Introduction
Primary pulmonary amyloidosis is an uncommon
disease, characterized by amyloid deposits localized to the
respiratory system (1,2). Respiratory amyloidosis was first
described in 1877 by Lesser (3).
Although the number of reported cases has accumulated over past
years, the exact pathogenesis of the disease remains unclear. It is
believed that the misfolding of extracellular protein plays a
prominent role in the molecular mechanism of amyloidosis.
Respiratory impairment is uncommon and can be classified as three
forms: Diffuse interstitial deposits, single or multiple pulmonary
nodules and submucosal tracheobronchial deposits (4). Amyloid deposition in the
tracheobronchial airways is rare and constitutes ~1% of benign
tumors (5,6), with only 12 cases of solitary thoracic
amyloidomas reported in the literature, as reviewed by Cresner
et al (7). The natural
history of this disorder and the efficacy of potential therapies
have not been clearly defined. Thorough evaluation of respiratory
tract amyloidosis is required to determine the type and need for
treatment. Broadly, systemic chemotherapy is indicated for systemic
amyloidosis and local intervention for its localised forms. The
management of tracheobronchial amyloidosis is also largely
dependent upon symptoms and may involve intermittent bronchoscopic
resection, surgical resection, carbon dioxide laser ablation and
neodymium-doped yttrium, aluminum and garnet laser therapy
(7). The manifestations, clinical
significance and prognosis of respiratory tract amyloidosis vary
considerably depending on its etiology and anatomical distribution.
Therefore, each patient requires thorough evaluation to determine
their optimal management (8). The
clinical presentation of pulmonary amyloidosis varies between cases
and the symptoms are non-specific, causing diagnosis to be
problematic. The predominant differential diagnoses based on
imaging are lung cancer, lung metastasis, tuberculoma and
cryptococcosis. The ability to differentiate pulmonary amyloidosis
from other disorders by diagnostic imaging as an alternative to
invasive tests, such as bronchoscopy and biopsy under CT guidance,
would be beneficial (4,9). Therefore, in the present study, biopsy
under CT guidance was chosen, and shown to be effective.
Positron emission tomography (PET) with
18F-fluoro-deoxyglucose (18F-FDG) is widely used in the diagnosis
of indeterminate solitary pulmonary nodules (SPNs) on computed
tomography (CT) imaging (10). In
particular, the standardized uptake values (SUVs) measured by
dual-time-point (DTP) or delayed PET/CT imaging have been proposed
to be helpful indicators in distinguishing malignant from benign
SPN (11). To the best of our
knowledge, only one reported case of pulmonary amyloid lesions has
evaluated the disorder by DTP PET/CT imaging. Tan et al
(12) presented a case of
amyloidosis exhibiting significantly increased 18F-FDG accumulation
in the right lung and the left lung lesions on the 1-hour early
phase (maximum SUV was not shown). However, on the 2-h delayed
images, compared with the levels of the 1-h images (maximum SUV was
not shown), the metabolic activity of these lesions was markedly
reduced. Therefore, it has been hypothesized that pulmonary amyloid
lesions may potentially be distinguished from malignancy when
utilizing dual phase FDG PET/CT imaging (12). Therefore, the dual phase FDG PET/CT
imaging was performed in the present study.
The present study reports one case of primary
pulmonary amyloidosis in a 59-year-old Chinese female, initially
misdiagnosed as malignancy on DTP PET/CT imaging. The patient
provided written informed consent for the study.
Case report
A 59-year-old Chinese female was referred to the
chest clinic of the First Affiliated Hospital, College of Medicine,
Zhejiang University (Hangzhou, China) in April 2011 due to a
two-month history of cough, hemoptysis and general fatigue. A CT
scan of the chest revealed a nodule in the left lower lung with
mild enhancement in the lesion. The nodule progressed during the
two-month follow-up period under treatment with anti-infection
drugs. No other significant history was noted. The patient’s
physical examination was unremarkable, and hematological and
biochemical parameters were within normal limits.
A dual phase FDG PET/CT scan was performed following
six hours of fasting. FDG (5.5 MBq/kg) was injected intravenously
through an antecubital vein while the patient remained at rest.
Image acquisition was subsequently conducted using a Siemens
Biograph 16 PET-CT scanner (Siemens Medical Solutions USA, Inc.,
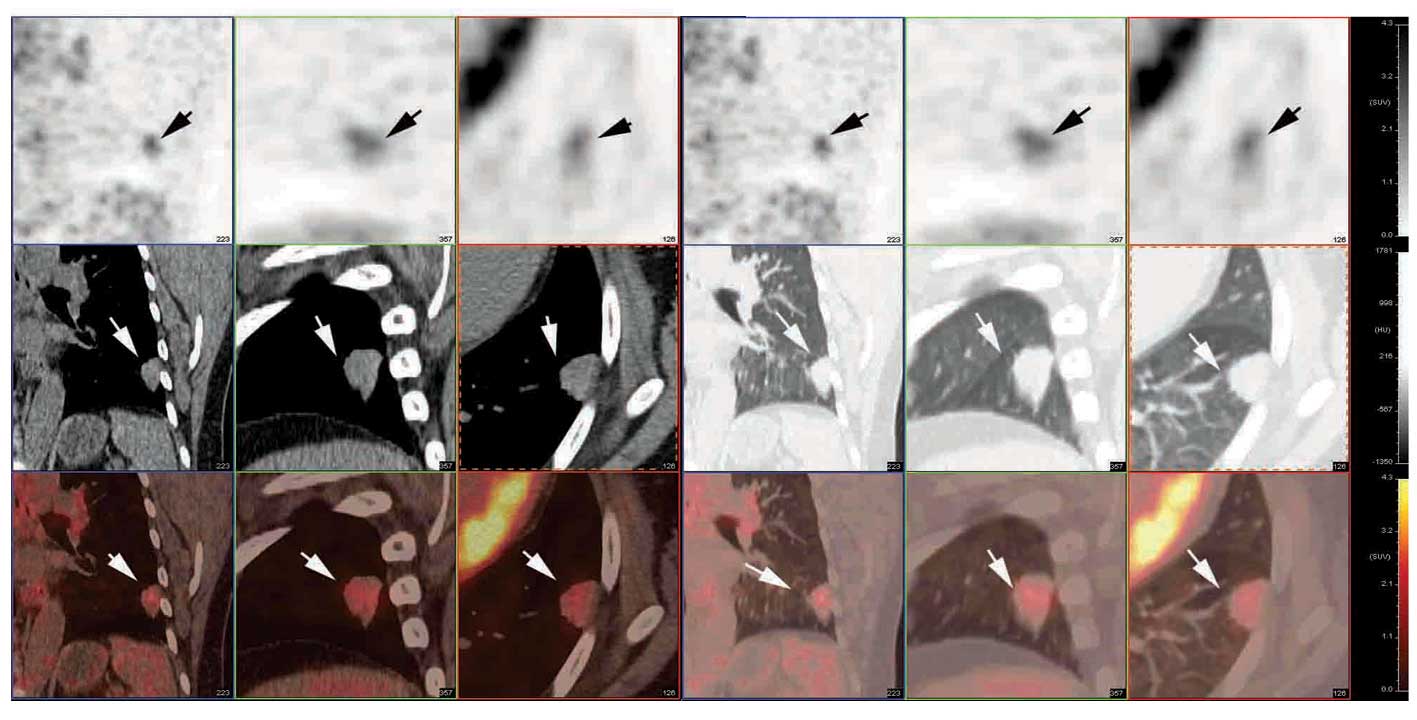
Malvern, PA, USA). The PET/CT images revealed a 1.83×1.40 cm
slightly lobulated nodule, with burr-like margins in the left lower
lung, exhibiting moderately increased F-18 FDG uptake (maximum SUV
of 2.6) in the initial images (1 h following the FDG injection),
and more intense FDG uptake with an SUV of 3.5 (26.9% increase) in
the delayed images (2 h following the injection) (Fig. 1). Based on the dual phase FDG PET/CT
imaging findings, morphological features, contrast-enhanced chest
CT imaging and medical history (progression of the nodule during
the two-month follow-up period), lung malignancy was highly
suspected. A percutaneous CT-guided thoracoscopic biopsy was
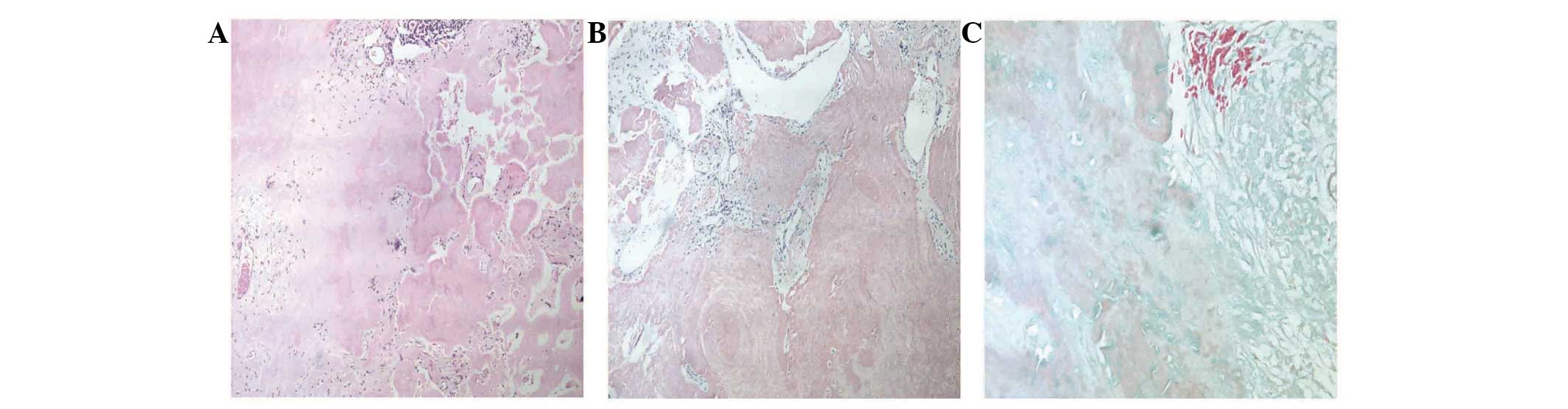
subsequently performed. Histological examination revealed that the
specimens contained amorphous, homogeneous material with a number
of polyclonal plasma cells, lymphocytes and giant cells.
Eosinophilic material exhibited apple-green birefringence under
polarizing microscopy. Immunohistochemically, congo red staining
was positive, and trichrome staining was negative, confirming the
deposition of amyloid within the specimen (Fig. 2). Therefore, a diagnosis of primary
nodular parenchymal pulmonary amyloidosis was determined, and the
patient was discharged without chemotherapy and other treatment.
After May 2011, the patient was followed up every 3 months and was
in good clinical condition at the time of writing.
Discussion
Pulmonary localized nodular amyloidosis,
characterized by the deposition of various proteins that form
insoluble β-pleated sheets in the lung parenchyma, is a rare
disorder and is not associated with primary systemic amyloidosis
(1,2,13).
Integrated PET/CT provides both metabolic and
morphological information for the characterization of nodules, and
is rapidly becoming a front-line modality in the evaluation of SPNs
(14). The diagnostic accuracy of
FDG avidity has been demonstrated by numerous studies to be ~90%
for the assessment of SPNs (15–17)
and the application of DTP imaging in particular has been reported
to potentially improve diagnostic accuracy. For differentiation
between benign and malignant lesions in the thorax, Demura et
al (18) estimated the
sensitivity and specificity to be 74% [37/50; 95% confidence
interval (CI), 0.60–0.85] and 50% (15/30; CI, 0.31–0.69),
respectively, for initial imaging, and 98% (49/50; CI, 0.89–1.00)
and 67% (20/30; CI, 0.47–0.83), respectively, for DTP imaging in 80
patients with thoracic nodular lesions. The authors concluded that
DTP imaging was more accurate compared with single-time-point
scanning, with the exception of use in patients with active
granulomatous diseases. Matthies et al (19) reported 36 patients with 38 known or
suspected malignant pulmonary nodules who underwent PET of the
thorax at two time points: Scan one at 70 min (range, 56–110 min)
and scan two at 123 min (range, 100–163 min) after the intravenous
injection of 2.5 MBq 18F-FDG per kilogram of body weight. The
sensitivity and specificity were found to be 80% (16/20; CI,
0.56–0.94) and 94% (17/18; CI, 0.73–1.00), respectively, for
initial imaging, and 100% (20/20; CI, 0.83–1.00) and 89% (16/18;
CI, 0.65–0.99) for DTP imaging in the detection of malignant lung
tumors. Though there are differences in the sensitivity and
specificity data between these two studies, which may be due to the
different sample sizes, it has been suggested that DTP FDG PET/CT
imaging was high value in the diagnosis or differentiation between
malignancy and benign for the patients with the SPNs.
To the best of our knowledge, few studies have
reported the evaluation of pulmonary amyloid lesions by FDG PET,
and each case has exhibited different FDG uptake activity (9–1§).
Ishii et al (9) reported a
nodular shadow in the right middle lobe with no FDG uptake observed
on FDG PET imaging. The nodule was circular and smooth, with
clearly demarcated borders, indicating benign nodules, which is
significantly different from the present case. Benign nodules are
generally well-defined and have smooth margins, whereas the
majority of malignant SPNs have irregular and spiculated margins
(20). Zhang et al (21) reported a 44-year-old male with
different sized nodules in both lungs detected on a chest CT scan.
As metastases were suspected in the multiple lung nodules, FDG
PET/CT was conducted to characterize the nodules and to detect a
possible primary malignancy. The PET/CT revealed that the nodules
had a mild uptake of 18F-FDG suggestive of malignancy, with a
maximum SUV of 1.19. Khan et al (22) and Soussan et al (23) revealed intense tracheobronchial FDG
uptake (maximum SUV of 4.6) associated with an intense uptake in
mediastinal fat, particularly in the surrounding aorta, which made
it difficult to differentiate between malignanct and benign
disease. DTP FDG PET/CT imaging may be an effective modality in the
evaluation of these lesions and, thus, it was performed in the
present study. However, only one case of a pulmonary amyloid lesion
has been reported with identification via DTP FDG PET/CT imaging.
Tan et al (12) presented a
case of amyloidosis exhibiting increased 18F-FDG accumulation upon
PET imaging. The 2-h delayed images revealed significantly reduced
metabolic activity in these lesions, and the authors concluded that
pulmonary amyloid lesions can potentially be distinguished from
malignancies using a FDG PET/CT scan. DTP FDG PET/CT imaging was
suggested as a discriminator between benign and malignant diseases,
with images being obtained 1 and 2 h after the administration of
18F-FDG. Malignant lesions showed a significant increase in SUV
over time, and those benign lesions showed a decrease over time
(11,24).
The present case demonstrates that nodular
amyloidosis may be indistinguishable from tumors due to
similarities in DTP 18F-FDG PET images and in morphological
changes. The DTP FDG PET scan revealed high FDG uptake in the
initial images, and more intense FDG uptake (an increase of 26.9%)
in the delayed images, which is consistent with the FDG uptake
characteristics of malignancy (13). Morphological evaluations can aid in
the differentiation between benign and malignant nodules only when
they have typically benign or malignant features. Determining the
growth rate of lung nodules by comparing current and prior CT
images is an important and cost-effective step in the evaluation of
SPNs (8). SPNs usually grow at
constant rates, expressed as the doubling time. A nodule with a
doubling time between 20 and 400 days is usually malignant, whereas
benign nodules usually have a doubling time of >400 days
(25). In the present case, a
lobulated nodule with burr-like margins was discovered in the left
lower lung, and exhibited progression during the two-month
follow-up period. Based on the DTP FDG PET/CT imaging findings,
morphological features, and medical history, lung malignancy was
highly suspected, however, histological evaluation revealed that
this diagnosis was incorrect and confirmed the lesions to be a
result of pulmonary amyloidosis.
In conclusion, this case study indicates that
localized nodular amyloidosis with increased FDG uptake on DTP FDG
PET imaging must be considered during the differential diagnosis of
growing lung nodules, and that a histological examination must be
performed to distinguish this disorder from lung malignancies.
Further prospective investigations on a larger sample of cases are
required to better define the potential benefits of DTP 18F-FDG PET
imaging in the diagnosis of primary pulmonary amyloidosis.
References
|
1
|
Utz JP, Swensen SJ and Gertz MA: Pulmonary
amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann
Intern Med. 124:407–413. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eguchi T, Yoshida K, Kobayashi N, et al:
Localized nodular amyloidosis of the lung. Gen Thorac Cardiovasc
Surg. 59:715–717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lesser A: Ein Fall von Enchondroma
osteiodes mixtum der Lunge mit partieller amyloid Entotung.
Virchows Arch (Pathol Anat). 69:404–408. 1877. View Article : Google Scholar
|
|
4
|
Ding L, Li W, Wang K, Chen Y, Xu H, Wang H
and Shen H: Primary tracheobronchial amyloidosis in China: analysis
of 64 cases and a review of literature. J Huazhong Univ Sci
Technolog Med Sci. 30:599–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiorelli A, Accardo M, Galluccio G and
Santini M: Tracheobronchial amyloidosis treated by endobronchial
laser resection and self expanding Y stent. Arch Bronconeumol.
49:303–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O’Regan A, Fenlon HM, Beamis JF Jr, et al:
Tracheobronchial amyloidosis. The Boston University experience from
1984 to 1999. Medicine (Baltimore). 79:69–79. 2000. View Article : Google Scholar
|
|
7
|
Cresner R, Mahmood S, Chen J, et al:
Thoracic amyloidomas: Two case reports of an evasive diagnosis.
JRSM Open. 5:20542704145272802014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gillmore JD and Hawkins PN: Amyloidosis
and the respiratory tract. Thorax. 54:444–451. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishii S, Kubota K, Minamimoto R, et al:
Lung amyloid nodule detected by 99mTc-aprotinin scintigraphy. Ann
Nucl Med. 26:522–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong YJ, Yi CA and Lee KS: Solitary
pulmonary nodules: detection, characterization, and guidance for
further diagnostic workup and treatment. AJR Am J Roentgenol.
188:57–68. 2007. View Article : Google Scholar
|
|
11
|
Lan XL, Zhang YX, Wu ZJ, Jia Q, Wei H and
Gao ZR: The value of dual time point (18)F-FDG PET imaging for the
differentiation between malignant and benign lesions. Clin Radiol.
63:756–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan H, Guan Y, Zhao J and Lin X: Findings
of pulmonary amyloidosis on dual phase FDG PET/CT imaging. Clin
Nucl Med. 35:206–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berk JL, O’Regan A and Skinner M:
Pulmonary and tracheobronchial amyloidosis. Semin Respir Crit Care
Med. 23:155–165. 2002. View Article : Google Scholar
|
|
14
|
Orlacchio A, Schillaci O, Antonelli L,
D’Urso S, et al: Solitary pulmonary nodules: morphological and
metabolic characterisation by FDG-PET-MDCT. Radiol Med.
112:157–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gould MK, Maclean CC, Kuschner WG, Rydzak
CE and Owens DK: Accuracy of positron emission tomography for
diagnosis of pulmonary nodules and mass lesions: a meta-analysis.
JAMA. 285:914–924. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong YJ, Lee KS and Kwon OJ: Diagnosis
and management of solitary pulmonary nodules. Expert Rev Respir
Med. 2:767–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SK, Allen-Auerbach M, Goldin J, et al:
Accuracy of PET/CT in characterization of solitary pulmonary
lesions. J Nucl Med. 48:214–220. 2007.PubMed/NCBI
|
|
18
|
Demura Y, Tsuchida T, Ishizaki T, et al:
18F-FDG accumulation with PET for differentiation between benign
and malignant lesions in the thorax. J Nucl Med. 44:540–548.
2003.PubMed/NCBI
|
|
19
|
Matthies A, Hickeson M, Cuchiara A and
Alavi A: Dual time point 18F-FDG PET for the evaluation of
pulmonary nodules. J Nucl Med. 43:871–875. 2002.PubMed/NCBI
|
|
20
|
Ooi GC, Khong PL and Yau YY: Advances in
imaging of the solitary pulmonary nodule. Hong Kong Med J.
10:107–116. 2004.PubMed/NCBI
|
|
21
|
Zhang LN, Xue XY, Wang N and Wang JX:
Mimicking pulmonary multiple metastatic tumors: A case of primary
nodular parenchymal pulmonary amyloidosis with review of the
literature. Oncol Lett. 4:1366–1370. 2012.PubMed/NCBI
|
|
22
|
Khan AM, Manzoor K, Jain V, Mahadevia P
and Berman A: Detection of nodular pulmonary amyloid by PET
positive scan - deception for lung cancer. Rev Port Pneumol.
18:299–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soussan M, Ouvrier MJ, Pop G, et al:
Tracheobronchial FDG uptake in primary amyloidosis detected by
PET/CT. Clin Nucl Med. 36:723–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alkhawaldeh K, Bural G, Kumar R and Alavi
A: Impact of dual-time-point (18)F-FDG PET imaging and partial
volume correction in the assessment of solitary pulmonary nodules.
Eur J Nucl Med Mol Imaging. 35:246–252. 2008. View Article : Google Scholar
|
|
25
|
Erasmus JJ, Connolly JE, McAdams HP and
Roggli VL: Solitary pulmonary nodules: Part I. Morphologic
evaluation for differentiation of benign and malignant lesions.
Radiographics. 20:43–58. 2000. View Article : Google Scholar : PubMed/NCBI
|