Introduction
Gastric cancer (GC) is the fourth most common
malignancy, and the third leading cause of cancer-related mortality
among males and the fifth leading cause of cancer-related mortality
among females worldwide, with ~1,000,000 new patients diagnosed
every year (1). Curative resection
is the most successful treatment modality for locally confined GC
(2), and the five-year relative
survival rate is ~29% (3).
Recurrence of GC generally occurs early, predominantly within the
initial two years following gastrectomy. Late recurrence is
uncommon and is extremely rare >10 years after gastrectomy
(4). The majority of advanced GC
patients eventually develop cachexia and peritoneal carcinomatosis,
and succumb to multiple organ failure within five years (4). Advanced-stage cancer patients
demonstrate a significantly higher incidence of bone cancer
recurrence, and the majority develop combined recurrences, most
commonly lymph node metastasis and peritoneal carcinomatosis
(5). Solitary bone metastases are
only observed in one-third of cases (6–10). In
the present study, we report a rare case of relapse from advanced
GC with extensive vertebral metastases and bone marrow infiltration
at an 11-year follow-up. The theory of tumour dormancy may explain
this phenomenon. Written informed consent for the publication of
this study was obtained from the patient.
Case report
Clincial history
In September 2001, a 49-year-old female was admitted
to the IX Division of General Surgery of the Second University of
Naples (Naples, Italy) presenting with locally advanced GC. The
patient underwent total gastrectomy with Billroth II anastomosis
and D2 lymphadenectomy. The postoperative course was normal.
Histological analysis identified an infiltrated mucinous
adenocarcinoma with the presence of signet ring cells and
metastases were identified in 2/32 dissected lymph nodes. Stage III
cancer was diagnosed according to the fifth edition of the American
Joint Committee on Cancer classification system (11) and the patient received an adjuvant
chemotherapy treatment according to the ELFE regimen (60
mg/m2 epirubicin on day 1; 100 mg/m2
leucovorin and 375 mg/m2 fluorouracil on days 1–5; and
80 mg/m2 etoposide on days 1–3). This regimen was
repeated every three weeks for six cycles (12). The patient was regularly followed-up
at the outpatient clinic of our department every three months for
the first two years and every six months until the fifth year.
Furthermore, the patient underwent annual upper endoscopy, and
chest and abdomen computed tomography (CT) scans. After five years,
the patient was subjected to annual physical examinations, chest
radiographs and abdominal ultrasound scans.
Clinical presentation and diagnosis
In November 2012, the patient developed severe
progressive paraparesis with the inability to maintain an upright
posture, as well as retention of the sphincters. In addition, the
patient complained of severe pain in the spine and left sciatica in
the previous 2–3 weeks. Physical examinations conducted three
months prior to the present symptoms did not reveal chest,
abdominal or central nervous system indicators of relapse. Upon
clinical examination, paraparesis with reduced tone and areflexia
of the lower limbs was identified. Muscle power was determined to
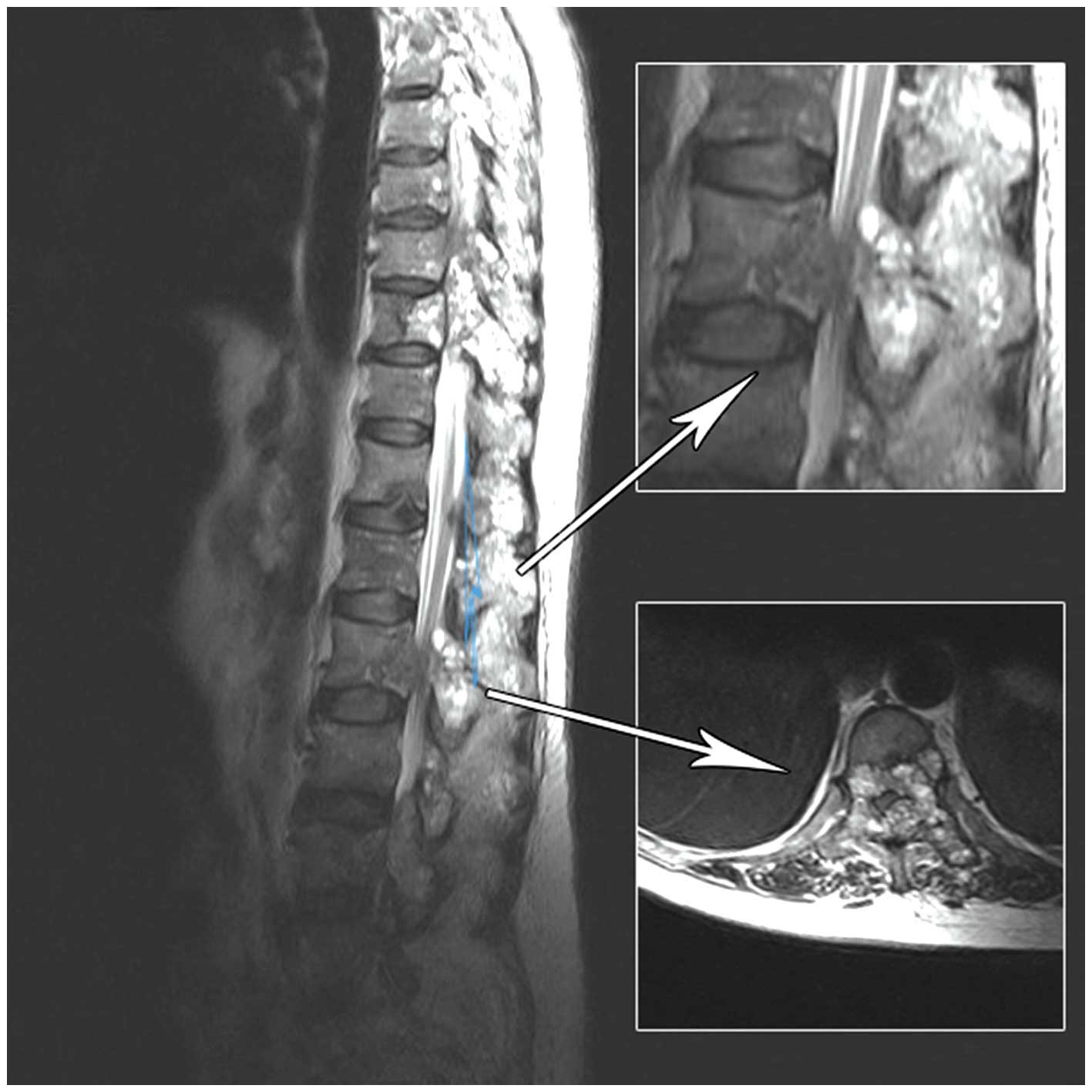
be 1/5 in the left and right legs. Magnetic resonance imaging (MRI)
of the entire spine revealed numerous abnormal vertebrae; in
particular, the thoracic (T4–T6, T9–T10), lumbar (L2–L4) and sacral
(S2) vertebra. Furthermore, several vertebral bodies exhibited
marrow infiltration, particularly L2 and L4 (Fig. 1), and a soft tissue mass was
identified in the spinal cord canal from L2 to L4. The dural sac
was displaced anteriorly with marked compression at the L2 level.
Brain, chest, abdomen and pelvis CT scans were performed for tumour
staging, which detected no signs of disease. A CT-guided biopsy of
the soft tissue at the L2 level revealed metastatic adenocarcinoma
with signet ring cells; specifically, the biopsy cells positively
stained for mucin, cytokeratins 7 and 20, and carcinoembryonic
antigen, whereas the cells were negatively stained oestrogen and
progesterone receptors, as well as cancer antigen (CA) 125. These
histological features were consistent with metastasis from gastric
mucinous adenocarcinoma. Thus, human epidermal growth factor
receptor 2 (HER2) expression levels were assessed in biopsy and
resected gastric cancer tissue samples obtained from the patient in
2001; however, no HER2 overexpression was identified. The
performance status was determined as two according to the Eastern
Cooperative Oncology Group scale (http://www.ecog.org/general/perf_stat.html) and, with
the exception of the bone marrow infiltration, no haematological
disorders were detected.
Treatment
Initial management was targeted to the control the
patient’s severe back pain symptoms; thus, 20 Gy radiotherapy
targeted to the L2–L4 vertebral bodies was performed. Subsequently,
systemic chemotherapy according to FOLFOX-4 regimen commenced: 85
mg/m2 oxaliplatin; 200 mg/m2 leucovorin; and
400 mg/m2 fluorouracil (5-FU), intravenous bolus,
followed by continuous infusion of 5-FU (2,400 mg/m2)
over 46 h every two weeks (13).
After eight cycles, a further progression of the disease occurred,
with the patient exhibiting superimposed lung metastases. In
consideration of the poor clinical condition of the patient,
supportive care was administered. The patient succumbed one month
later.
Discussion
The first novel aspect of the present report is the
uncommon location of the secondary lesions. GC usually metastasizes
to the liver, peritoneum, lymph nodes and lungs, whereas bone
metastases are uncommon, occurring in ~13.4% of autopsy cases in a
Japanese study, and are rarely detected as isolated lesions
(6–10). The metastatic path of neoplastic GC
cells is generally hematogenous through the bone marrow, as the
gastric mucosa has a rich capillary network and the bone marrow
does not contain lymphatic vessels (14). This hypothesis is supported by the
observation that a higher incidence of bone metastasis occurs in
the axial skeleton, such as the spine, pelvic bones or the sternum,
where there is a higher content of hematopoietic bone marrow in
adults (15). Therefore, the bone
marrow, rather than the bone tissue, is the target in bone
metastasis.
The prognosis of patients exhibiting bone metastases
from GC is worse compared with other solid tumours, with a mean
survival period of <5 months and the longest survival period
reported in the literature, 3.5 years (6). Early detection of bone metastases is
difficult as, according to the majority of important international
guidelines (16), skeletal
examinations are only performed upon presentation of bony pain
symptoms.
No standard treatment for bone metastases with
marrow infiltration has been established and local approaches
represent the most feasible therapeutic approaches. In the present
report, in consideration of the multiple vertebral metastases, a
palliative radio-chemotherapy approach was implemented. In the
metastatic setting, treatment aims to control patient symptoms,
improve patient quality of life and prolong patient survival;
however, current chemotherapeutic approaches have limited efficacy
and specific approaches exhibit unfavourable toxicity profiles.
Fluoropyrimidine, taxanes and platinum-based regimens are the most
commonly used chemotherapeutic approaches, providing response rates
of 30–50% and a median overall survival of ~1 year (17). These data support the requirement
for the development of novel therapeutic strategies based on
targeted agents. Trastuzumab, a monoclonal antibody against HER2,
has demonstrated a survival advantage when applied as a
chemotherapeutic agent in various types of HER2-positive GC
patients (13,18). However, the current patient was
determined to be HER2-negative and, thus, did not benefit from the
treatment.
The second novel aspect of the present report is the
long disease-free period experienced by the patient. Relapse from
GC usually occurs within five years after surgery and the median
recurrence period is 28 months (range, 4–111 months) following
surgery (2,5). Advanced cancer and poorly
differentiated adenocarcinoma are associated with a high risk of
relapse (6), hence, late
recurrences are uncommon; <10% of patients recur after five
years and <1% recur after 10 years (19). However, few studies of isolated bone
recurrences ≥9 years after detection of the primary tumour have
been reported (20). Recent studies
on GC reported a 0.4–3.8% incidence of bone metastasis (20–23).
The time elapsed between surgical resection and the onset of bone
metastasis may be explained by the tumour dormancy theory, which
proposes that a period of tumour progression exists in which the
presence of tumour cells in distant organs does not increase the
tumour burden. This condition is clinically translated as a long
disease-free interval between primary tumour resection and relapse
(21,22). Single dormant cells are defined as
cells undergoing cycle arrest that have the ability to develop
mechanisms to evade immune surveillance (24,25).
Only a small proportion of dormant cells (~2%) develop into
micrometastases, and an even smaller proportion (~0.02%) develop
into macroscopic tumours (26). In micrometastatic dormancy, a state
of balance exists between apoptosis and cell proliferation,
resulting in no increase of tumour burden (27). Tumour dormancy
ends after variable periods, up to years after the diagnosis of the
primary tumour; subsequently, the cancer cells start to proliferate
and late relapse occurs. The regulation of tumour dormancy entry
and exit remains poorly understood, however, various factors may be
involved, including genetic, and epigenetic changes, angiogenic
switching, immune evasion and the microenvironment. The prevalence
of clinical dormancy has been reported in numerous solid tumours,
such as breast, renal, thyroid and prostate cancer, as well as
melanoma, however, clinical dormancy has rarely been observed in GC
(26).
In conclusion, vertebral metastases with bone marrow
infiltration represent an uncommon occurence in GC, and their
treatment can prove difficult and usually aims to manage the
symptoms. A period of 11 years elapsed between the surgical
resection of the tumor and the onset of bone metastasis observed in
the present patient. This could be explained by the tumor dormancy
theory, which is a poorly understood process observed in several
solid neoplasms, regulated by genetic and epigenetic changes, that
requires further studies to be completely comprehended.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statics. CA Cancer J Clin.
61:1342011. View Article : Google Scholar
|
|
2
|
Lehnert T, Rudek B, Buhl K and Golling M:
Surgical therapy for loco-regional recurrence and distant
metastasis of gastric cancer. Eur J Surg Oncol. 28:455–461. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park JM, Song KY, O JH, et al: Bone
recurrence after curative resection of gastric cancer. Gastric
Cancer. 16:362–369. 2013. View Article : Google Scholar
|
|
4
|
Shiraishi N, Inomata M, Osawa N, et al:
Early and late recurrence after gastrectomy of gastric carcinoma.
Univariate and multivariate analyses. Cancer. 89:255–261. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song KY, Park SM, Kim SN and Park CH: The
role of surgery in the treatment of recurrent gastric cancer. Am J
Surg. 196:19–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moon YW, Jeung HC, Rha SY, et al: Changing
patterns of prognosticators during 15-year follow-up of advanced
gastric cancer after radical gastrectomy and adjuvant chemotherapy:
a 15-year follow-up study at a single Korean institute. Ann Surg
Oncol. 14:2730–2737. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishidoi H and Koga S: Clinicopathological
study of gastric cancer with bone metastasis. Gan To Kagaku Ryoho.
14:1717–1722. 1987.(In Japanese). PubMed/NCBI
|
|
8
|
Crivellari D, Carbone A, Sigon R, et al:
Gastric cancer with bone marrow invasion at presentation:
case-report and review of the literature. Tumori. 81:74–76.
1995.PubMed/NCBI
|
|
9
|
Noda N, Sano T, Shirao K, et al: A case of
bone marrow recurrence from gastric carcinoma after a nine-year
disease-free interval. Jpn J Clin Oncol. 26:472–475. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abrams HL, Spiro R and Goldstein N:
Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer.
3:74–85. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH1 and Fleming ID: TNM
Classification of Malignant Tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Vita F, Giuliani F, Orditura M, et al:
Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil
and etoposide regimen in resected gastric cancer patients: a
randomized phase III trial by the Gruppo Oncologico Italia
Meridionale (GOIM 9602 Study). Ann Oncol. 18:1354–1358. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Vita F, Giuliani F, Silvestris N, et
al: Human epidermal growth factor receptor 2 (HER2) in gastric
cancer: a new therapeutic target. Cancer Treat Rev. 3653(Suppl 3):
S11–S15. 2010. View Article : Google Scholar
|
|
14
|
Kobayashi M, Okabayashi T, Sano T and
Araki K: Metastatic bone cancer as a recurrence of early gastric
cancer - characteristics and possible mechanisms. World J
Gastroenterol. 11:5587–5591. 2005.PubMed/NCBI
|
|
15
|
Galasko C: The anatomy and pathways of
skeletal metastases. Bone Metastases. Weiss L and Gilbert A: GK
Hall; Boston, MA: pp. 49–63. 1981
|
|
16
|
Ajani JA, Bentrem DJ, Besh S, et al;
National Comprehensive Cancer Network. Gastric cancer, version
2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc
Netw. 11:531–546. 2013.PubMed/NCBI
|
|
17
|
Field K, Michael M and Leong T: Locally
advanced and metastatic gastric cancer: current management and new
treatment developments. Drugs. 68:299–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng XY, Chen YB, Wang W, et al:
Time-varying pattern of recurrence risk for gastric cancer
patients. Med Oncol. 30:5142013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blanchette P, Lipton JH, Barth D and
Mackay H: Case report of very late gastric cancer recurrence. Curr
Oncol. 20:e161–e164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Willis RA: Secondary tumors of bones. The
spread of tumors in the human body. 3rd edition.
Butterworth-Heinemann; London: pp. 229–250. 1973
|
|
22
|
Yoshikawa K and Kitaoka H: Bone metastasis
of gastric cancer. Jpn J Surg. 13:173–176. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turkoz FP, Solak M, Kilickap S, et al:
Bone metastasis from gastric cancer: the incidence,
clinicopathological features, and influence on survival. J Gastric
Cancer. 14:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naumov GN, MacDonald IC, Weinmeister PM,
et al: Persistence of solitary mammary carcinoma cells in a
secondary site: a possible contributor to dormancy. Cancer Res.
62:2162–2168. 2002.PubMed/NCBI
|
|
25
|
Naumov GN, MacDonald IC, Chambers AF and
Groom AC: Solitary cancer cells as a possible source of tumour
dormancy? Semin Cancer Biol. 11:271–276. 2001. View Article : Google Scholar : PubMed/NCBI
|