Introduction
Globally, gastric cancer (GC) is the predominant
cause of gastrointestinal cancer and is the second leading cause of
cancer-related mortality (1,2).
Despite the use of curative resection for the treatment of
resectable GC, recurrence rates remain high following surgery.
Furthermore, the majority of patients are diagnosed at an advanced
stage of GC, at which surgical resection is no longer feasible;
although palliative radiotherapy and chemotherapy provide some
benefit, the prognosis of advanced GC remains poor (3–6).
Various attempts have been made to improve the
objective response rate to GC treatment, including the development
of biologically targeted agents in combination with traditional
chemotherapy regimens; for example, trastuzumab in combination with
a cisplatin (DDP)-based chemotherapy regimen. Trastuzumab is a
humanized monoclonal anti-human epidermal growth factor receptor 2
(HER2) antibody treatment. The first phase III, prospective,
randomized, multicenter trial to evaluate its efficacy and safety
for HER2-positive GC treatment was the Trastuzumab for Gastric
Cancer (ToGA) study (7–9). Although trastuzumab (Herceptin™) in
combination with a DDP-based chemotherapy regimen produced a
significant overall survival benefit, the benefit was modest and
the mechanism involved was not clearly addressed.
A number of in vitro and in vivo
studies have demonstrated that the administration of trastuzumab in
combination with chemotherapeutic agents produces an additive
effect, a synergistic effect or both in breast cancer (10–13).
Furthermore, previous in vitro studies demonstrated that
trastuzumab in combination with DDP (14) and doxorubicin (15) produces a synergistic effect in human
HER2-overexpressing GC cells; however, the mechanisms of
this synergistic anticancer activity are yet to be fully
explored.
Chromosome ends consist of specialized structures
called telomeres that are critical to chromosome integrity. In
vertebrates, telomeres consists of thousands of T2AG3 hexamer
repeats; the nucleophilic sites of these repeats are able to react
with platinum agents to form DNA adducts, principally at adjacent
deoxyguanines (GpG). The DNA adducts formed are 1,2-intrastrand
cross-links, which result in the efficient inhibition of DNA
replication, RNA transcription, cell cycle arrest or apoptosis.
Subject to the presence of two or more tandem guanines, platinum
agents exhibit maximal targeting of the DNA; thus, telomeric
repeats are a good target for platinum agents (16). Furthermore, a previous in
vitro study demonstrated that telomere dysfunction may increase
DDP sensitivity in melanoma cells (17) and an increasing number of proteins
have been discovered to interact with telomere DNA repeats; for
example, telomere protection, function, and length appear to depend
on the shelterin protein complex [telomeric repeat binding factor 1
(TRF1), TRF2, TPP1 (formerly known as TINT1, PTOP and PIP),
protection of telomere 1 (POT1), TRF1-interacting nuclear factor
(TIN2), and TRF2-interacting protein 1 (TRF2IP)] (18). The ToGA study indicated that
trastuzumab in combination with a DDP-based chemotherapy regimen
resulted in a significant overall survival benefit (9). In the present study, we hypothesize
that trastuzumab may additionally affect the expression levels of
the abovementioned telomere-associated proteins and, thus, the
sensitivity of GC cells to platinum agents.
The present preclinical study was undertaken to
investigate the effect of low-dose trastuzumab on the sensitivity
of GC cells to platinum agents, and to elucidate the possible
mechanisms involved in the interaction between the trastuzumab and
platinum agents. In addition, the protein and mRNA expression
levels of telomere-associated genes and proteins was investigated
in GC cells following treatment with trastuzumab and platinum
agents, alone and in combination.
Methods
Cell lines and cell culture
The effects of trastuzumab, Oxa, DDP, 5-fluorouracil
(FU) and taxol administration (alone and in combination) on
malignant cell growth were studied in a panel of five human GC cell
lines (AGS, NCI-N87, MGC-803, HGC and MKN45) obtained from the
Shanghai Institute of Cell Biology (Shanghai, China). Of these,
NCI-N87 is an HER2-amplified cell line (19). All of the cell lines were cultured
in RPMI-1640 medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% bovine serum (Invitrogen Life Technologies,
Carlsbad, CA, USA), 100 mg/ml streptomycin (Sichuan Pharmaceutical
Co., Ltd., Sichuan, China), 100 U/ml penicillin (Sichuan
Pharmaceutical Co., Ltd.), insulin (Tonghua Dongbao Pharmaceutical
Co., Ltd., Jilin, China), glutamine and pyruvate (Invitrogen Life
Technologies) at 37°C in a 5% CO2 water-saturated
atmosphere.
Platinum agents
Prior to each experiment, the dilutions of all of
the reagents were freshly prepared. Trastuzumab was obtained from
the University of California Pharmaceutical Services (Los Angeles,
CA, USA) and was prepared from a stock concentration of 20 mg/ml.
Oxa, DDP, 5-FU and Taxol were supplied by the Jiangsu Hengrui
Medicine Co., Ltd. (Lianyungang, China). A CellTiter 96®
AQueous One Solution Cell Proliferation Assay kit was purchased
from Promega Corporation (Madison, WI, USA) and the
Annexin-V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
was purchased from Invitrogen Life Technologies.
Cell viability assay
The CellTiter 96 kit (Promega Corporation) was used
to determine cytotoxicity, according to the manufacturer’s
instructions. Briefly, the five human GC cell lines were grown to
the log phase, trypsinized, seeded into 96-well plates at a density
of 2×103 cells/well and incubated overnight to allow
cell adherence. Subsequently, the medium in each well was replaced
with fresh (platinum agent-free) medium or medium containing
various concentrations (1×10−4, 1×10−3,
1×10−2, 1×10−1, 1, 10 and 100 μg/ml) of
platinum agents (Oxa and DDP) and was incubated for an additional
48 h. CellTiter 96 AQueous One solution was added to each well at
one fifth of the mixture volume and the plates were incubated for 3
h. Absorbance was determined at an wavelength of 490 nm using a
microplate reader (Bio-Rad Laboratories, Hercules, CA, USA), with
blank control wells to zero the absorbance. For each experiment, 10
control wells were allocated for platinum agent-free medium and a
minimum of six replicate wells were allocated for each
concentration of platinum agent-containing medium. Using the
background-corrected absorbance values, the inhibition rate [I (%)]
was calculated by the following equation: I (%) = 100 ×
(Auntreated control well − Aexperimental
well)/Auntreated control well. The half maximal
inhibitory concentration (IC50) was defined as the
concentration of platinum agent required for 50% inhibition of cell
growth.
Apoptosis assay
The number of apoptotic cells was quantified using
an Annexin V-FITC Apoptosis Detection kit (Invitrogen Life
Technologies), according to the manufacturer’s instructions.
Briefly, the NCI-N87 cells were grown to 75–80% confluence in 60-mm
Petri dishes, exposed to trastuzumab (1.0 μg/ml) and platinum
agents (Oxa, 5 μg/ml; DDP, 2.5 μg/ml) alone or in combination for
48 h, and compared with the untreated control cells. To quantify
the apoptosis, the cells were collected, resuspended in 500 μl
binding buffer, treated with 5 μl Annexin V-FITC and 5 μl propidium
iodide (PI), and analyzed using a FACSCalibur™ flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Briefly, the three human GC cell lines, NCI-N87,
HGC27 and MKN45, were grown to the log phase, trypsinized, washed
with phosphate-buffered saline (PBS) and collected by performing
centrifugation for 5 min at 174 × g. Total RNA was extracted from
each cell line using the SV Total RNA isolation system (Promega
Corporation), according to the manufacturer’s instructions. The
purity and quality of the extracted mRNA were determined using a
Bio-visible spectrophotometer at 260 and 280 nm (Eppendorf,
Hamburg, Germany); and the integrity of the extracted mRNA was
determined by performing agarose gel electrophoresis on a 1% gel.
Reverse transcriptase from the reverse transcription system was
used according to the manufacturer’s instructions (Promega
Corporation) to synthesize a total volume of 20 μl complementary
DNA for each cell line, and the iCycler iQ™ Multi-Color Real Time
PCR detection system (Bio-Rad Laboratories, Inc.) was used to
perform RT-qPCR of the target genes and the internal control
(β-actin). Applied Biosystems Life Technologies (Foster
city, CA, USA) supplied the primers [1X; Assay IDs: Hs00819517_mH
(TRF1); Hs01554305_g1 (TIN2); Hs00194619_m1
(TRF2); Hs00368526_g1 (TPP1); Hs00430292_m1
(TRF2IP); Hs00209984_m1 (POT1); and Hs99999903_m1
(β-actin)] and probe mixture, and AbGene Ltd. (Surrey, UK)
supplied the ABsolute qPCR mix (1X) used to produce the 20-μl PCR
reaction mixture. The PCR conditions were as follows: 50°C for 2
min, 95°C for 15 min, followed by 45 cycles at 95°C for 15 sec and
60°C for 1 min. Using β-actin as an endogenous control and
commercial human total RNA samples (Clontech Laboratories, Inc.,
Mountainview, CA, USA) as calibrators, the relative gene expression
levels were quantified according to the comparative Ct method,
employed the formula 2−ΔΔCt to determine the final
results (20). Following PCR, the
10-μl product was loaded onto a 1.5% agarose gel and visualized by
ethidium bromide staining (Sigma-Aldrich, Munich, Germany).
Western blot analysis
Total cell lysates were prepared in RIPA lysis
buffer (Pierce Biotechnology, Inc., Rockford, IL, USA). Following
determination of protein concentration using a bicinchoninic acid
protein assay kit (Pierce Biotechnology, Inc.), an aliquot of
lysate containing 50 μg of each protein was subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to
polyvinylidene fluoride membranes, blocked with blocking buffer
(PBS Tween-20 containing 5% non-fat milk) for 2 h at room
temperature and incubated overnight at 4°C with the following
specific primary antibodies: monoclonal mouse anti-human TPP1 (ACD;
cat. no. TA504406), monoclonal rabbit anti-human POT1 (cat. no.
TA310771), polyclonal rabbit anti-human TRF1 (cat. no. TA322887),
rabbit anti-human monoclonal TRF2 (cat. no. TA307200), rabbit
anti-human polyclonal TIN2 (cat. no. TA315321), rabbit anti-human
polyclonal TRF2IP (cat. no. TA324532 ) and rabbit anti-human
polyclonal GAPDH (cat. no. TA308884) (1:10,000, all primary
antibodies; OriGene Technologies, Inc., Rockville, MD, USA).
Subsequent incubation with the appropriate horseradish
peroxidase-conjugated goat anti-rabbit POT1, TRF1, TRF2, TIN2,
TRF2IP, GAPDH (cat. no. SP 9001) and goat anti-mouse TPP1 (cat. no.
SP9002) secondary antibodies (1:1,000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) was performed for 2 h at room
temperature. Signals were then detected using enhanced
chemiluminescence reagents (Thermo Fisher Scientific, Waltham, MA,
USA), and a FluorChem SP imaging system (Alpha Innotech, San
Leandro, CA, USA) was used for image capture.
Statistical analysis
Values are expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). Statistical
comparison was performed using Student’s t-test and P<0.05 was
considered to indicate a statistically significant difference.
Results
Trastuzumab renders HER2-amplified cancer
cells sensitive to platinum agents
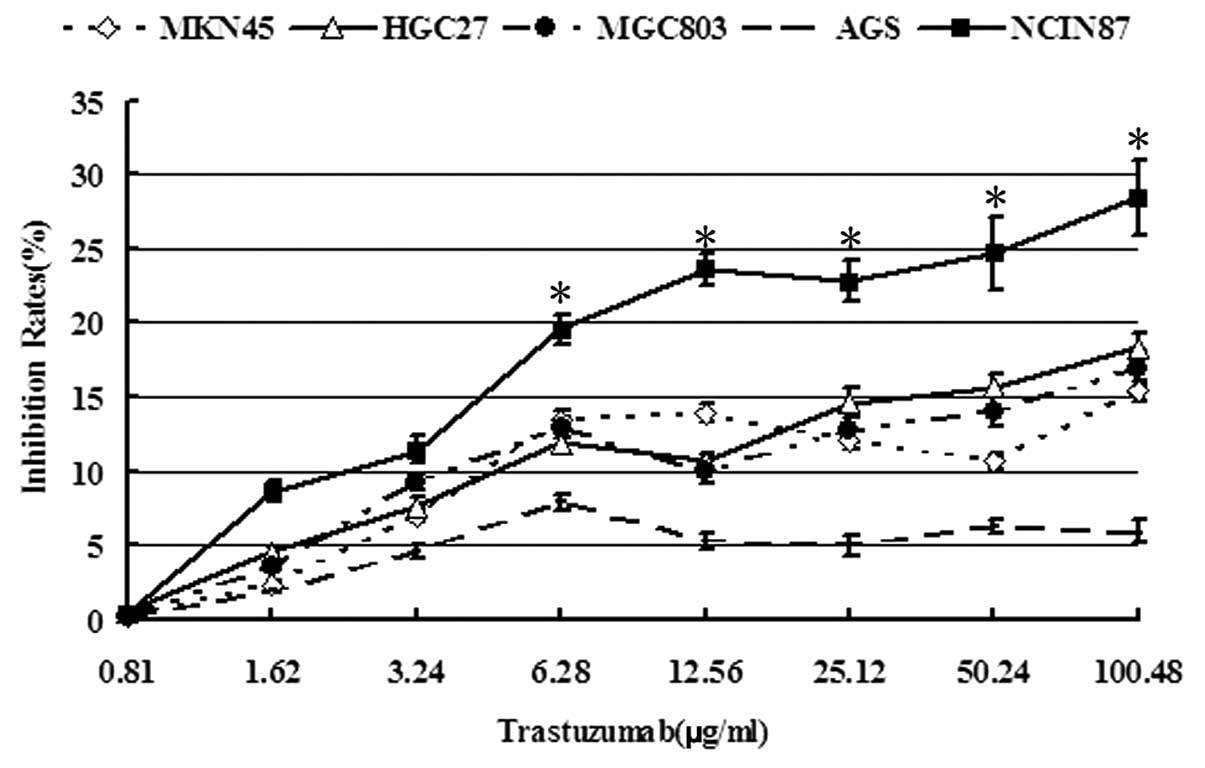
The cytotoxicity of trastuzumab to five GC cell
lines was initially analyzed using a CellTiter 96 Aqueous One
Solution Cell Proliferation Assay kit. It was identified that
trastuzumab (6.28, 12.56, 25.12, 50.24 and 100.48 μg/ml)rendered
more significant cytotoxicity to the NCI-N87 cell line with
HER2 amplification compared with the four other cell lines
(P<0.05). At 0.81–100.48 μg/ml trastuzumab, the inhibition rates
to NCI-N87 cells were not >30% and 0.81–1.62 μg/ml trastuzumab
was not obviously cytotoxic to cancer cells (survival rate,
>90%; Fig. 1). Thus, as
treatment of the cells with 1.0 μg/ml trastuzumab exhibited no
significant effect on cell viability, this concentration was used
for subsequent analyses.
Additionally, 1.0 μg/ml trastuzumab was administered
to five GC cell lines and the effect on the sensitivity of the cell
lines to various platinum agents was investigated. It was
identified that pretreatment with trastuzumab significantly
increased the sensitivity of only the NCI-N87 cell line to platinum
agents. As indicated in Table I,
the IC50 of Oxa and DDP was decreased to ~3.29 (P=0.001)
and 6.91 times (P=0.002) in NCI-N87 cells, respectively; however,
trastuzumab did not alter the IC50 of Oxa or DDP in the
other four cell lines. Subsequently, it was identified that
simultaneous treatment with low-dose trastuzumab and platinum
agents may significantly increase the sensitivity of NCI-N87 cells
to platinum agents; for example, the IC50 of Oxa and DDP
were reduced by ~2.67 (P=0.001) and 4.56 times (P=0.003),
respectively. Thus, these data indicate that low-dose trastuzumab
may increase platinum sensitivity in NCI-N87 cells.
 | Table IEffects of trastuzumab on the
IC50 of DDP and Oxa in five gastric cancer cell
lines. |
Table I
Effects of trastuzumab on the
IC50 of DDP and Oxa in five gastric cancer cell
lines.
| Gastric cancer cell
line (IC50, mean ± standard deviation) |
|---|
|
|
|---|
| Agent | NCI-N87a | HGC27 | MGC803 | AGS | MKN45 |
|---|
| DDP | 12.86±1.41 | 10.23±1.94 | 15.26±1.85 | 9.39±1.77 | 26.44±2.72 |
| DDP
(trastuzumab)b | 1.86±0.55c | 10.97±2.36 | 14.38±1.63 | 10.19±3.02 | 25.44±4.70 |
| Oxa | 21.53±1.96 | 15.07±3.30 | 27.26±3.39 | 19.21±1.85 | 35.77±3.82 |
| Oxa
(trastuzumab)b | 6.53±1.10d | 14.66±2.66 | 26.69±4.47 | 20.39±3.70 | 37.64±6.66 |
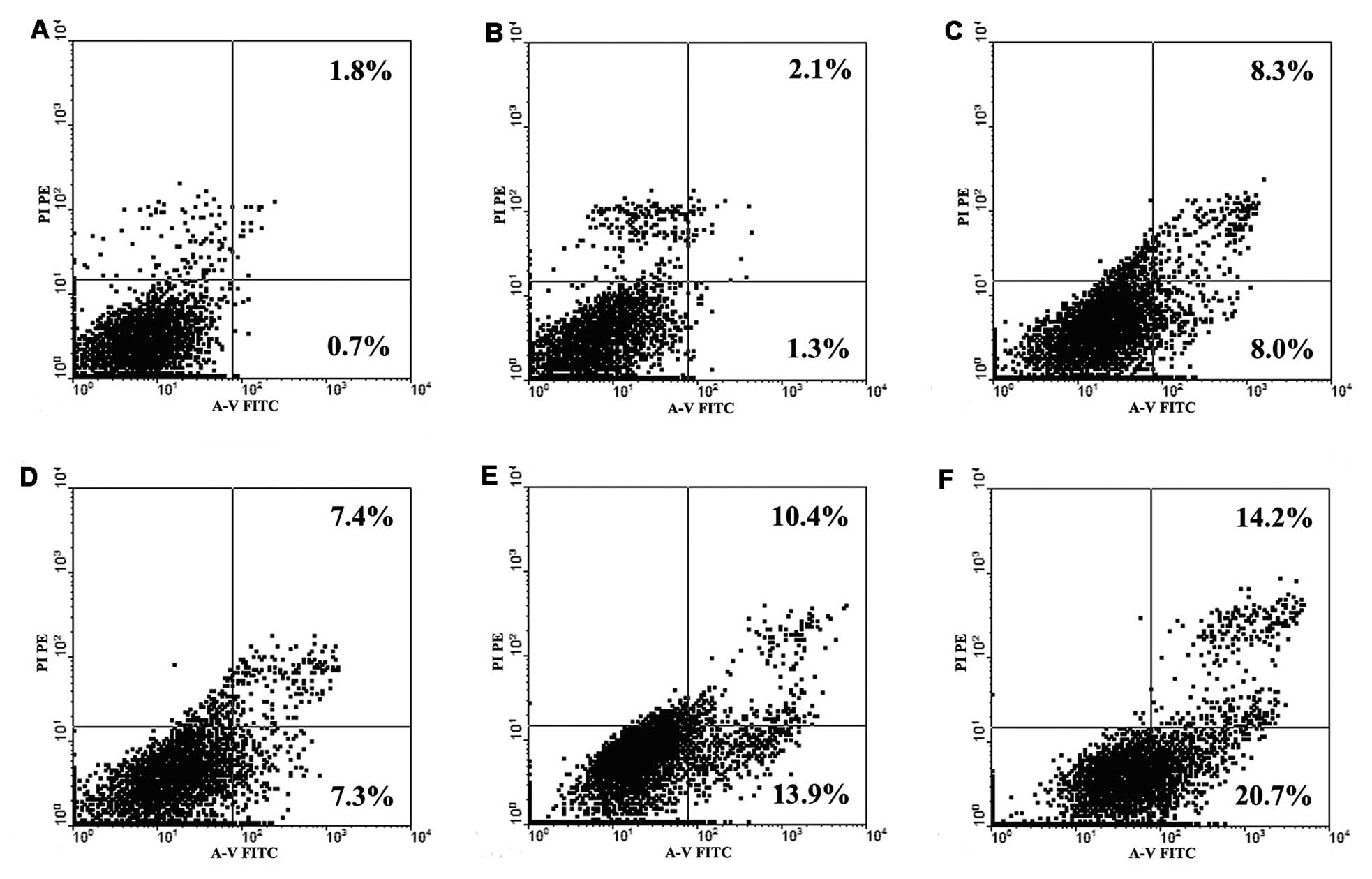
Trastuzumab treatment increases platinum
agent-induced apoptosis in NCI-N87 cells
To further investigate whether low-dose trastuzumab
increases the sensitivity of NCI-N87 cells to platinum agents, GC
cells were double-stained with Annexin V-FITC and PI to detect
early apoptosis induced by treatment with platinum agents and
trastuzumab, alone or in combination. The doses of Oxa and DDP
selected were 5 μg/ml and 2.5 μg/ml, respectively, as they were
close to the 20% inhibitory concentrations (IC20) of
NCI-N87 cells. The dose of trastuzumab used was 1.0 μg/ml, as
previously determined. For the groups treated with two agents in
combination, the cells were pretreated with trastuzumab for 48 h,
followed by the addition of platinum agents for 48 h. As indicated
in Fig. 2, the percentage of early
apoptosis induced by independent Oxa and DDP administration in
NCI-N87 cells was 8.0 and 7.3%, respectively; however, this
increased to 13.9 and 20.7% upon treatment with trastuzumab in
combination with Oxa and DDP, respectively. Furthermore, treatment
with trastuzumab alone (1.0 μg/ml) did not induce significant
apoptosis in the cancer cells. Thus, it appears that low-dose
trastuzumab administration may increase platinum agent-induced
apoptosis in NCI-N87 cells and subsequently result in the increased
cytotoxicity of platinum agents (Fig.
1).
Trastuzumab downregulates mRNA and
protein expression levels of telomere-associated genes in NCI-N87
cells
To understand the mechanisms by which trastuzumab
may increase the sensitivity of NCI-N87 cells to platinum agents,
we hypothesized that trastuzumab may affect the expression levels
of telomere-associated genes or proteins in NCI-N87 cells, thus,
influencing their sensitivity to platinum agents. Following
incubation with 1.0 μg/ml trastuzumab alone, 2.5 μg/ml DDP alone or
the two agents in combination, the mRNA expression of various
telomere-associated genes and their proteins were assessed in
NCI-N87 cells by performing RT-qPCR and western blot analysis. As
demonstrated in Fig. 3A, the mRNA
expression levels of TRF1, TRF2, POT1,
TPP1 and TRF2IP were significantly downregulated
following treatment with trastuzumab alone or DDP in combination
with trastuzumab in NCI-N87 cells (P<0.05), and DDP alone only
downregulated the expression level of TRF2 (P<0.05).
However, in the HGC27 and MKN45 cell lines, treatment wth low dose
trastuzumab alone or in combination with DDP did not significantly
downregulate the mRNA expression levels of any of the
telomere-associated genes (P>0.05) (data not shown). Western
blot analysis indicated that treatment with trastuzumab alone and
in combination with DDP significantly downregulated the protein
expression levels of TRF2, POT1 and TPP1 in NCI-N87 cells, and DDP
alone did not downregulate the protein expression level of any of
the telomere-associated genes investigated (data not shown). The
results indicated that low-dose trastuzumab administration may
downregulate the mRNA and protein expression levels in an area of
the telomere-associated genes that may be involved in
chemosensitivity NCI-N87 cells to platinum agents.
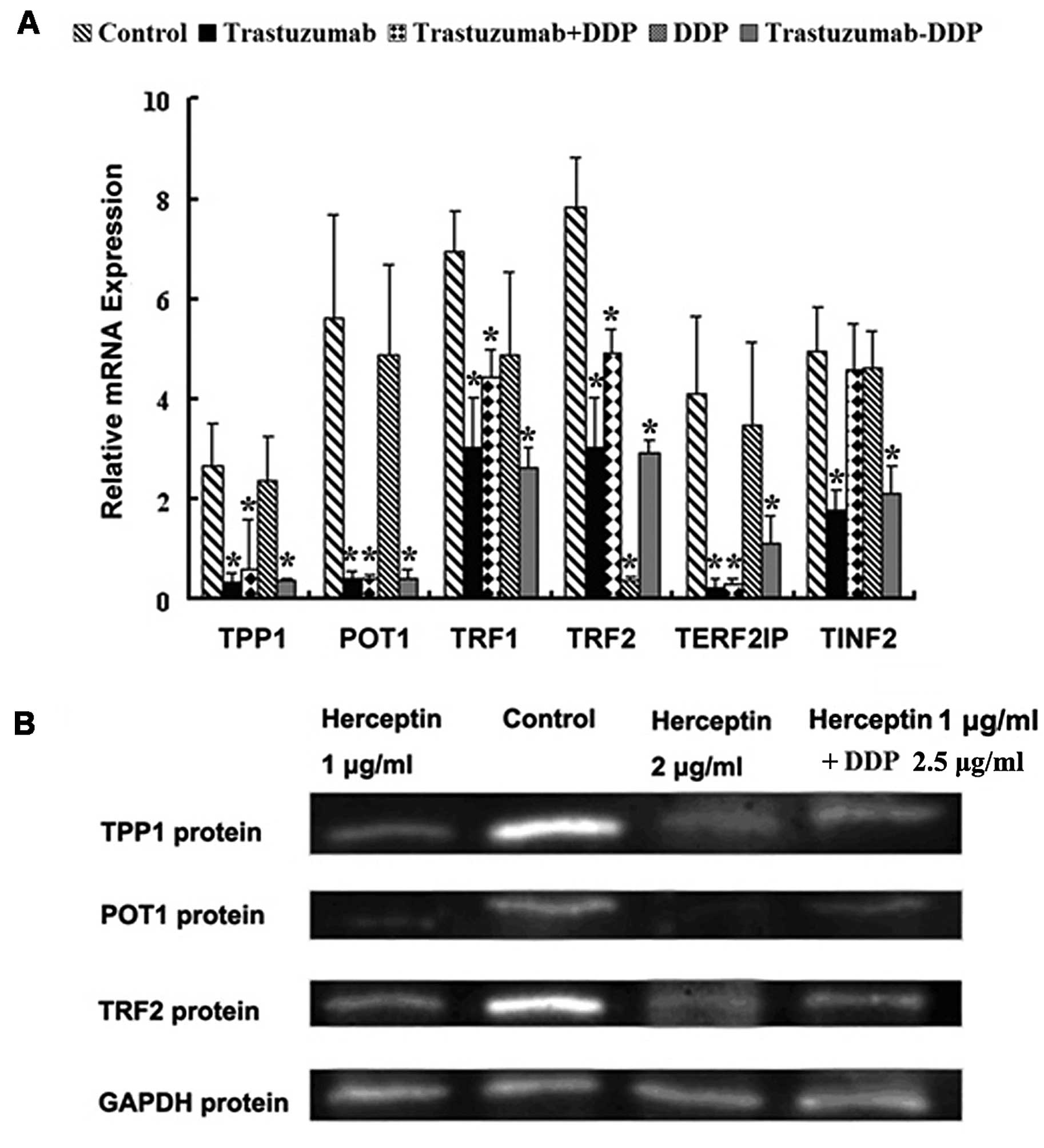 | Figure 3Effect of trastuzumab administration
on the expression of telomere-associated genes and proteins in
NCI-N87 cells. (A) Reverse transcription-quantitative polymerase
chain reaction was used to determine the levels of TPP1,
POT1, TRF1, TRF2, TERF2IP and
TINF2 mRNA expression in NCI-N87 cells treated with
trastuzumab and cisplatin (alone or in combination). Error bars
indicate the standard deviation from the mean.
*P<0.05, vs. control cells. (B) Western blot analysis
determined that the protein expression levels of TPP1, POT1 and
TRF2 in NCI-N87 cells were significantly inhibited by various
concentrations of trastuzumab alone and low-dose trastuzumab in
combination with cisplatin. TPP1 (formerly known as TINT1, PTOP and
PIP); POT1, protection of telomere 1; TRF2, telomeric repeat
binding factor 2; Control, untreated NCI-N87 cells. |
Discussion
Overexpression and activation of HER2 has
previously been associated with chemotherapy resistance in
HER2 gene-amplified cancer cells (21–23),
and telomere dysfunction has been associated with platinum
sensitivity in cancer cells (17).
The aim of the present study was to investigate whether the
antitumor activity of platinum agents commonly used in the
treatment GC could be enhanced by the addition of low-dose
trastuzumab and to discuss the possible mechanisms of platinum
agent-resistance in GC cells, which may involve telomere-associated
proteins. First, it was investigated whether low-dose trastuzumab
could increase the sensitivity of five GC cell lines to platinum
agents by performing cell proliferation and early apoptosis
analysis. The findings of the present study indicate that treatment
with low-dose trastuzumab may reduce the IC50 of
platinum agents and increase the early apoptosis rates induced by
platinum agents in NCI-N87 cells, which overexpress HER2
gene; however, this increase in sensitivity was not observed in the
other four cell lines. Additionally, the expression levels of
telomere-associated genes appeared to be downregulated in NCI-N87
cells following treatment with low-dose trastuzumab alone or
trastuzumab in combination with DDP. These results indicate that
pretreatment with low-dose trastuzumab followed by platinum agent
administration may be a promising method the treatment of
HER2-positive advanced GC. Thus, a possible, partial mechanism for
the increase in platinum agent sensitivity may be the
downregulation of the expression of telomere-related genes.
HER-2/neu or c-erbB-2 is a member of the HER family
of growth factors (endothelial growth factor receptor, erbB-2,
erbB-3 and erbB-4), which exhibit intrinsic protein tyrosine kinase
activity. Increased HER-2 activity is the assumed mechanism
underlying cell cycle control, proliferation, differentiation,
motility, apoptosis, metastasis and transformation (14,24–26)
and HER-2 overexpression is observed in numerous human
carcinomas, including breast, ovarian, gastric, colon and non-small
cell lung cancer. Trastuzumab, a humanized monoclonal antibody
(mAb) against the extracellular domain of HER2, has been approved
by the Food and Drug Administration for the treatment of patients
with invasive HER2-overexpressing breast cancer (27). Additionally, HER2-amplified
GC is the most probable candidate for responding to trastuzumab
treatment, as various studies have demonstrated that an antitumor
effect occurs when trastuzumab is added to HER2-amplified GC
cells or the corresponding xenograft models (19,28,29).
Additionally, the results of the present study demonstrated
significant cytotoxicity of trastuzumab on the
HER2-amplified NCI-N87 cell line; however, the inhibition
rates were not >30%, even at the highest concentration. These
results are consistent with those of a previous in vitro
study (30), which indicated that
the administration of trastuzumab alone may not exhibit a
significant antitumor effect.
Numerous in vitro and in vivo studies
have demonstrated that additive and synergistic effects occur when
trastuzumab is combined with other chemotherapeutic agents to treat
breast cancer and GC (10–14). The mechanisms by which this occurs
include a reduction in DNA repair activity following
chemotherapeutic agent-induced DNA damage (31,32),
inhibition of the unscheduled DNA synthesis (33), and increased apoptosis via the
activation of antibody responses and reduced expression of
anti-apoptotic genes (34,35).
In the present study, it was identified that
low-dose trastuzumab administration may significantly decrease the
IC50 of platinum agents and increase the early apoptosis
rates induced by them in NCI-N87 cells, which overexpress the
HER2 gene, but not in other four cell lines. These results
indicated that the HER2/neu signaling pathway may participate in
the mechanisms of platinum agent chemosensitivity in
HER2-amplified GC. The results obtained in the present study
are partially consistent with those reported by Yu and Hung
(36), Funato et al
(37), and Järvinen and Liu
(38). For example, Yu and Hung
(36) reported that the
trastuzumab-induced increase in paclitaxel sensitivity in
HER2-overexpressing breast cancer cells may occur by
reversing the anti-apoptotic function of HER2. In the present
study, the administration of trastuzumab alone at 1.0 μg/ml did not
induce significant apoptosis in the NCI-N87 cells; however, when
the NCI-N87 cells were treated with trastuzumab in combination with
DDP or Oxa for 48 h, the early apoptotic rates were markedly
increased to 20.7 and 13.9%, respectively. Thus, it appears that
trastuzumab may increase platinum agent-induced apoptosis in
NCI-N87 cells, resulting in the increased cytotoxicity of platinum
agents.
Telomeres are nucleoprotein complexes located at the
ends of chromosomes. In vertebrates, telomeres consists of tandem
repeats of the T2AG3 hexamer, a G-rich motif and associated
proteins (39). Due to the presence
of guanine triplets, telomeric DNA is considered to be a
preferential target for DDP (16).
Furthermore, telomeres are critical for genomic stability as they
provide a mechanism for the maintenance and protection of
chromosomal ends by folding into a structure termed the T-loop. The
T-loop is characterized by a single-stranded overhang of 30
nucleotides, which is sequestered by invasion of a duplex region of
the telomere (39). TRF1, TRF2,
POT1, TRF2IP, TPP1 and TIN2 form a six-protein complex termed
shelterin (18), which contributes
to the formation of the T-loop as well as the telomere protection
and length regulation. Within shelterin, TRF1, TRF2 and POT1 bind
directly to the telomeric DNA; TRF1 forms a complex with a number
of proteins, including tankyrase, TIN2, TPP1 and POT1 (40–43);
and TRF2 interacts with various proteins, including TRF2IP, TIN2
and POT1 (41,42,44–46).
The uncapping of telomeres due to dysfunctional telomeric proteins
or telomere shortening can disrupt their protective function and
activate a DNA damage response (47,48).
The loss of telomere protection, whether induced by telomere
shortening or via the disruption of telomere structure, is commonly
referred to as telomere dysfunction, and has been reported as the
principal determinant governing chemosensitivity against agents
that induce double-strand DNA breaks (49,50).
In conclusion, the present study identified that the
mRNA and protein expression levels of TRF2, POT1 and TPP1 were
significantly downregulated following treatment with trastuzumab
alone or trastuzumab in combination with DDP. Thus, it is proposed
that the HER2/neu signaling pathway modulates the expression of a
number of telomere-associated proteins, resulting in telomere
dysfunction. Telomere dysfunction may represent a physiological
trigger of the DNA damage or apoptotic response, analogously to
other genotoxic insults that introduce chromosome breaks.
Concurrently, telomere dysfunction may additionally contribute to
sensitizing the platinum agents in HER2-amplified GC cells.
The mechanisms by which trastuzumab influences the expression of
these telomere-associated proteins remain unclear; however, a
possible mechanism may involve changes in numerous signal
transduction pathways associated with drug-gene or drug-protein
interactions. Thus, additional studies are required to analyze the
effects of platinum agents on the gene and protein expression
profiles of various signaling pathways.
Acknowledgements
The abstract was presented at the 2013
Gastrointestinal Cancers Symposium and published as abstract no. 53
in J Clin Oncol 31 (suppl 4; abstr 53), 2013. The present authors
thank the personnel at the Changzhou Tumor Hospital (Changzhou,
China) who were involved in the present study. The current study
was supported by the Science and Technology Planning Project of
Changzhou Health Bureau (Jiangsu, China; grant nos. QN201106 and
ZD201203), the Natural Science Foundation of China (grant nos.
81071799, 81372212, BK2011251 and BL2013012), the Research Project
of the Health Department of Jiangsu Province (grant no. Z201221),
the Science and Technology Planning Project of Jiangsu (grant no.
CE20135051), the Science and Technology Planning Project of
Traditional Chinese Medicine, Jiangsu (grant no. LZ13143), the 333
Talents Training Project of Jiangsu Province (grant no. 2011-0635),
the Key Medical Innovation Talents Training Project of Changzhou
(grant no. 2010368), and the Project of Jiangsu Province Sanitation
Innovation Team (grant no. LJ201157).
Reference
|
1
|
Leung WK, Wu MS, Kakugawa Y, et al; Asia
Pacific Working Group on Gastric Cancer. Screening for gastric
cancer in Asia: current evidence and practice. Lancet Oncol.
9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hundahl SA, Phillips JL and Menck HR: The
National Cancer Data Base Report on poor survival of U.S. gastric
carcinoma patients treated with gastrectomy: Fifth Edition American
Joint Committee on Cancer staging, proximal disease, and the
“different diseas” hypothesis. Cancer. 88:921–932. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al; MAGIC Trial Participants. Perioperative
chemotherapy versus surgery alone for resectable gastroesophageal
cancer. New Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macdonald JS, Smalley SR, Benedetti J,
Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA,
Gunderson LL, Jessup JM and Martenson JA: Chemoradiotherapy after
surgery compared with surgery alone for adenocarcinoma of the
stomach or gastroesophageal junction. New Engl J Med. 345:725–730.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smalley SR, Benedetti JK, Haller DG,
Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson
JA, Jessup JM, et al: Updated analysis of SWOG-directed intergroup
study 0116: a phase III trial of adjuvant radiochemotherapy versus
observation after curative gastric cancer resection. J Clin Oncol.
30:2327–2333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hede K: Gastric cancer: trastuzumab trial
results spur search for other targets. J Natl Cancer Inst.
101:1306–1307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jørgensen JT: Targeted HER2 treatment in
advanced gastric cancer. Oncology. 78:26–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al; ToGA Trial Investigators. Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): a phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pegram MD, Konecny GE, O’Callaghan C,
Beryt M, Pietras R and Slamon DJ: Rational combinations of
trastuzumab with chemotherapeutic drugs used in the treatment of
breast cancer. J Natl Cancer Inst. 96:739–749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pegram M, Hsu S, Lewis G, et al:
Inhibitory effects of combinations of HER-2/neu antibody and
chemotherapeutic agents used for treatment of human breast cancers.
Oncogene. 18:2241–2251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pegram MD, Pienkowski T, Northfelt DW, et
al: Results of two open-label, multicenter phase II studies of
docetaxel, platinum salts, and trastuzumab in HER2-positive
advanced breast cancer. J Natl Cancer Inst. 96:759–769. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robert N, Leyland-Jones B, Asmar L, et al:
Randomized phase III study of trastuzumab, paclitaxel, and
carboplatin compared with trastuzumab and paclitaxel in women with
HER-2-overexpressing metastatic breast cancer. J Clin Oncol.
24:2786–2792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SY, Kim HP, Kim YJ, et al: Trastuzumab
inhibits the growth of human gastric cancer cell lines with HER2
amplification synergistically with cisplatin. Int J Oncol.
32:89–95. 2008.
|
|
15
|
Gong SJ, Jin CJ, Rha SY and Chung HC:
Growth inhibitory effects of trastuzumab and chemotherapeutic drugs
in gastric cancer cell lines. Cancer Lett. 214:215–224. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burstyn JN, Heiger-Bernays WJ, Cohen SM
and Lippard SJ: Formation of cis-diamminedichloroplatinum(II)
1,2-intrastrand cross-links on DNA is flanking-sequence
independent. Nucleic Acids Res. 28:4237–4243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biroccio A, Gabellini C, Amodei S, et al:
Telomere dysfunction increases cisplatin and ecteinascidin-743
sensitivity of melanoma cells. Mol Pharmacol. 63:632–638. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Lange T: Shelterin: the protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanner M, Hollmén M, Junttila TT, et al:
Amplification of HER-2 in gastric carcinoma: association with
Topoisomerase IIα gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Modi S, DiGiovanna MP, Lu Z, et al:
Phosphorylated/activated HER2 as a marker of clinical resistance to
single agent taxane chemotherapy for metastatic breast cancer.
Cancer Invest. 23:483–487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai CM, Chang KT, Perng RP, et al:
Correlation of intrinsic chemoresistance of non-small-cell lung
cancer cell lines with HER-2/neu gene expression but not with ras
gene mutations. J Natl Cancer Inst. 85:897–901. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calikusu Z, Yildirim Y, Akcali Z, et al:
The effect of HER2 expression on cisplatin-based chemotherapy in
advanced non-small cell lung cancer patients. J Exp Clin Cancer
Res. 28:972009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klapper LN, Glathe S, Vaisman N, et al:
The ErbB-2/HER2 oncoprotein of human carcinomas may function solely
as a shared coreceptor for multiple stroma-derived growth factors.
Proc Natl Acad Sci USA. 96:4995–5000. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moasser MM: Targeting the function of the
HER2 oncogene in human cancer therapeutics. Oncogene. 26:6577–6592.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hung MC and Lau YK: Basic science of
HER-2/neu: a review. Semin Oncol. 26(Suppl 12): 51–59.
1999.PubMed/NCBI
|
|
27
|
Hudis CA: Trastuzumab - mechanism of
action and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui Y, Inomata M, Tojigamori M, Sonoda
K, Shiraishi N and Kitano S: Suppression of tumor growth in human
gastric cancer with HER2 overexpression by an anti-HER2 antibody in
a murine model. Int J Oncol. 27:681–685. 2005.PubMed/NCBI
|
|
29
|
Fujimoto-Ouchi K, Sekiguchi F, Yasuno H,
Moriya Y, Mori K and Tanaka Y: Antitumor activity of trastuzumab in
combination with chemotherapy in human gastric cancer xenograft
models. Cancer Chemother Pharmacol. 59:795–805. 2007. View Article : Google Scholar
|
|
30
|
Wainberg ZA, Anghel A, Desai AJ, et al:
Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively
inhibits HER2-amplified human gastric cancer cells and is
synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res.
16:1509–1519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pietras RJ, Fendly BM, Chazin VR, Pegram
MD, Howell SB and Slamon DJ: Antibody to HER-2/neu receptor blocks
DNA repair after cisplatin in human breast and ovarian cancer
cells. Oncogene. 9:1829–1838. 1994.PubMed/NCBI
|
|
32
|
Boone JJ, Bhosle J, Tilby MJ, Hartley JA
and Hochhauser D: Involvement of the HER2 pathway in repair of DNA
damage produced by chemotherapeutic agents. Mol Cancer Ther.
8:3015–3023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pietras RJ, Pegram MD, Finn RS, Maneval DA
and Slamon DJ: Remission of human breast cancer xenografts on
therapy with humanized monoclonal antibody to HER-2 receptor and
DNA-reactive drugs. Oncogene. 17:2235–2249. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clynes RA, Towers TL, Presta LG and
Ravetch JV: Inhibitory Fc receptors modulate in vivo cytotoxicity
against tumor targets. Nat Med. 6:443–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grazette LP, Boecker W, Matsui T, et al:
Inhibition of ErbB2 causes mitochondrial dysfunction in
cardiomyocytes: implications for herceptin-induced cardiomyopathy.
J Am Coll Cardiol. 44:2231–2238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu D and Hung MC: Role of erbB2 in breast
cancer chemosensitivity. Bioessays. 22:673–680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Funato T, Kozawa K, Fujimaki S, Miura T
and Kaku M: Increased sensitivity to cisplatin in gastric cancer by
antisense inhibition of the her-2/neu (c-erbB-2) gene.
Chemotherapy. 47:297–303. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Järvinen TA and Liu ET: Effects of
HER-2/neu on chemosensitivity of tumor cells. Drug Resist Updat.
3:319–324. 2000. View Article : Google Scholar
|
|
39
|
Griffith JD, Comeau L, Rosenfield S, et
al: Mammalian telomeres end in a large duplex loop. Cell.
97:503–514. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Loayza D and De Lange T: POT1 as a
terminal transducer of TRF1 telomere length control. Nature.
423:1013–1018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Houghtaling BR, Cuttonaro L, Chang W and
Smith S: A dynamic molecular link between the telomere length
regulator TRF1 and the chromosome end protector TRF2. Curr Biol.
14:1621–1631. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu D, O’Connor MS, Qin J and Songyang Z:
Telosome, a mammalian telomere-associated complex formed by
multiple telomeric proteins. J Biol Chem. 279:51338–51342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye JZ, Donigian JR, van Overbeek M, et al:
TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2
complex on telomeres. J Biol Chem. 279:47264–47271. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li B, Oestreich S and de Lange T:
Identification of human Rap1: implications for telomere evolution.
Cell. 101:471–483. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim SH, Beausejour C, Davalos AR, Kaminker
P, Heo SJ and Campisi J: TIN2 mediates functions of TRF2 at human
telomeres. J Biol Chem. 279:43799–43804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Q, Zheng YL and Harris CC: POT1 and
TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol.
25:1070–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van Steensel B, Smogorzewska A and de
Lange T: TRF2 protects human telomeres from end-to-end fusions.
Cell. 92:401–413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Karlseder J, Smogorzewska A and de Lange
T: Senescence induced by altered telomere state, not telomere loss.
Science. 295:2446–2449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wong KK, Chang S, Weiler SR, Ganesan S,
Chaudhuri J, Zhu C, Artandi SE, Rudolph KL, Gottlieb GJ, Chin L, et
al: Telomere dysfunction impairs DNA repair and enhances
sensitivity to ionizing radiation. Nat Genet. 26:85–88. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee KH, Rudolph KL, Ju YJ, Greenberg RA,
Cannizzaro L, Chin L, Weiler SR and DePinho RA: Telomere
dysfunction alters the chemotherapeutic profile of transformed
cells. Proc Natl Acad Sci USA. 98:3381–3386. 2001. View Article : Google Scholar : PubMed/NCBI
|