ responsiveness to the thyroid hormone. The precise
incidence of RTH is unclear. A study observed that high blood T4
levels were present in one case per 40,000 in neonatal screening
(1). Patients with RTH exhibit
elevated serum free thyroxine (FT4), free triiodothyronine (FT3)
and normal or elevated serum thyroid stimulating hormone (TSH)
levels. The characteristic clinical features vary, including an
absence of the usual symptoms of hyperthyroidism/hypothyroidism,
hyperthyroidism or hypothyroidism, with or without goiter (2). The majority of cases are related to
thyroid hormone receptor β (THRB) mutations, a few cases are caused
by thyroid hormone receptor α (THRA) mutations, and even fewer
cases have no THR mutation, which may be associated with post
transcriptional regulation (3–8).
Primary thyroid lymphoma (PTL) is a rare form of
thyroid cancer, it accounts for 1–5% of all thyroid malignancies
and 1–2% of all extra-nodal lymphomas. Typically patients present
with a rapidly enlarging thyroid as opposed to other thyroid
malignancies, about 30–50% of patients have complications with
hoarseness, stridor, dysphagia and a pressure sensation in the neck
(9).
Recent studies have reported that RTH is associated
with certain types of thyroid cancer, including papillary thyroid
carcinoma and papillary microcarcinoma (10–14).
In the current study, we report a case of RTH with thyroid
non-Hodgkin’s lymphoma.
Case report
Written informed consent was obtained from the
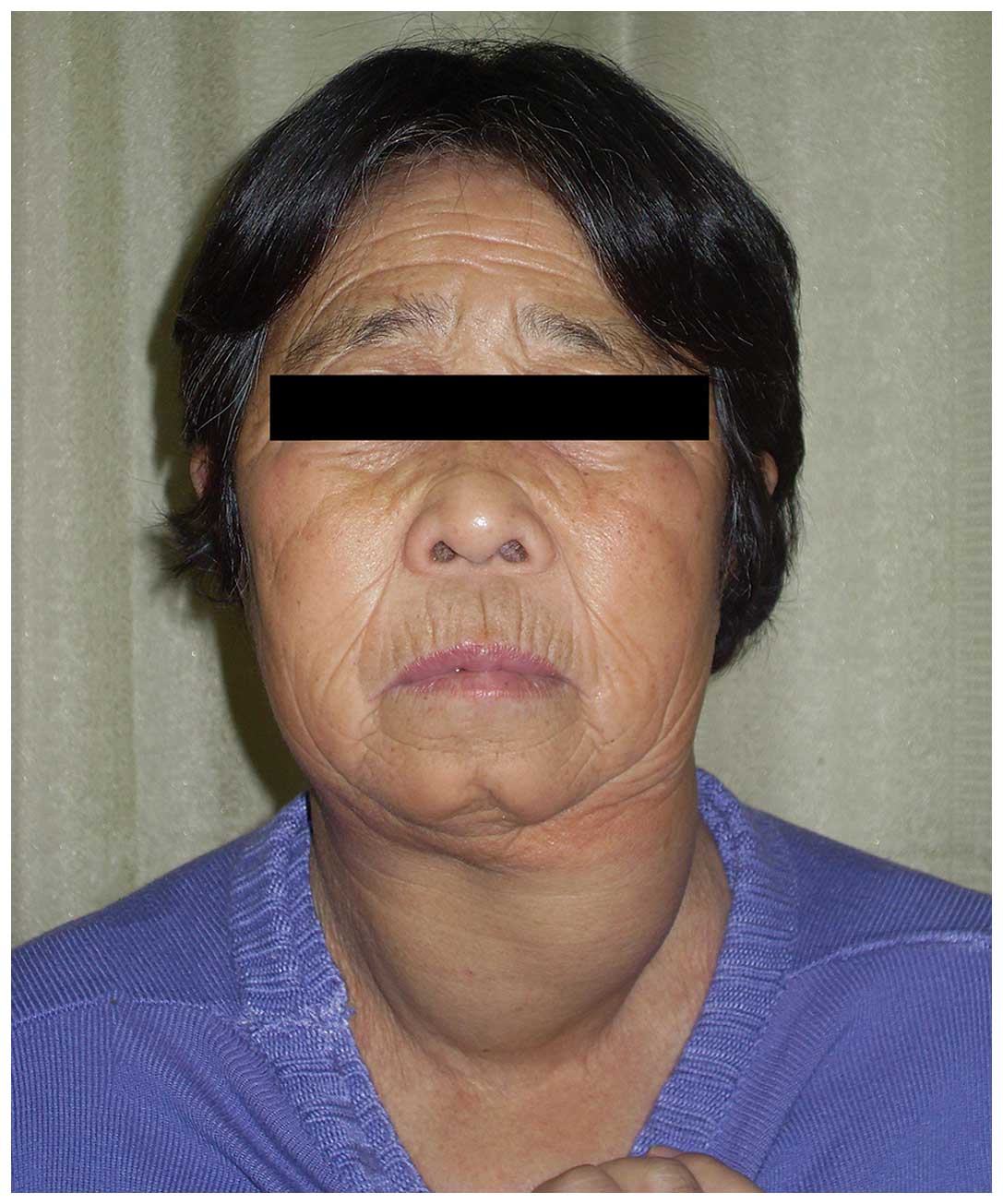
patient and the patient’s family. A 67-year-old female was referred
to the Third Xiangya Hospital of Central South University
(Changsha, China) in December 2012 with a neck that had become
gradually enlarged over the previous two years, with rapid
enlargement in the previous two months, accompanied by a slight
sensation of shortness of breath (Fig.
1). During the two years, no hyperthyroidism or hypothyroidism
symptoms such as sensitivity to heat, irritability, tremors or
sensitivity to the cold, fatigue and edema were experienced. No
history of irradiation or family history of thyroid disease was
reported. On admission, pulse rate was 82 bpm, regular blood
pressure was 145/59 mmHg and body temperature was 36.6°C. Physical
examination revealed a third degree enlargement of the left lateral
lobe of the thyroid; the right lateral lobe thyroid was normal and
no proptosis was present. Laboratory investigations revealed
significantly elevated levels of serum TSH [33.63 μIU/ml; normal
range (N), 0.27–4.2], total T3 (TT3; 3.11 nmom/l; N, 1.3–3.1),
total T4 (TT4; >320 nmom/l; N, 66–181), thyroperoxidase (TPO)
antibody (23.8 IU/ml; N, 0–34), TSH receptor antibody (TRAB;
<0.3 IU/l; N, 0–1.75). In the outpatient clinic the following
day, the thyroid hormone examination was repeated, yielding serum
TSH values of 32.28 μIU/ml, TT3 of 3.58 nmom/l, TT4 of more than
320 nmom/l, free T3 (FT3) of 9.57 pmom/l (N, 3.1–6.8 pmom/l), free
T4 (FT4) of >100 pmom/l (N, 12–22 pmom/l), TPO antibody of 13.56
IU/ml, TRAB of 1.3 IU/l, thyroglobulin antibody (TgAB) of 141.2
IU/ml (N, 0–115), thyroprotein (TG) of 192.3 ng/ml (N, 1.4–7.8).
Blood cell count showed a white blood cell level of
13.8×109/l (N, 4.0–10.0), hemoglobin level of 92 g/l (N,
110–150), platelet level of 233×109/l (N, 100–300) and
serum albumin of 34.2 g/l (N, 35.0–50.0). The thyroid color Doppler
ultrasound scan revealed a hypoechoic mass on the left lateral lobe
of the thyroid, while the right lateral lobe of the thyroid had an
uneven echo. The additional color Doppler ultrasound results of the
liver, gallbladder, pancreas, spleen, retroperitoneal lymph node,
uterus and ovary, kidney, ureter, bladder and bilateral adrenal
were all normal. A chest X-ray revealed a widened mediastinum,
tracheal compression and cardiac enlargement (primarily an enlarged
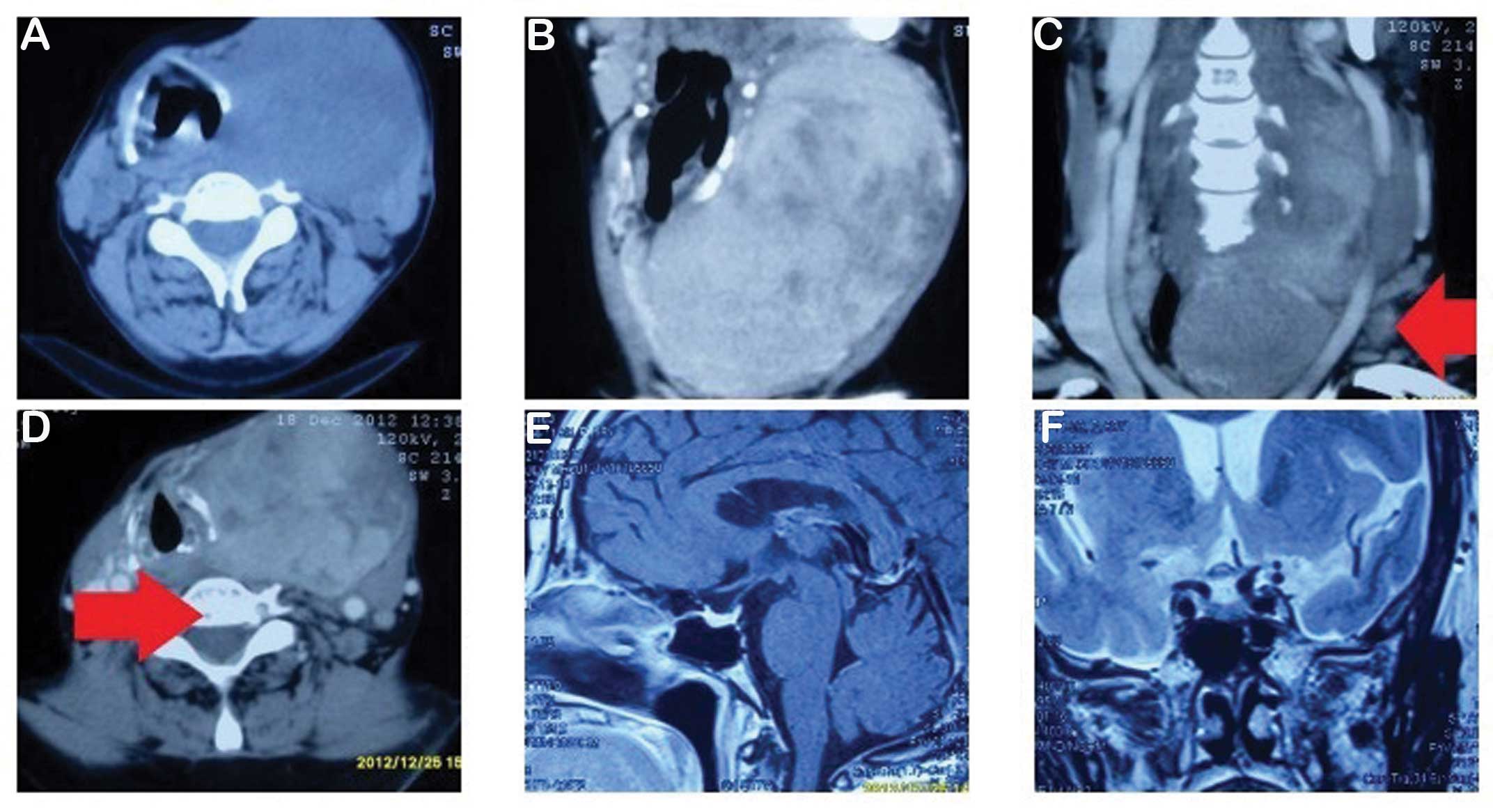
left ventricle). A cervical computed tomography (CT) showed a
thyroid left lateral lobe tumor, considered to be thyroid cancer
and possible bilateral neck metastases, multiple mediastinal lymph
node metastases and cervical vertebra centrum bone shifts (Fig. 2A–D). Single photon emission computed
tomography (SPECT) of the thyroid showed that the volume of the
left lateral lobe had increased significantly and there were
multiple bilateral thyroid nodules. Therefore, the possibility of a
malignant tumor was considered. Bone marrow cytology examination
showed obviously active bone marrow hyperplasia and normal cells in
different stages. Magnetic resonance imaging (MRI) of the pituitary
did not reveal any pathological findings (Fig. 2E–F).
A dexamethasone suppression test (2 mg q6h for two
days) showed that the level of TSH had decreased from 35.53 to 16.3
μIU/ml, a thyroid hormone suppression test (thyroid tablets 100 mg
qd for two days) revealed that the TSH had decreased from 32.58
μIU/ml to 18.26 μIU/ml. It was not possible to measure L-T3.
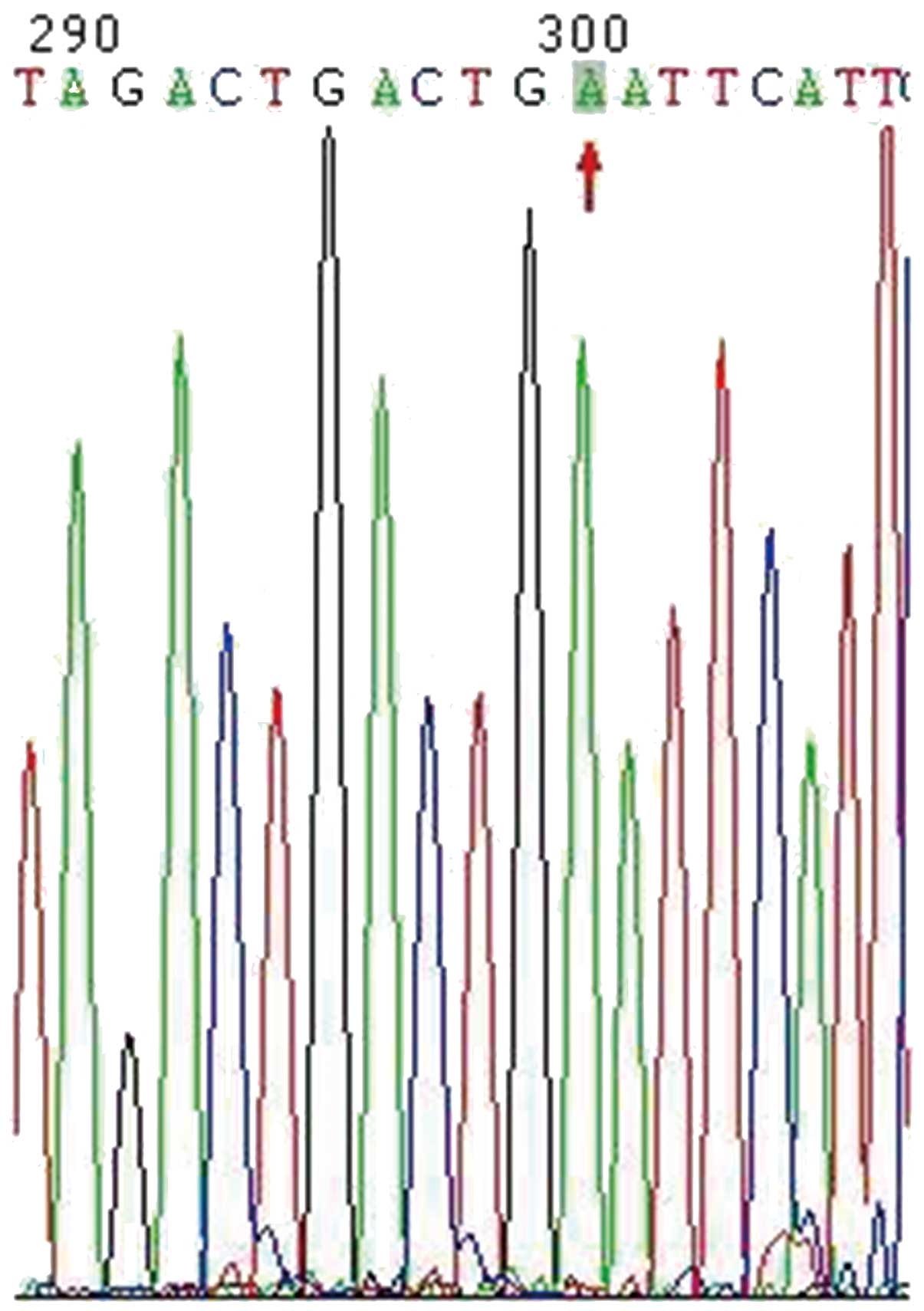
The genetic analysis presented in Fig. 3 revealed that exons 1–9 were normal,
and that exon 10 had a novel point mutation of THRB (g1680 G to A);
the same mutation was identified in the patient’s son and daughter.
To exclude the mutation as a THRB polymorphism, the sequences of
100 healthy controls were analyzed and the mutation was not found
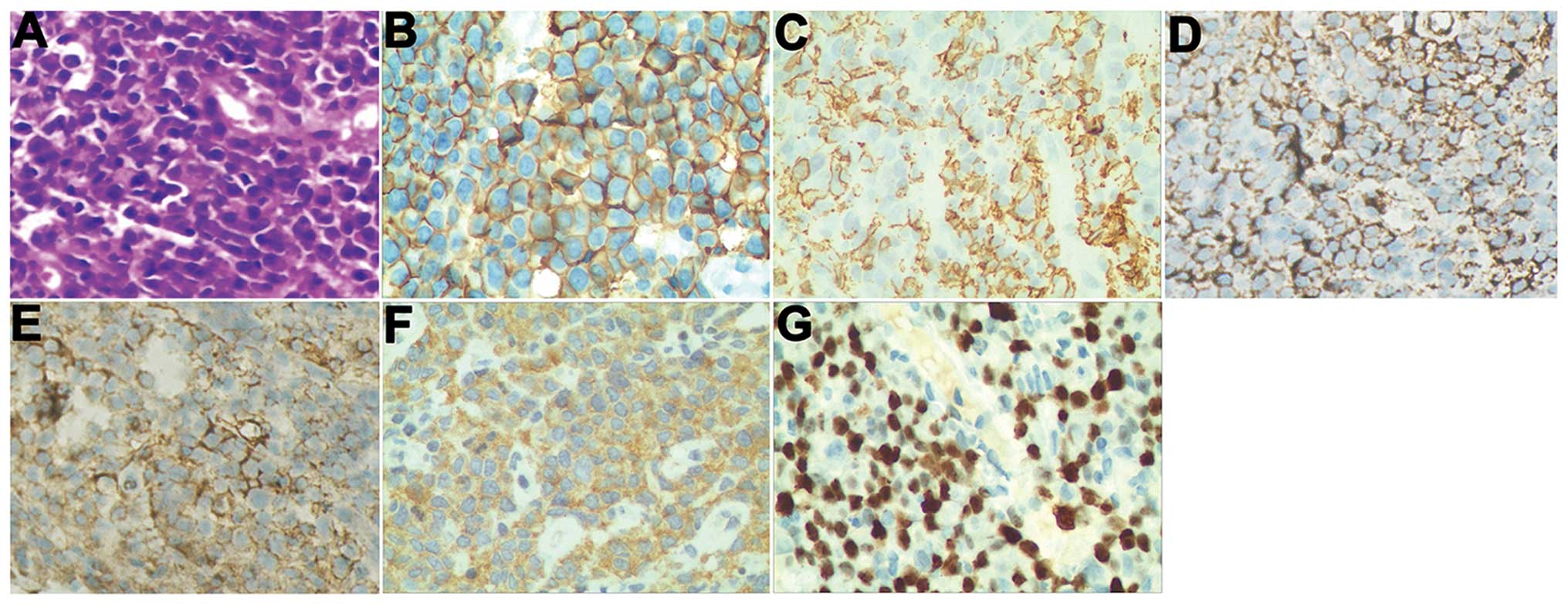
(data not shown). A core biopsy, stained with hematoxylin and eosin
(HE), showed round and polygon poorly differentiated tumor cells
(Fig. 4A). The results of
immunohistochemical staining were as follows: CD20 (+), CD38 (+),
immunoglobulin light chain [Kappa (+) and Lambda (+)], BCL-2 (+),
CD79α (−), CD34 (−), CD3 (−), CD5 (−), CD10 (−), CKPan (−), EMA
(−), CD30 (−) and TdT (−) (Fig. 4).
Ki67 stained ~40% of the cells. These results indicated a
non-Hodgkin’s lymphoma [lymphoplasmacytic lymphoma (LPL)]. The
patient’s serum showed an IgA level of 1.05 g/l (N, 0.5–5 g/l), an
IgE level of 31 IU/ml (N, 0–385 IU/ml), an IgG level of 7.8 g/l (N,
5.16–14.25 g/l) and an IgM level of 1.13 g/l (N, 0.3–2.09 g/l); no
M protein was identified by protein electrophoresis.
The patient was transferred to the department of
hematology for CHOP + nimustine chemotherapy, and, following a
course of treatment, the neck swelling was significantly reduced
and shortness of breath was improved. The patient and son refused
the subsequent treatment and the collection of blood samples from
other family members, therefore no clinical and genetic data could
be obtained for the family.
Discussion
Due to a low morbidity and complicated clinical
manifestations, RTH is often misdiagnosed. Prior to diagnosis of
RTH, alternative causes of elevated TT4 and TSH must be excluded,
for example, raised serum binding proteins, non-thyroidal illness
(including acute psychiatric disorders), drug use (amiodaron,
heparin), familial dysalbuminemic hyperthyroxinemia and TSH
secreting pituitary adenoma (TSH-oma); the most difficult
distinction is between RTH and TSH-oma (15). In the current case, non-thyroidal
illness, increased albumin and drugs were excluded. The patient’s
TT4, TG and TGA levels were significantly increased, however, the
levels of FT3 and FT4 were also increased, which aided in excluding
elevated TT4 due to increased serum TGB. A thyroid suppression test
showed that TSH was reduced by 32%, pituitary MRI was negative and
further the genetic analysis supported the view that the patient
had RTH.
RTH is a rare autosomal, hereditary disease,
consisting of 75–85% familial cases and 15–25% sporadic cases, with
THRB mutations causing RTH in ~90% of cases (16). In the current study, the patient,
and the patient’s son and daughter all possessed a novel point
mutation in exon 10 (g1680 G to A) of THRB. The point mutation was
located in the 3′-untranslated region (UTR) rather than in the
coding sequence (CDS), however, this mutation may affect the
post-transcription processing of THRB.
Patient pathology revealed that this case did not
belong to the most common type of PTL, rather, it was indicated to
be a LPL (17). LPL is an extremely
rare subtype of non-Hodgkin’s lymphoma, which is a low-grade,
B-cell neoplasm composed of small lymphocytes, plasmacytoid
lymphocytes and plasma cells that typically involve the bone
marrow, lymph node or spleen. However, a few cases do not originate
in the bone marrow but are extramedullary. If the extramedullary
tumor infiltrates bone marrow, it may cause pancytopenia,
organomegaly and hyperviscosity; the majority of cases are
asymptomatic or present with anemia. The pathological features
usually present as eccentric nuclei and a more abundant basophilic
cytoplasm. The typical immunophenotype of LPL shows expression of
CD19, CD20, CD22, FMC7, BCL2, CD38 and CD79a; however, CD5, CD10,
and CD23 are usually absent (18).
In the current case a primary tumor was not derived from bone
marrow and non-Hodgkin’s lymphoma did not infiltrate the bone
marrow; although the CT scan indicated possible cervical bone
metastases, only mild anemia was detected, the bone marrow cytology
did not suggest lymphocyte and plasma cell infiltration.
Additionally, M protein was not detected and the Ig M level was
within the normal range. The immunohistochemical staining supported
the diagnosis of thyroid LPL (10–14).
A number of studies of RTH with thyroid cancer have
been previously reported (10–14).
In an animal study, mutation of THRB significantly increased the
morbidity of spontaneous thyroid carcinoma through the activation
of TSH-mediated signaling pathways (19). Whether or not the mutation of THRB
increases the morbidity of non-Hodgkin’s lymphoma or LPL remains
unclear.
In conclusion, to the best of our knowledge this is
the first report of a case of RTH with thyroid non-Hodgkin’s
lymphoma. The pathology may be considered as LPL, which is an
extremely rare subtype of non-Hodgkin’s lymphoma. The present case
indicated that the point mutantion of THRB in 3′-UTR might be an
important indicator for RTH or non-Hodgkin’s lymphoma. Further
functional studies will be performed in order to confirm the
function of this THRB mutation in RTH or non-Hodgkin’s
lymphoma.
References
|
1
|
Lafranchi SH, Snyder DB, Sesser DE, et al:
Follow-up of newborns with elevated screening T4 concentrations. J
Pediatr. 143:296–301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olateju TO and Vanderpump MP: Thyroid
hormone resistance. Ann Clin Biochem. 43:431–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zavacki AM and Larsen PR: RTHalpha, a
newly recognized phenotype of the resistance to thyroid hormone
(RTH) syndrome in patients with THRA gene mutations. J Clin
Endocrinol Metab. 98:2684–2686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schoenmakers N, Moran C, Peeters RP,
Visser T, Gurnell M and Chatterjee K: Resistance to thyroid hormone
mediated by defective thyroid hormone receptor alpha. Biochim
Biophys Acta. 1830:4004–4008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pohlenz J, Weiss RE, Macchia PE, et al:
Five new families with resistance to thyroid hormone not caused by
mutations in the thyroid hormone receptor beta gene. J Clin
Endocrinol Metab. 84:3919–3928. 1999.PubMed/NCBI
|
|
6
|
Fozzatti L, Lu C, Kim DW, et al:
Resistance to thyroid hormone is modulated in vivo by the nuclear
receptor corepressor (NCOR1). Proc Natl Acad Sci USA.
108:17462–17467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bottcher Y, Paufler T, Stehr T, Bertschat
FL, Paschke R and Koch CA: Thyroid hormone resistance without
mutations in thyroid hormone receptor beta. Med Sci Monit.
13:CS67–CS70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Işık E, Beck Peccoz P, Campi I, et al:
Thyroid hormone resistance: a novel mutation in thyroid hormone
receptor beta (THRB) gene- case report. Turk J Pediatr. 55:322–327.
2013.
|
|
9
|
Walsh S, Lowery AJ, Evoy D, McDermott EW
and Prichard RS: Thyroid lymphoma: recent advances in diagnosis and
optimal management strategies. Oncologist. 18:994–1003. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Unluturk U, Sriphrapradang C, Erdogan MF,
et al: Management of differentiated thyroid cancer in the presence
of resistance to thyroid hormone and TSH-secreting adenomas: a
report of four cases and review of the literature. J Clin
Endocrinol Metab. 98:2210–2217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramos-Prol A, Antonia Perez-Lazaro M,
Isabel del Olmo-Garcia M, et al: Differentiated thyroid carcinoma
in a girl with resistance to thyroid hormone management with
triiodothyroacetic acid. J Pediatr Endocrinol Metab. 26:133–136.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paragliola RM, Lovicu RM, Locantore P, et
al: Differentiated thyroid cancer in two patients with resistance
to thyroid hormone. Thyroid. 21:793–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugita M, Harada H and Yamamoto T:
Perioperative management of a patient with thyroid hormone
resistance who underwent total thyroidectomy for thyroid cancer. J
Anesth. 26:595–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HK, Kim D, Yoo EH, et al: A case of
resistance to thyroid hormone with thyroid cancer. J Korean Med
Sci. 25:1368–1371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agrawal NK, Goyal R, Rastogi A, Naik D and
Singh SK: Thyroid hormone resistance. Postgrad Med J. 84:473–477.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeda K, Sakurai A, DeGroot LJ and
Refetoff S: Recessive inheritance of thyroid hormone resistance
caused by complete deletion of the protein-coding region of the
thyroid hormone receptor-beta gene. J Clin Endocrinol Metab.
74:49–55. 1992.PubMed/NCBI
|
|
17
|
Naderi N and Yang DT: Lymphoplasmacytic
lymphoma and Waldenstrom macroglobulinemia. Arch Pathol Lab Med.
137:580–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin P, Molina TJ, Cook JR and Swerdlow SH:
Lymphoplasmacytic lymphoma and other non-marginal zone lymphomas
with plasmacytic differentiation. Am J Clin Pathol. 136:195–210.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao L, Zhu X, Won Park J, Fozzatti L,
Willingham M and Cheng SY: Role of TSH in the spontaneous
development of asymmetrical thyroid carcinoma in mice with a
targeted mutation in a single allele of the thyroid hormone-beta
receptor. Endocrinology. 153:5090–5100. 2012. View Article : Google Scholar : PubMed/NCBI
|