Introduction
Pancreatic cancer is the sixth most common cause of
cancer-related mortality in China (1,2).
Pancreatic cancer exhibits an extremely poor prognosis, possibly as
it is often diagnosed at an advanced stage, despite recent advances
in diagnostic methods. At present, no adjuvant chemotherapy
regimens have been identified for the treatment of pancreatic
cancer, due to problems regarding drug resistance and limited
efficacy. Curative surgical approaches remain the standard
treatment; however, the prognosis of pancreatic cancer remains poor
with regard to postsurgical five-year survival rates (3). Thus, the identification of novel
prognostic markers for pancreatic cancer is critical to assess
invasion and metastasis and to aid with the management of
postoperative treatment for high-risk patients.
CD97 is an important member of the epidermal growth
factor-seven transmembrane family (4,5), which
is associated with aggressiveness and lymph node involvement in
thyroid tumors, colorectal cancer, oral squamous cell carcinomas
and primary gallbladder carcinoma (6–9). Based
on its expression pattern and structure, CD97 has been hypothesized
to be involved in cellular adhesion via interactions with other
cell-surface and extracellular matrix proteins (10). Through its epidermal growth factor
domain region, CD97 binds to CD55, which affects cancer invasion
and metastasis (6,11,12).
CD55 is also termed complement decay accelerating factor, as it
mediates the complement activation pathway (4,13). The
complement immune system consists of a number of glycoproteins,
which are involved in an enzymatic cascade, that upon activation
results in the formation of the membrane attack complex that leads
to cell lysis (4,7). A number of studies have demonstrated
that CD97 and CD55 are involved in tumor dedifferentiation,
migration, invasiveness and metastasis (14). However, the association between CD97
and CD55 expression in pancreatic cancer has not yet been
systemically investigated. Therefore, in this study, CD97 and CD55
expression in pancreatic cancer tissues and adjacent normal
pancreatic tissues was investigated with regard to tumor
aggressiveness and prognosis.
Materials and methods
Patients and tissue samples
Surgical pancreatic cancer and adjacent normal
pancreatic tissue specimens were obtained from 37 patients at The
First College of Clinical Medical Sciences, Three Gorges University
(Yichang, China) between January 2009 and December 2010. Written
informed consent was obtained from all patients. A total of 18 male
and 19 female patients were included, with a mean age of 56.7 years
(range: 47–64 years). No patients had received preoperative
radiotherapy or chemotherapy. Histologically, all 37 cancer
specimens were stained using hematoxylin and eosin and were
subsequently diagnosed as pancreatic adenocarcinomas. The tumor
stage of the specimens was classified according to the American
Joint Committee on Cancer staging manual (15). All protocols were approved by the
institutional review board of The First College of Clinical Medical
Sciences, Three Gorges University. The cancer specimens were graded
as well-, moderately or poorly differentiated adenocarcinoma
according to the National Comprehensive Cancer Network
classification (16). The majority
of the cancers (32/37) were moderately differentiated, four were
well-differentiated and one was poorly differentiated. The 37
patients included in this study were followed up for three years.
This study was approved by the Human Research Ethics Committees at
The First College of Clinical Medical Sciences, Three Gorges
University.
Immunohistochemistry
Paraffin sections (3-μm) were incubated overnight at
4°C with primary antibodies against CD55 (1:50; goat polyclonal
antibody; cat. no. sc-7067; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and CD97 (1:100; rabbit polyclonal antibody; cat.
no. sc-98577; Santa Cruz Biotechnology, Inc.), washed and incubated
with biotinylated goat anti-rabbit IgG (1:50; goat anti-rabbit
monoclonal antibody; cat. no. BA1003; Boster Biotechnology, Inc.,
Wuhan, China) for 30 min. Next, sections were washed three times
with phosphate-buffered saline, stained with diaminobenzidine and
examined by light microscopy (Olympus BX52; Olympus Corporation,
Tokyo, Japan). Six randomly selected fields from each sample were
examined by two pathologists, who were blinded to patient diagnosis
and outcome. Areas positively expressing CD55 and CD97, and the
average optical density were recorded and analyzed using Image-Pro
Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
software.
Statistical analysis
Results are presented as the mean ± standard
deviation. The treatment groups were compared using independent
sample t-tests. One way analysis of variance was used for two-sided
pair-wise multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference. All analyses were
performed using SPSS software, version 17.0 (SPSS Inc., Chicago,
IL, USA).
Results
Clinicopathological characteristics of
CD97 expression in pancreatic cancer
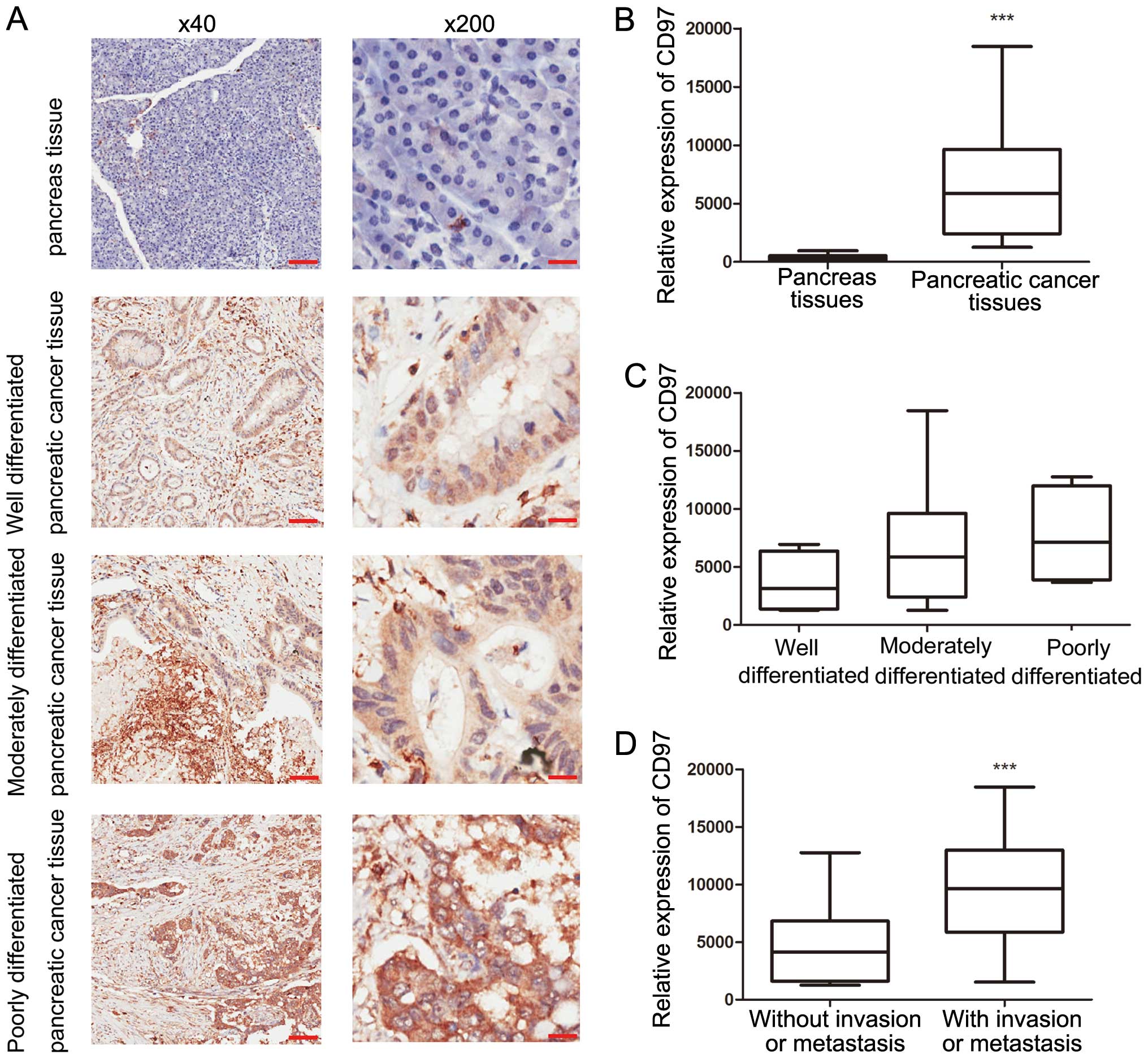
CD97 expression in pancreatic cancer and adjacent
normal pancreatic tissues was investigated. In adjacent normal
pancreatic tissues, CD97 staining was absent or weakly positive and
was significantly localized in the cytoplasm of tumor cells,
particularly in tumors with lymph node involvement or distant
metastases (Fig. 1A). All 37
pancreatic cancer tissue specimens were CD97+, including
15 tumors with extremely strong CD97+ staining and four
tumors with uniform CD97 staining. All 15 tumors exhibiting high
CD97 expression had lymph node involvement and of the four tumors
with uniform CD97 staining, two exhibited vascular invasion and one
had distant metastasis. Based on the Image-Pro Plus analysis
results, CD97 expression in pancreatic cancer tissues was
significantly higher than that of the adjacent normal pancreatic
tissues (P<0.0001; Fig. 1B).
Furthermore, tumors with lymph node involvement, vascular invasion
or distant metastasis exhibited a significantly higher level of
CD97, when compared with that of carcinomas without (P=0.0018;
Fig. 1D). Notably, no significant
difference was identified between CD97 expression and clinical
parameters, including age, gender and differentiation (Fig. 1C).
Clinicopathological characteristics of
CD55 expression in pancreatic cancer
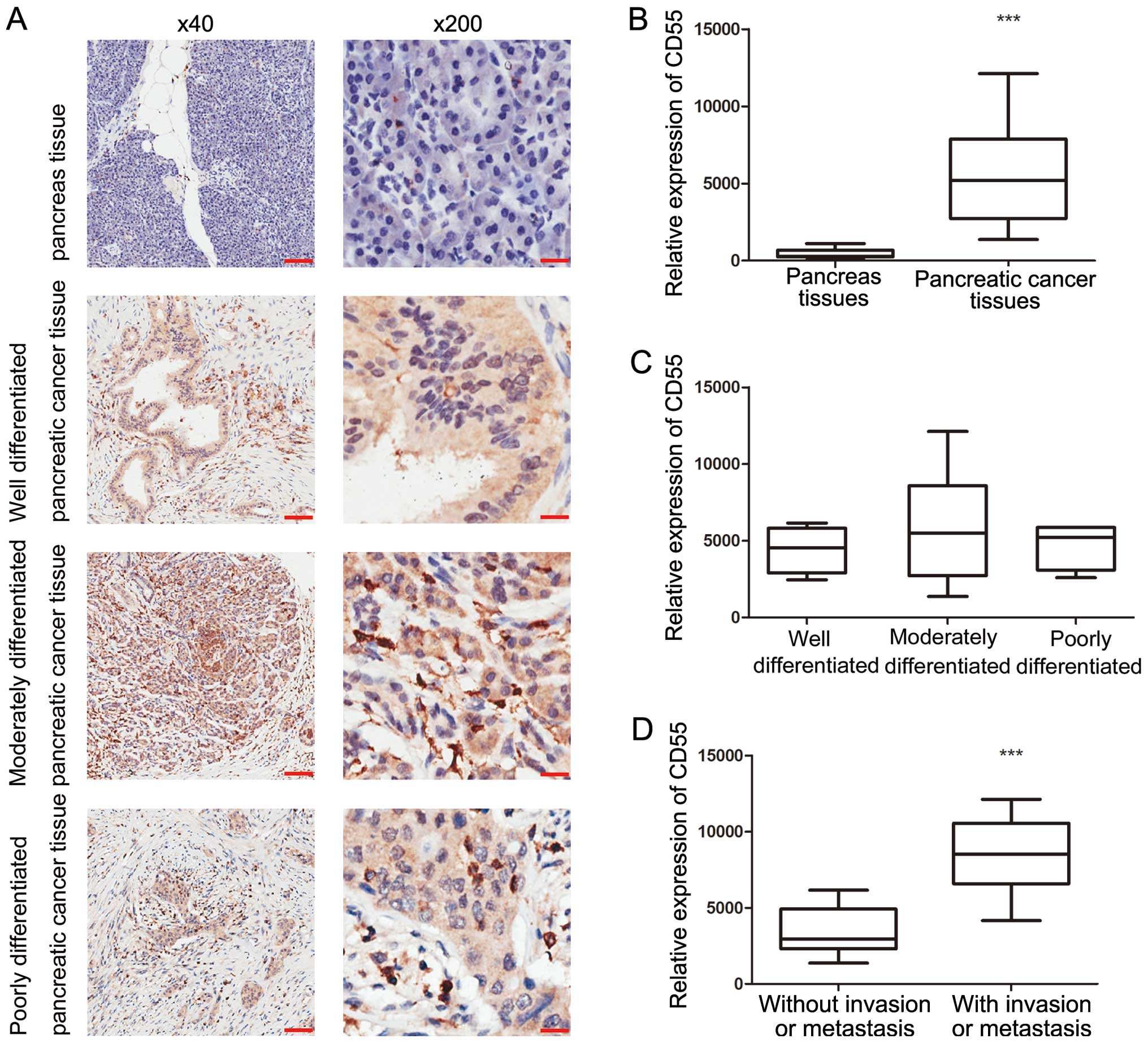
The expression of CD55 in pancreatic cancer and
adjacent normal pancreatic tissues was investigated. CD55 staining
was absent or weakly positive in normal pancreatic tissues.
However, strong CD55+ expression was exhibited in the
cell membranes in pancreatic cancer tissues (Fig. 2A). All 37 pancreatic cancer tissue
specimens were CD55+, including 13 tumors with strong
CD55 expression and four tumors with uniform CD55+
staining. All 13 CD55+ tumors had lymph node
involvement, and of the four tumors with uniform CD55+
staining, two exhibited vascular invasion and one exhibited distant
metastasis. Based on the Image-Pro Plus analysis results, CD97
expression levels in pancreatic cancer tissues are significantly
higher than that of adjacent normal pancreatic tissues
(P<0.0001; Fig. 2B). In tumors
with lymph node involvement, vascular invasion or distant
metastasis, carcinomas exhibited a significantly higher level of
CD97+ expression when compared with carcinomas without
(P<0.0001; Fig. 2D). Notably, no
significant differences between clinical parameters, including age,
gender and differentiation, and CD97 expression were identified
(Fig. 2C).
Correlation between CD97 and CD55
expression and tumor aggressiveness and prognosis
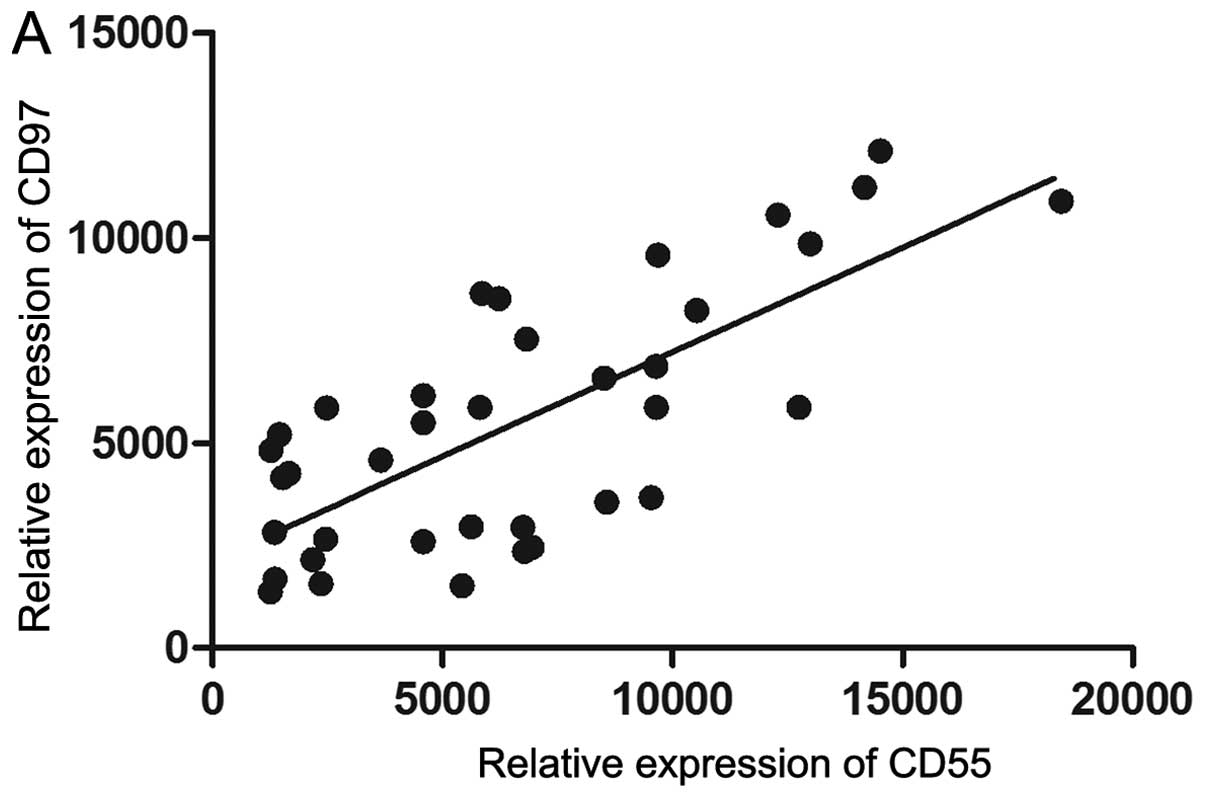
The expression of CD97 was found to significantly
correlate with CD55 expression (r2=0.5422;
P<0.0001; Fig. 3A). A total of
thirteen patients had a significantly stronger expression of CD97
and CD55 than the other 24 patients. These 13 patients were defined
as the CD97/CD55high expression group and the remaining
24 patients were the CD97/CD55low expression group. The
co-expression of CD97 and CD55 was associated with lymph node
involvement, metastasis and vascular invasion, which are hallmarks
of aggressive tumors. Therefore, the aforementioned groups were
used to analyze the association between tumor aggressiveness and
CD97/CD55 expression. The tumors of the CD97/CD55high
group were more aggressive than tumors of the
CD97/CD55low group.
To further investigate the clinical importance of
CD97 and CD55 expression in pancreatic cancer, the correlation
between overall survival (OS) and CD97/CD55 expression was
investigated. CD97/CD55high expression was found to
significantly correlate with a shorter OS. Kaplan-Meier plots
demonstrated significantly poorer survival rates at all times
points for the CD97/CD55high group when compared with
the CD97/CD55low group (P<0.01; Fig. 3B). The overall three-year survival
for all patients was 46%. However, the CD97/CD55high
group exhibited a significantly worse survival rate (16%) than the
CD97/CD55low group (63%). These results indicated that a
progressive and significant deterioration in prognosis occurs with
increased CD97/CD55 expression.
Discussion
Pancreatic cancer is one of the most challenging
types of cancer worldwide, and is a markedly aggressive disease
with a poor overall prognosis and an incidence rate that
approximately equals its mortality rate (3). Previous studies have indicated that
adjuvant chemotherapy may improve survival in pancreatic cancer
patients. Specific signaling pathways are already involved in
therapeutic approaches or considered as potential therapeutic
targets in pancreatic cancer (17,18).
Recently, signaling pathways that mediate desmoplastic stromal
response and tumor immunity have received attention as they may
present promising therapeutic targets for future therapy (18). However, which patients would benefit
from such therapy remains unclear.
In the present study, histological analysis of
pancreatic cancer tissue specimens revealed a significant increase
in CD97+/CD55+ epithelial cells, as
percentage and intensity of expression were significantly higher in
pancreatic cancer tissues when compared with that of adjacent
normal pancreatic tissues. The involvement of CD97 in malignant
tumor behavior may occur due to the interactions between CD97 and
its receptor, CD55 (4). Recently,
CD55 was identified as the ligand for CD97, which is a
seven-transmembrane epidermal-like growth factor receptor that is
involved in cell-cell and cell-matrix adhesion (6). In addition, previous studies have
indicated that CD97 and CD55 are associated with malignancy and
contribute to invasion and metastasis (8,19). Our
results revealed that the tumor tissues with
CD97/CD55high expression were more aggressive than the
tumor tissues exhibiting CD97/CD55low expression. The
upregulation of CD55 and the possible role of the CD55-CD97
interaction in invasion and metastasis promotion have led to the
development of compounds which target CD55, as an immunotherapeutic
approach for cancer treatment (14,20).
Sutavani et al (20)
demonstrated that CD55 is a potent costimulator and activator of
human naive CD4+ cells, resulting in the differentiation
of a discrete Tr1 population that inhibits T-cell function in an
IL10-dependent manner and maintains the Tr1 phenotype upon
re-stimulation. CD97 and CD55 have been shown to be upregulated in
a variety of solid tumors, including gastric and colorectal cancers
(8,21–23).
The expression levels of CD97 and CD55 in the abovementioned tumors
were associated with the severity of the tumor, and were
independent predictors of shorter OS in these patients.
CD97 and CD55 have been found in the tumor
microenvironment and contribute to metastasis and invasion, leading
to a poor prognosis (8). Although
CD97 and CD55 are expressed by cells to protect them from the
complement immune system, the presence of a small population that
strongly expresses CD97 and CD55 predicts poor prognosis in a
number of cancers. For example, Durrant et al (21) prospectively analyzed the correlation
between CD55 expression and seven-year survival in 136 patients
with colorectal cancer, and found that patients with tumors with
high CD55 expression exhibited a significantly worse survival than
patients with tumors exhibiting low CD55 expression. Furthermore,
Wu et al (24) revealed that
CD97 and CD55 are upregulated in human gallbladder carcinoma. The
expression levels of CD97 and CD55 in gallbladder carcinoma were
associated with tumor severity. In addition, levels of CD97 and
CD55 expression were independent predictors of shorter OS in
patients with gallbladder carcinoma (24). Mustafa et al (9) identified CD97 as a novel marker for
dedifferentiated oral squamous cell carcinoma. The interaction
between CD97 and CD55 may facilitate the adhesion of OSCC cells to
the surrounding surfaces, which may lead to metastasis. The present
study identified a significant correlation between the intensity of
CD97 and CD55 expression and tumor aggressiveness and prognosis. A
progressive and significant deterioration in prognosis as CD97 and
CD55 expression increases has been shown.
In conclusion, the present study indicated that CD97
and its ligand CD55 are upregulated in pancreatic cancers and are
closely associated with lymph node involvement, metastasis and
vascular invasion. We propose that analysis of the expression of
both CD97 and CD55 has a prognostic value for pancreatic
cancer.
Acknowledgements
The authors would like to thank the Pathological
Examination Center of The First College of Clinical Medical
Sciences, Three Gorges University (Yichang, China) for technical
support during pathological examinations.
References
|
1
|
Ma C, Jiang YX, Liu SZ, et al: Trend and
prediction on the incidence of pancreatic cancer in China. Zhonghua
Liu Xing Bing Xue Za Zhi. 34:160–163. 2013.(In Chinese). PubMed/NCBI
|
|
2
|
Ji BT, Silverman DT, Dosemeci M, et al:
Occupation and pancreatic cancer risk in Shanghai, China. Am J Ind
Med. 35:76–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolfgang CL, Herman JM, Laheru DA, et al:
Recent progress in pancreatic cancer. CA Cancer J Clin. 63:318–348.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamann J, Vogel B, van Schijndel GM and
van Lier RA: The seven-span transmembrane receptor CD97 has a
cellular ligand (CD55, DAF). J Exp Med. 184:1185–1189. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKnight AJ and Gordon S: The EGF-TM7
family: unusual structures at the leukocyte surface. J Leukoc Biol.
63:271–280. 1998.PubMed/NCBI
|
|
6
|
Wobus M, Vogel B, Schmucking E, Hamann J
and Aust G: N-glycosylation of CD97 within the EGF domains is
crucial for epitope accessibility in normal and malignant cells as
well as CD55 ligand binding. Int J Cancer. 112:815–822. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin HH, Stacey M, Saxby C, et al:
Molecular analysis of the epidermal growth factor-like short
consensus repeat domain-mediated protein-protein interactions:
dissection of the CD97-CD55 complex. J Biol Chem. 276:24160–24169.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han SL, Xu C, Wu XL, et al: The impact of
expressions of CD97 and its ligand CD55 at the invasion front on
prognosis of rectal adenocarcinoma. Int J Colorectal Dis.
25:695–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mustafa T, Eckert A, Klonisch T, et al:
Expression of the epidermal growth factor seven-transmembrane
member CD97 correlates with grading and staging in human oral
squamous cell carcinomas. Cancer Epidemiol Biomarkers Prev.
14:108–119. 2005.PubMed/NCBI
|
|
10
|
Abbott RJ, Spendlove I, Roversi P, et al:
Structural and functional characterization of a novel T cell
receptor co-regulatory protein complex, CD97-CD55. J Biol Chem.
282:22023–22032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamann J, Stortelers C, Kiss-Toth E, et
al: Characterization of the CD55 (DAF)-binding site on the
seven-span transmembrane receptor CD97. Eur J Immunol.
28:1701–1707. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamann J, Wishaupt JO, van Lier RA, et al:
Expression of the activation antigen CD97 and its ligand CD55 in
rheumatoid synovial tissue. Arthritis Rheum. 42:650–658. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian YM, Haino M, Kelly K and Song WC:
Structural characterization of mouse CD97 and study of its specific
interaction with the murine decay-accelerating factor (DAF, CD55).
Immunology. 98:303–311. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karpus ON, Veninga H, Hoek RM, et al:
Shear stress-dependent downregulation of the adhesion-G
protein-coupled receptor CD97 on circulating leukocytes upon
contact with its ligand CD55. J Immunol. 190:3740–3748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge SB, Byrd DR, Compton CC, et al:
Exocrine and endocrine pancreas. AJCC Cancer Staging Manual. 7th
edition. Springer; New York, NY: pp. 241–249. 2010
|
|
16
|
Tempero MA, Behrman S, Ben-Josef E, et al:
Pancreatic adenocarcinoma: Clinical Practice Guidelines in
Oncology. J Natl Compr Canc Netw. 3:598–626. 2005.PubMed/NCBI
|
|
17
|
Barton MK: Thromboembolism is common and
influences prognosis in patients with pancreatic cancer, study
reports. CA Cancer J Clin. Jan 18–2012.(Epub ahead of print).
View Article : Google Scholar
|
|
18
|
Yang GY, Wagner TD, Fuss M and Thomas CJ:
Multimodality approaches for pancreatic cancer. CA Cancer J Clin.
55:352–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mustafa T, Klonisch T, Hombach-Klonisch S,
et al: Expression of CD97 and CD55 in human medullary thyroid
carcinomas. Int J Oncol. 24:285–294. 2004.PubMed/NCBI
|
|
20
|
Sutavani RV, Bradley RG, Ramage JM, et al:
CD55 costimulation induces differentiation of a discrete T
regulatory type 1 cell population with a stable phenotype. J
Immunol. 191:5895–5903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Durrant LG, Chapman MA, Buckley DJ, et al:
Enhanced expression of the complement regulatory protein CD55
predicts a poor prognosis in colorectal cancer patients. Cancer
Immunol Immunother. 52:638–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Chen L, Peng SY, Chen ZX and
Hoang-Vu C: Role of CD97 (stalk) and CD55 as molecular markers for
prognosis and therapy of gastric carcinoma patients. J Zhejiang
Univ Sci B. 6:913–918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loberg RD, Wojno KJ, Day LL and Pienta KJ:
Analysis of membrane-bound complement regulatory proteins in
prostate cancer. Urology. 66:1321–1326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu J, Lei L, Wang S, Gu D and Zhang J:
Immunohistochemical expression and prognostic value of CD97 and its
ligand CD55 in primary gallbladder carcinoma. J Biomed Biotechnol.
2012:5876722012. View Article : Google Scholar : PubMed/NCBI
|