Introduction
The causal factors for hemangiomas have remained
unclear until recently. However, it is widely hypothesized that
vascular endothelial growth factor (VEGF) is a major factor in the
formation of hemangiomas. Shima et al (1) proposed that a shortage of oxygen
initially prompts an organism to produce VEGF (1), and VEGF is a type of vessel growth
factor, which stimulates the formation of hemangiomas and has
previously been investigated in detail (2). Its primary biological functions are as
follows: i) Selectively enhancing vascular endothelial cell
proliferation and promoting the formation of blood vessels; and ii)
participating in the mechanism that increases the permeability of
blood vessels, particularly microvessels (3,4). By
facilitating the ability to exude large molecules of plasma from a
vessel into the surrounding tissue, VEGF promotes the growth of
tumor cells, accelerates the formation of novel blood vessels and
provides nutrition for these novel vessels (5).
There are various treatment methods for hemangiomas,
including surgery, laser treatment, cryotherapy, partial radiation
therapy and local injections. Local injections of therapeutic
agents are administered directly into the blood vessels within the
hemangioma. The agents commonly administered in clinical practice
include the following: Corticosteroids, 5% morrhuate sodium,
pingyangmycin and pure alcohol, amongst others. Pure alcohol is a
traditional Chinese medicinal compound and its primary ingredients
are gal, alum, glycerin, chlorobutanol and stabilizer compounds.
Pure alcohol damages the cells of the endothelium of a hemangioma,
resulting in clumping of the red blood cells, protein coagulation
and thrombosis (6). These effects
result in thrombus formation in the lumina of hemangiomas.
Therefore, the method of injecting pure alcohol is simple, there is
less damage to the patient and the range of applications is
wide.
Enzyme-linked immunosorbent assay (ELISA) was used
to determine the concentrations of serum VEGF in 10 healthy
subjects and 10 hemangioma patients. The 10 hemangioma patients
were injected with pure alcohol one week prior to the collection of
blood samples, and eight of the hemangioma patients were
subsequently injected with pure alcohol one month later. The
association between VEGF and the morbidity resulting from
hemangiomas was examined. Furthermore, the clinical efficacy of the
method of injecting pure alcohol into hemangiomas was observed. In
addition, the clinical efficacy of all of the treatment methods for
hemangiomas that are adopted at the Department of Oral and
Maxillofacial Surgery, the Second Affiliated Hospital of Harbin
Medican University (Harbin, China), including laser therapy and
surgical removal, are provided based on the experiments that are
presented in the current study.
Materials and methods
Experimental principles
The present study employed the double antibody
sandwich ELISA method (Shenzhen Jingmei Biological Engineering Co.,
Ltd., Shenzhen, China). A rat anti-human VEGF monoclonal antibody
(Shenzhen Jingmei Biological Engineering Co., Ltd.) was coated onto
an ELISA plate, and standard and biotinylated detection antibody,
double antibody, and specimen and standard VEGF samples were added,
which formed an ‘antibody-VEGF-antibody’ compound. The ingredients
that did not form part of the compound were washed off. Horseradish
peroxidase-labeled avidin and biotin were added to achieve
avidin-specific binding. Again, the ingredients that did not
combine were washed off and the color reagents,
H2O2 and 3,3′,5,5′-tetramethylbenzidine
(Shenzhen Jingmei Biological Engineering Co., Ltd.) were added. If
VEGF was present, the reagent turned blue. A terminating agent was
added and the reaction color changed to yellow. The optical density
(OD) was measured at 450 nm; the level of VEGF is proportionate to
OD450, whereby a high density of VEGF results in a low OD. This
enabled the calculation of the VEGF concentration of the samples by
constructing a standard curve.
Specimen collection
Ten normal, healthy subjects (five males and five
females; mean age, 46.4 years; range, 20–63 years) were selected at
random and served as the control group, with 10 individuals with
hemangiomas (six males and four females; mean age, 48.8 years)
forming the treatment group (pathological classification: Cavernous
hemangioma of; the lower lip [n=3], the tongue [n=1], the upper lip
[n=3] and of the cheek [n=3]). However, two of the 10 hemangioma
patients did not complete the follow-up treatment. The treatment
process was as follows: Pure alcohol was injected into the tumors
of the hemangioma patients using 5-ml disposable syringes following
routine sterilization. When the color of the tumor turned pale the
injection was stopped. Each of the 10 hemangioma patients was
injected with pure alcoho1 once. Blood samples were obtained prior
to the injections, as well as one week and one month after the
injections. The blood samples were collected from the patients when
they were in a fasted state. Peripheral venous blood (4 ml) was
extracted (without anticoagulant) into a centrifuge tube, which was
frozen at −112°F in a freezer. One week following the injection of
absolute alcohol, the samples were stained with hematoxylin and
eosin and observed using a microscope (BX53; Olympus Corporation,
Tokyo, Japan). Written informed consent was obtained from all
patients and this study was approved by the ethics committee of the
Second Affiliated Hospital of Harbin Medical University.
Experimental procedure
ELISA was used in the present study and the human
VEGF kits were purchased from Shenzhen China Crystal Co. Ltd
(Shenzhen, China). The double antibody sandwich ELISA method was
used, which included the following steps. The cleaning mixture was
diluted with deionized water at a ratio of 1:20. The reference
material comprised of the following: 1.0 ml Standard/sample diluent
was placed into 20 ng of the reference material (at a concentration
of 20,000 pg/ml) using a 1,000-μl pipette. The mixture was allowed
to dissolve completely for 15 min at room temperature, then the
reference material was confected at the following concentrations:
1,000, 500, 250, 125, 62.5, 31.25, and 0 pg/ml. To analyze the
blood samples, they were removed from the −112°F freezer and they
dissolved completely at room temperature. The blood samples were
centrifuged (LD5-10B; Beijing Jingli Centrifuge Co., Ltd., Beijing,
China) at a speed of 80 × g for 10 min. The 96-well reaction plates
(Shenzhen Jingmei Biological Engineering Co., Ltd.) were coated
with the blood samples, and 100 μl standard or sample serum was
then added to the corresponding well using a 100-μl micropipette.
The plate was incubated at 98.6°F for 90 min and washed four times
with prepared washing solution. A total of 200 μl biotinylated
antibody working solution (20,000 pg/ml) was added to each well and
then the the reaction wells were covered with seal adhesive tape,
incubated at 98.6°F for 60 min, and washed four times with the
prepared washing solution. Enzyme conjugate (100 μl) was added to
the working solution in each well and the reactions were incubated
at 98.6°F for 30 min, followed by four sequential washes with the
prepared washing solution. The color reagent (100 μl) was added to
the working solution in each well, and the reactions were incubated
at 98.6°F for 2 min and maintained in the dark. Stopping solution
(100 μl) was added to each well and the reaction solution was
mixed. The absorbance was then measured using an ELISA analyzer
(9606; Perlong Medical Equipment Co., Ltd., Beijing, China) at a
wavelength of 450 nm (completed within 5 min) and the results were
automatically printed and revealed the OD values (Table I). The results were subsequently
converted to units of pg/ml (Table
II), and a bilateral t-test was performed as part of the
statistical analysis (Table
III).
 | Table IOptical density of patient blood
samples measured at a wavelength of 450 nm. |
Table I
Optical density of patient blood
samples measured at a wavelength of 450 nm.
|
Optical
density |
|---|
|
|
|---|
| Group | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
|---|
| Healthy subjects | 0.048 | 0.057 | 0.043 | 0.046 | 0.048 | 0.034 | 0.031 | 0.052 | 0.045 | 0.044 |
| Hemangioma
patients | 0.337 | 0.424 | 0.445 | 0.365 | 0.441 | 0.420 | 0.351 | 0.414 | 0.410 | 0.400 |
| Post pure alcohol
injection |
| 1 week | 0.415 | 0.455 | 0.445 | 0.363 | 0.350 | 0.420 | 0.425 | 0.401 | 0.415 | 0.450 |
| 1 month | 0.058 | 0.051 | 0.050 | 0.042 | 0.045 | 0.059 | 0.060 | 0.054 | | |
| Control | 0.0 | | | | | | | | | |
 | Table IILevels of VEGF (optical density
converted to pg/ml). |
Table II
Levels of VEGF (optical density
converted to pg/ml).
|
VEGF level,
pg/ml |
|---|
|
|
|---|
| Group | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
|---|
| Healthy subjects | 97.3 | 115.1 | 86.5 | 92.7 | 95.3 | 68.3 | 63.6 | 103.3 | 90.2 | 87.3 |
| Hemangioma
patients | 650.5 | 849.3 | 890.4 | 730.6 | 881.3 | 840.7 | 701.5 | 828.2 | 820.3 | 800.2 |
| Post pure alcohol
injection |
| 1 week | 830.7 | 909.5 | 890.4 | 725.4 | 700.9 | 840.8 | 849.7 | 801.9 | 830.8 | 900.9 |
| 1 month | 115.4 | 102.3 | 100.8 | 83.5 | 89.9 | 118.9 | 120.7 | 108.7 | | |
| Control | 0.0 | | | | | | | | | |
 | Table IIIVEGF levels in the different groups
(presented as the mean ± standard deviation). |
Table III
VEGF levels in the different groups
(presented as the mean ± standard deviation).
| Group | Cases, n | VEGF, pg/ml |
|---|
| Healthy subjects | 10 | 97.3±15.1 |
| Hemangioma
patients |
| Prior to
injection | 10 | 799.3±84.1a |
| 1 week after
injection | 10 | 828.1±68.8a |
| 1 month after
injection | 8 | 105.6±13.5 |
The treatment evaluation criteria included the
following: i) The outcome was considered excellent if the tumor
completely disappeared, there was normal skin and mucosa, no
dysfunction and the tongue movement test was negative; ii) the
outcome was considered valid if the tumor regression was >25%
(but did not completely disappear), deformity improved, and the
posture mobility test was either negative or positive; and iii) the
outcome was considered invalid if the tumor regression was <25%
or no signs of significant improvement were observed prior to and
following treatment.
Statistical analysis
Data were expressed as the mean ± standard
deviation. One-way analysis of variance was performed, and the post
hoc Student–Newman–Keuls test was used for multiple comparisons.
Statistics were calculated using SPSS version 15.0 for Windows
(SPSS Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristic features of a tongue
hemangioma
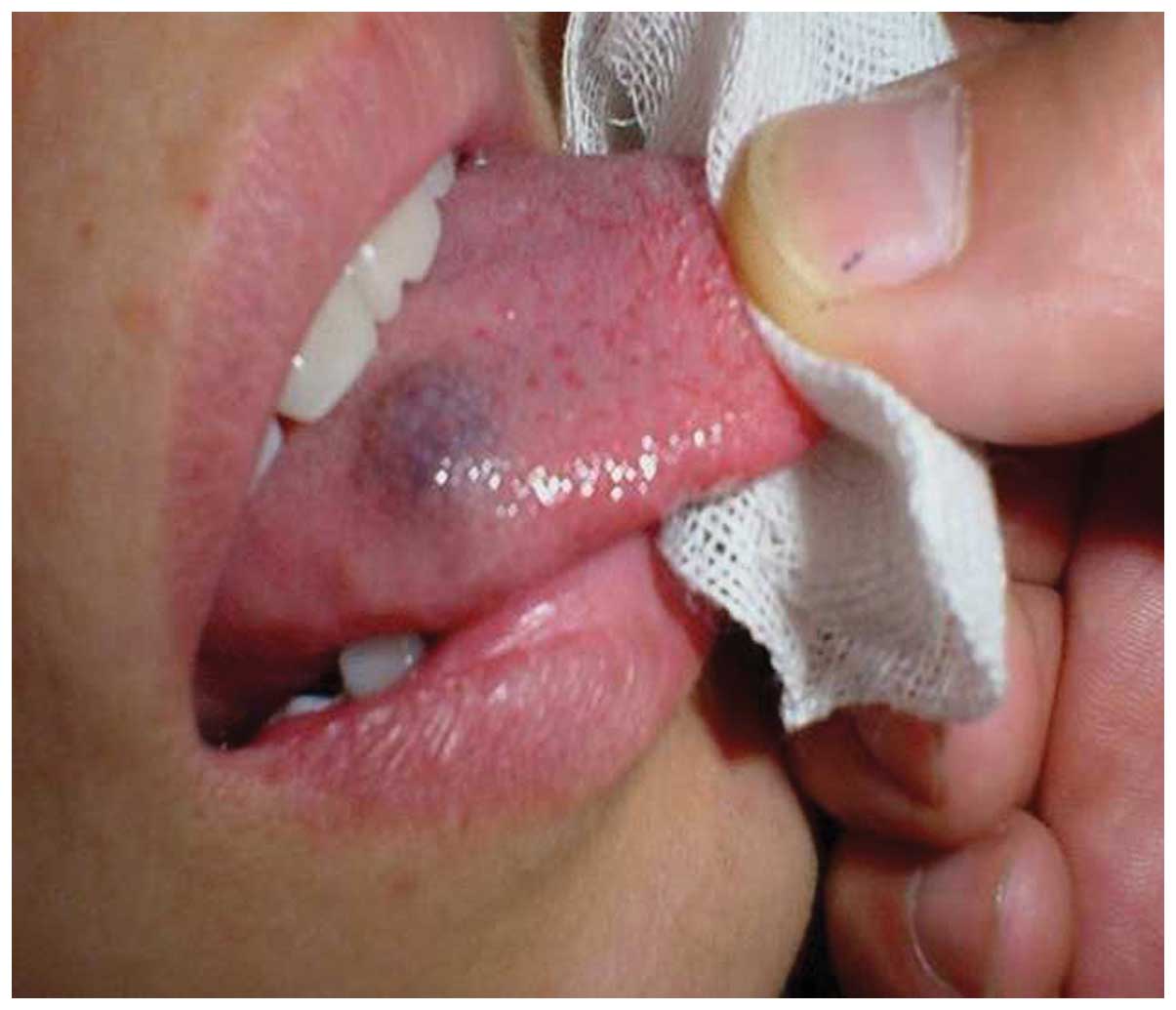
A tongue hemangioma is demonstrated in Fig. 1. The following characteristics of a
tongue hemangioma were observed: Raised mass, purple and soft on
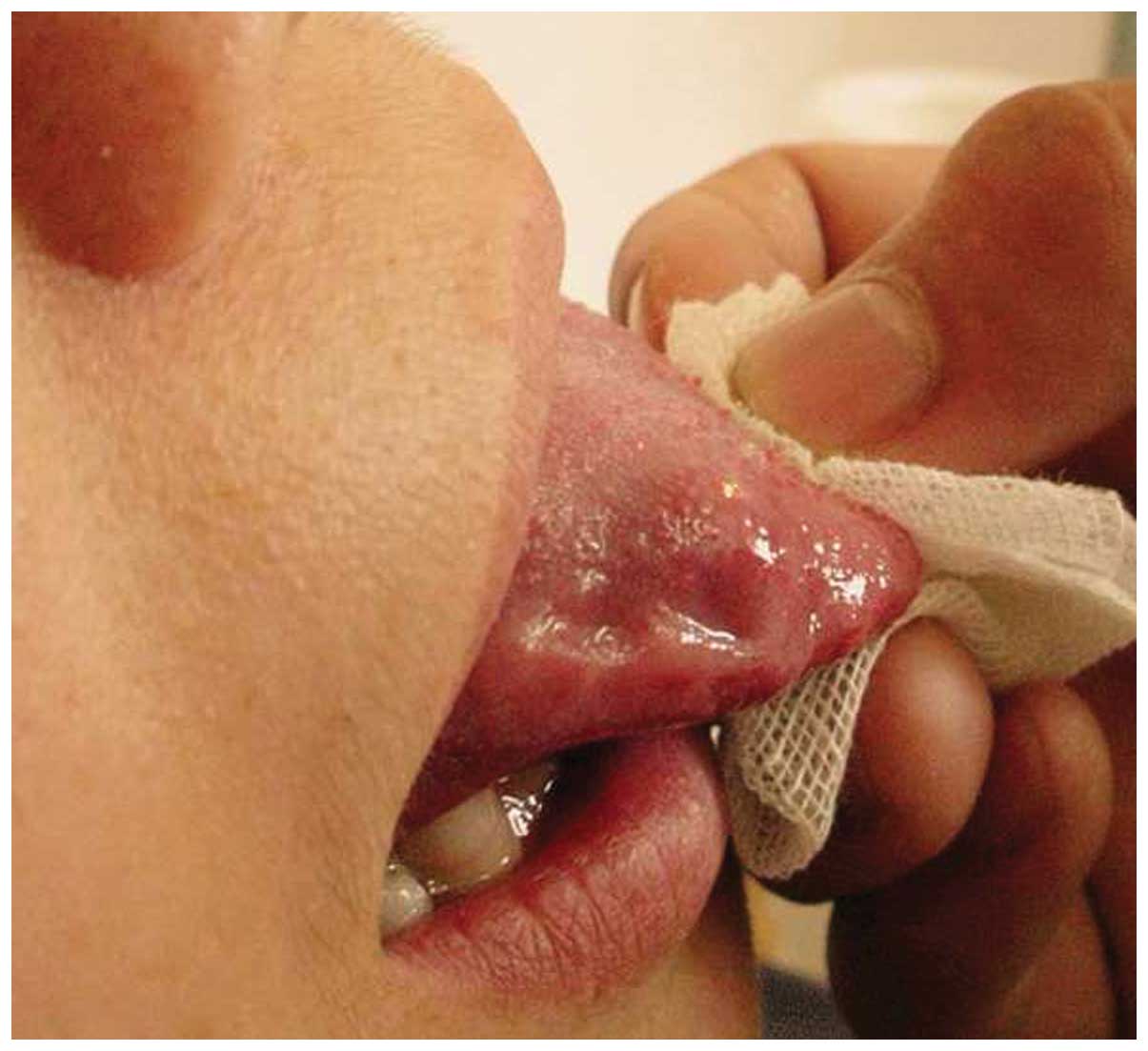
palpation. Fig. 2 shows the local
pathological changes that occurred one week after an injection of
pure alcohol. The local structure was no longer protruding, the
mucosal color had returned to normal, the tongue was freely
moveable without dysfunction, and the patient’s voice was
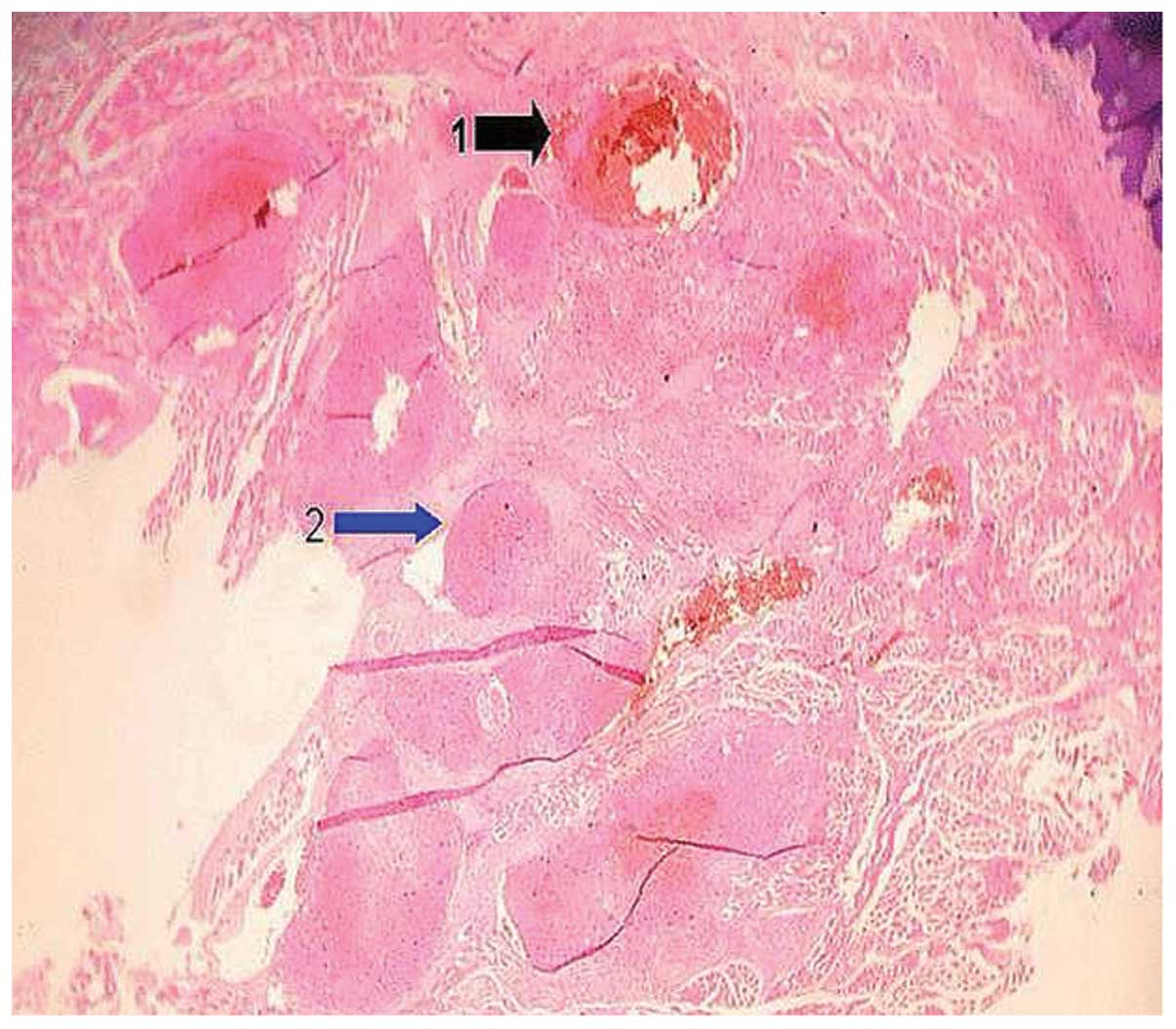
unchanged. Fig. 3 demonstrates the
local pathological changes that were obseved by optical microscopy
one week after the injection of pure alcohol. The injection of pure
alcohol led to the formation of a thrombus-like structure within
the lumen of the hemangioma. Certain regions of the lumen of the
hemangioma were blocked completely, whereas other regions were only
partially blocked. Such structural characteristics of the lumen
ensure a local blood supply and avoid the local formation of
necrotic tissue. These factors elucidate the reason that pure
alcohol is successful in the treatment of hemangiomas.
Comparison of serum VEGF levels
The serum VEGF levels of the 10 patients with
hemangioma were significantly higher when compared with those of
the healthy group (P<0.01). The serum VEGF levels of the 10
hemangioma patients one week after the pure alcohol injections were
not significantly different when compared with the values obtained
prior to treatment (P<0.01), however, were significantly
different from the healthy group. This result was due to VEGF
secretion by vascular endothelial cells and also by hematopoietic
stem cells, such as platelets, megakaryocytes, monocytes and
lymphocytes (7). The increase in
the VEGF concentration of the peripheral blood was due to the
stimulation of local oxidants, which resulted in the synthesis and
secretion of compensatory VEGF (8).
The serum VEGF levels of eight of the hemangioma patients one week
after the pure alcohol injections were not identified to be
significantly different than those of the healthy group. The serum
VEGF levels in the peripheral blood were close to normal levels.
This result was due to the disappearance of the body’s inflammatory
response one month after the pure alcohol injections, which had
metabolized the VEGF (9).
Discussion
Hemangiomas are a type of congenital benign tumor or
vascular malformation with the highest incidence rate observed in
infants and young children (10).
Hemangiomas, particularly facial lesions, may result in significant
psychological distress for the parents as well as the patients
(11). Hemangiomas occasionally
disappear with age; however, even in cases of complete regression,
38% of patients continue to present with residual alterations, such
as telangiectasias, yellowish discoloration, scarring or dermal
atrophy (12). A subset of
hemangiomas require active treatment, with the aim of therapy being
the reduction or eradication of the hemangioma while minimizing
infection, pain and scarring. Numerous treatment modalities have
been reported in the literature, ranging from pharmacological
therapies to electroacupuncture (13). Treatment options have expanded to
include systemic and intralesional corticosteroids, laser ablation,
open submucosal resection, and tracheostomy, with a concomitant
reduction in the mortality rate observed of 4% (14).
There are numerous methods for the treatment of
hemangiomas, such as surgery, laser treatment, cryotherapy,
interferon therapy, hormone therapy, partial radiation therapy, and
local injections, as well as others. Pure alcohol injection therapy
is frequently used in clinical applications. Currently, there is no
objective clinical indicator to assess the therapeutic effects and
prognoses following the application of these various treatment
strategies. Thus, the aim of the present study was to establish an
objective indicator to quantify the therapeutic effect. The exact
pathogenesis of hemangiomas remains unclear. The majority of
studies hypothesize that VEGF is a direct stimulus for hemangiomas
and that the continued elevated expression of it is a key factor in
the development of hemangiomas (15,16).
VEGF was characterized by Senger et al (17) in 1986 and was initially termed the
vascular permeability factor (18).
In humans, the VEGF-A gene contains a coding region of ~14 kb is on
chromosome 6p12. The gene has eight exons that are interspersed
with seven introns. Five distinct VEGF protein isoforms have been
identified; VEGF121, VEGF145, VEGF165, VEGF189 and VEGF206.
Although these VEGF proteins are structurally similar, they are
characterized by variations in function and differences in binding
specificity. There are important environmental effects of VEGF
expression, for example, hypoxia is the most potent agonist of VEGF
induction in vitro and in vivo (19). The expression of VEGF mRNA is
induced rapidly and reversibly by hypoxia in numerous cell types,
including normal, transformed and tumorigenic cells (20,21).
Furthermore, VEGF is a dipolymer composed of two identical subunits
(connected via disulfide bonds), with a molecular weight of
17,000–22,000 Da. Depending on alternative splicing during RNA
transcription, VEGF can be divided into five subtypes: VEGF121,
VEGF145, VEGF165, VEGF189 and VEGF208 (9). There are six VEGF variants, each with
similar proteins that are involved in the regulation and
differentiation of the vascular system, particularly in the blood
and lymph vessels (22). VEGF
exerts its effect via VEGF receptors that initially undergo
autophosphorylation and subsequently activate
phosphatidylcholine-specific phospholipase C (PLC-r). PLC-r
hydrolyzes phosphatidylinositol diphosphate, producing
diacylglycerol (DAG) and inositol triphosphate. DAG activates
protein kinase C, which is present in the cytoplasm, and binds it
to the membrane, inducing endothelial cell growth and increasing
vascular permeability.
In the present study, the VEGF levels in the
hemangioma patient group were significantly higher than those in
the healthy group (P<0.01). This finding indicates that the
serum concentrations of VEGF increased in the hemangioma patients.
The serum VEGF levels of the 10 hemangioma patients one week after
the pure alcohol injections were not identified to be significantly
different when compared with the levels observed prior to
treatment, however, were significantly different from the healthy
group. This result occurred as VEGF is expressed by vascular
endothelial cells, and by hematopoietic stem cells, such as
platelets, megakaryocyte, monocytes and lymphocytes (23). However, VEGF is also expressed by
endothelial cells, macrophages, and activated smooth muscle cells
in atherosclerotic lesions and is expressed as a result of in-stent
restenosis neovascularization (8,24,25).
The increase in VEGF concentration in the peripheral blood one week
after the injections was due to the stimulation of local oxidants,
resulting in the synthesis and secretion of compensatory VEGF. The
serum VEGF levels in the peripheral blood had almost returned to
baseline (similar to the levels of the control subjects) one month
after the pure alcohol injections. This result occurred due to the
disappearance of the body’s inflammatory response one month after
the pure alcohol injections, resulting in metabolization of excess
levels of VEGF.
In conclusion, the clinical effects of local pure
alcohol injections into hemangiomas in this study were consistent
with previous studies. The ELISA measurements of the serum VEGF
concentration in the peripheral blood accurately reflected the
concentrations of VEGF in the blood. Therefore, the serum VEGF
concentration in the peripheral blood may be used as a clinical
indicator of the efficacy of clinical treatment and to determine
the prognosis. The present study provides the basis for future
scientific research and clinical investigations.
References
|
1
|
Shima DT, Adamis AP, Ferrara N, Yeo KT,
Yeo TK, Allende R, et al: Hypoxic induction of endothelial cell
growth factors in retinal cells: identification and
characterization of vascular endothelial growth factor (VEGF) as
the mitogen. Mol Med. 1:182–193. 1995.PubMed/NCBI
|
|
2
|
Greenberger S, Boscolo E, Adini I,
Mulliken JB and Bischoff J: Corticosteroid suppression of VEGF-A in
infantile hemangioma-derived stem cells. N Engl J Med.
362:1005–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao X, Liu J and Sheng M: Synergistic
effect of estrogen and VEGF on the proliferation of hemangioma
vascular endothelial cells. J Pediatr Surg. 39:1107–1110. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleinman ME, Greives MR, Churgin SS,
Blechman KM, Chang EI, Ceradini DJ, et al: Hypoxia-induced
mediators of stem/progenitor cell trafficking are increased in
children with hemangioma. Arterioscler Thromb Vasc Bio.
27:2664–2670. 2007. View Article : Google Scholar
|
|
5
|
Tokuyama W, Mikami T, Masuzawa M and
Okayasu I: Autocrine and paracrine roles of VEGF/VEGFR-2 and
VEGF-C/VEGFR-3 signaling in angiosarcomas of the scalp and face.
Hum Pathol. 41:407–414. 2010. View Article : Google Scholar
|
|
6
|
Elmasri H, Karaaslan C, Teper Y, Ghelfi E,
Weng M, Ince TA, et al: Fatty acid binding protein 4 is a target of
VEGF and a regulator of cell proliferation in endothelial cells.
FASEB J. 23:3865–3873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haggstrom AN, Drolet BA, Baselga E,
Chamlin SL, Garzon MC, Horii KA, et al: Prospective study of
infantile hemangiomas: clinical characteristics predicting
complications and treatment. Pediatrics. 118:882–887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Span PN, Grebenchtchikov N, Geurts-Moespot
J, Westphal JR, Lucassen AM and Sweep CG: EORTC Receptor and
Biomarker Study Group Report: a sandwich enzyme-linked
immunosorbent assay for vascular endothelial growth factor in blood
and tumor tissue extracts. Int J Biol Markers. 15:184–191.
2000.PubMed/NCBI
|
|
9
|
Ozawa CR, Banfi A, Glazer NL, et al:
Microenviromental VEGF concentration, not total dose, determines a
threshold between normal and aberrant angiogenesis. J Clin Invest.
113:516–527. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mulliken JB and Glowacki J: Hemangiomas
and vascular malforations in infants and children: a classification
based on endothelial characteristics. Plast Reconstr Surg.
69:412–422. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanner JL, Dechert MP and Frieden IJ:
Growing up with a facial hemangioma: parent and child coping and
adaptation. Pediatrics. 101:446–452. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu LJ, Xiao C and Zhu X: New advances in
treatment of tumour using VEGF and its receptor. Oncology section
of foreign medicine science. 28:113–115. 2001.
|
|
13
|
Bruckner AL and Frieden IJ: Hemangiomas of
infancy. J Am Acad Dermatol. 48:477–493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bitar MA, Moukarbel RV and Zalzal GH:
Management of congenital subglottic hemangioma: trends and success
over the past 17 years. Otolaryngol Head Neck Surg. 132:226–231.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tischer E, Mitchell R, Hartman T, Silva M,
Gospodarowicz D, Fiddes JC and Abraham JA: The human gene for
vascular endothelial growth factor. Multiple protein forms are
encoded through alternative exon splicing. J Biol Chem.
266:11947–11954. 1991.PubMed/NCBI
|
|
16
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Senger DR, Perruzzi CA, Feder J and Dvorak
HF: A highly conserved vascular permeability factor secreted by a
variety of human and rodent tumor cell lines. Cancer Res.
46:5629–5632. 1986.PubMed/NCBI
|
|
18
|
Zachary I and Gliki G: Signaling
transduction mechanisms mediating biological actions of the
vascular endothelial growth factor family. Cardiovasc Res.
49:568–581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirito K and Kaushansky K: Thrombopoietin
stimulates vascular endothelial cell growth factor (VEGF)
production in hematopoietic stem cells. Cell Cycle. 4:1729–1731.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minchenko A, Bauer T, Salceda S and Caro
J: Hypoxic stimulation of vascular endothelial growth factor
expression in vitro and in vivo. Lab Invest. 71:374–379.
1994.PubMed/NCBI
|
|
21
|
Mahajan D, Miller C, Hirose K, McCullough
A and Yerian L: Incidental reduction in the size of liver
hemangioma following use of VEGF inhibitor bevacizumab. J Hepatol.
49:867–870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berse B, Brown LF, Van de Water L, Dvorak
HF and Senger DR: Vascular permeability factor (vascular
endothelial growth factor) gene is expressed differentially in
normal tissues, macrophages, and tumors. Mol Biol Cell. 3:211–220.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kastrup J, Jørgensen E, Rück A, Tägil K,
Glogar D, Ruzyllo W, et al; Euroinject One Group. Direct
intramyocardial plasmid vascular endothelial growth factor-A165
gene therapy in patients with stable severe angina pectoris. A
randomized double-blind placebo-controlled study: the Euroinject
One trial. J Am Coll Cardiol. 45:982–988. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Couffinhal T, Kearney M, Witzenbichler B,
Chen D, Murohara T, Losordo DW, et al: Vascular endothelial growth
factor/vascular permeability factor (VEGF/VPF) in normal and
atherosclerotic human arteries. Am J Pathol. 150:1673–1685.
1997.PubMed/NCBI
|
|
25
|
Inoue M, Itoh H, Ueda M, Naruko T, Kojima
A, Komatsu R, et al: Vascular endothelial growth factor (VEGF)
expression in human coronary atherosclerotic lesions: possible
pathophysiological significance of VEGF in progression of
atherosclerosis. Circulation. 98:2108–2116. 1998. View Article : Google Scholar : PubMed/NCBI
|