Introduction
Bladder cancer is one of the most common
malignancies worldwide. In males, bladder cancer is the fourth and
seventh most common malignancy in the United States and the Western
world, respectively, behind lung, prostate, colon, stomach, liver
and esophageal cancers. Furthermore, bladder cancer is the second
most common cause of mortality among genitourinary tumors, with
~72,570 new cases and 15,210 deaths occurring in males and females
in 2013 (1–3). Transitional cell carcinoma (TCC)
accounts for ~90% of cases of bladder cancer, whereas squamous cell
carcinoma accounts for ~5% and adenocarcinomas ~1–2% of cases
(4). At presentation, ~70% of cases
of bladder cancer are non-muscle invasive (stages Tis, Ta and T1)
which is typically low grade, multifocal, superficial and
papillary, and 30% are muscle invasive (stages T2, T3 and T4),
according to the TNM staging system (3). Numerous studies investigating the
etiology of bladder cancer have been conducted; however, current
understanding of the cellular and molecular mechanisms that
underlie the processes of bladder cancer carcinogenesis and
progression is poor.
The most common treatment for bladder cancer
patients is endoscopic resection (5,6).
Patients exhibiting non-invasive, low-grade bladder cancer
typically undergo a treatment strategy of simple resection and
fulguration of the tumor, followed by selective use of intravesical
chemotherapy (7). Patients
exhibiting the more aggressive muscle-invasive form of bladder
cancer are usually treated with radical cystectomy of the primary
tumor. However, despite this radical surgery, half of invasive
bladder cancer patients develop subsequent metastatic disease
(8). Therefore, bladder cancer
patients require monitoring for cancer recurrence or progression.
Furthermore, it is important to predict the prognosis of bladder
cancer patients to enable the implementation of the most effective
treatment strategy for prolonged survival.
Co-stimulation and -inhibition of T-cells is
primarily generated by interactions between B7 immune regulatory
ligands and cluster of differentiation (CD)28 receptors (9). Identification of new members of the
B7/CD28 families have expanded the B7 family to include: B7h
(CD275), B7× (B7-H4 or B7S1), B7-H3 (CD276), B7-1 (CD80), B7-2
(CD86), PD-L1 (CD274) and PD-L2 (CD273) (10). Phylogenetically, the B7 family is
divided into three groups (11):
Group I (B7-1, B7-2, and B7h) is involved in low-stage T-cell
responses, as well as T- and B-cell interactions in lymphoid
tissues (12); group II (PD-L1 and
PD-L2) is involved in peripheral immune tolerance and T-cell
impairment during chronic viral infections (13,14);
and group III [B7-H3 (B7RP-2) and B7× (B7-H4, B7S1)], which
contains the most recently identified members of the B7 family, is
considered to attenuate peripheral immune responses via
co-inhibition (9). The
counter-receptors and precise roles of B7 proteins in T-cell
regulation are yet to be defined; however, it is proposed that they
are significant factors in the interaction between tumors and the
immune system.
To the best of our knowledge, B7-H3 expression in
bladder cancer has not yet been examined. Thus, the present study
examines the mRNA and protein expression levels of B7-H3 in bladder
cancer by performing semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) and
immunohistochemical analysis, respectively, of clinical specimens
from TCC samples and their normal adjacent tissues (NATs). The mRNA
and protein expression levels of B7-H3 were significantly
upregulated in the TCC samples compared with the paired NAT
samples.
Materials and methods
Patients and clinical specimens
Seventeen tissue specimens were collected from
bladder cancer patients of the Yancheng City No. 1 People’s
Hospital (Yancheng, China). Following receipt of written informed
consent, tissues were obtained under a general tissue collection
protocol approved by the Institutional Review Board of Yancheng
City No. 1 People’s Hospital. Tissues were immediately snap-frozen
in liquid nitrogen.
RNA extraction and complementary (c)DNA
synthesis
Total RNA was extracted from the tissue samples
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) and treated with RNase-free DNase (Sangon Biotech Co., Ltd.,
Shanghai, China) to remove genomic DNA contamination, according to
the manufacturer’s instructions. The integrity and yield of RNA was
quantified spectrophotometrically (UV-1601; Shimadzu Corporation,
Kyoto, Japan) by measuring the absorbance at 260 nm (A260) and 280
nm (A280) to determine the A260/A280 ratio of pure RNA as ~1.8. RNA
was stored at −80°C for subsequent analysis and cDNA synthesis was
performed using the ReverTra Ace qPCR RT Kit (Toyobo Biotech Co.,
Ltd., Shanghai, China) in an Eppendorf RealPlex4S
Mastercycler® (Eppendorf, Hamburg, Germany).
Semi-quantitative RT-PCR
Total RNA was isolated and analyzed for B7-H3
expression by semi-quantitative RT-PCR. The primer sequences for
PCR were as follows: Forward, 5′-CAGGGCAGCCTATGACATTCCC-3′ and
reverse, 5′-GTGACCAGCACATGCTTCCGTG-3′ for B7-H3; and forward,
5′-ATTCAACGGCACAGTCAAGG-3′ and reverse, 5′-GCAGAAGGGGCGGAGATGA-3′
for the GAPDH internal control. The following PCR cycling
parameters were employed: 95°C for 5 min, followed by 42 cycles of
95°C for 45 sec, 56°C for 1 min and 72°C for 1 min, and then 72°C
for 7 min. Equal volumes of the PCR reaction were subjected to
electrophoresis on 2% agarose gels and PCR fragments were
visualized by ethidium bromide staining. Images were captured on a
gel documentation system and analyzed using an
AlphaImager® 2200 (Alpha Innotech Co., San Leandro, CA,
USA).
Immunohistochemical staining
At room temperature, the tissue samples were fixed
in 4% 3-heptanone in phosphate buffered saline (PBS) for 24 h,
embedded in paraffin, cut into sections (width, 5 μm) and mounted
onto poly-L-lysine-coated slides. Initially, the slides were heated
to 56°C in an oven for 2 h. Following dewaxing in xylene and
rehydration in gradient alcohols, the slides were heated to boiling
in 10 mmol/l sodium citrate buffer (pH 6.8) for 10 min using a
microwave. Following inactivation of endogenous peroxidase activity
with 3% hydrogen peroxide in methanol treatment, the samples were
blocked using blocking solution (5% goat serum in PBS) for 20 min
and incubated with primary mouse anti-human monoclonal B7-H3
antibodies (1:1,000; cat. no. BC062581; Bioworld Technology, Inc.,
St. Louis Park, MN, USA) overnight at 4°C. Following incubation,
secondary biotinylated goat anti-mouse antibodies (1:1,000; cat.
no. Bs10004; Bioworld Technology, Inc.) and ABC reaction solution
(Beyotime Institute of Biotechnology, Shanghai, China) were applied
sequentially and according to the manufacturer’s instructions. The
slides were counterstained with hematoxylin, briefly washed with 30
mmol/l ammonium hydroxide and mounted with permanent mounting
medium. Each experiment was performed in triplicate and conducted
under identical conditions.
To quantify the immunostaining intensity, the
integrated optical density (IOD) was calculated. Digitally fixed
images were analyzed at a magnification of ×400 using an AxioImager
A1 light microscope (Carl Zeiss AG, Oberkochen, Germany) equipped
with Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD,
USA). For each sample, the IOD was calculated for the same sized
arbitrary area. All data are presented as a mean value and
statistical analysis was conducted to compare the results of the
various experimental groups.
Statistical methods
Data are presented as the mean ± standard deviation,
and compared using a Student’s t-test in Stata software (version
8.2; StataCorp LP, College Station, TX, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
mRNA expression level of B7-H3 in TCC and
NAT samples
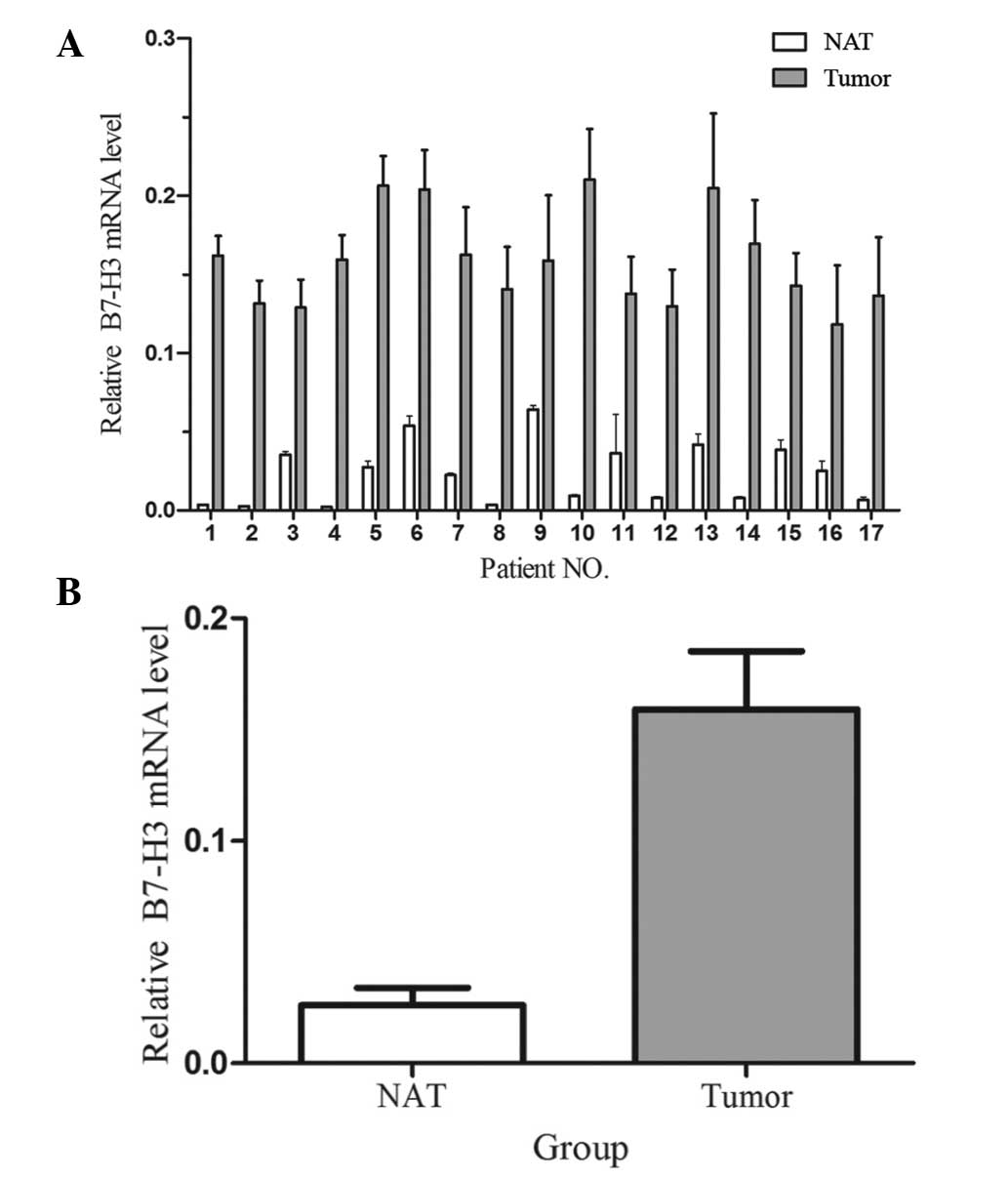
The relative mRNA expression levels of B7-H3 in 17
TCC and the paired NAT samples were examined using
semi-quantitative RT-PCR. In individual patients (Fig. 1A) and as a mean value (Fig. 1B), B7-H3 mRNA expression levels were
significantly higher in TCC samples compared with the paired NAT
samples (P<0.05).
Protein expression level of B7-H3 in TCC
and NAT samples
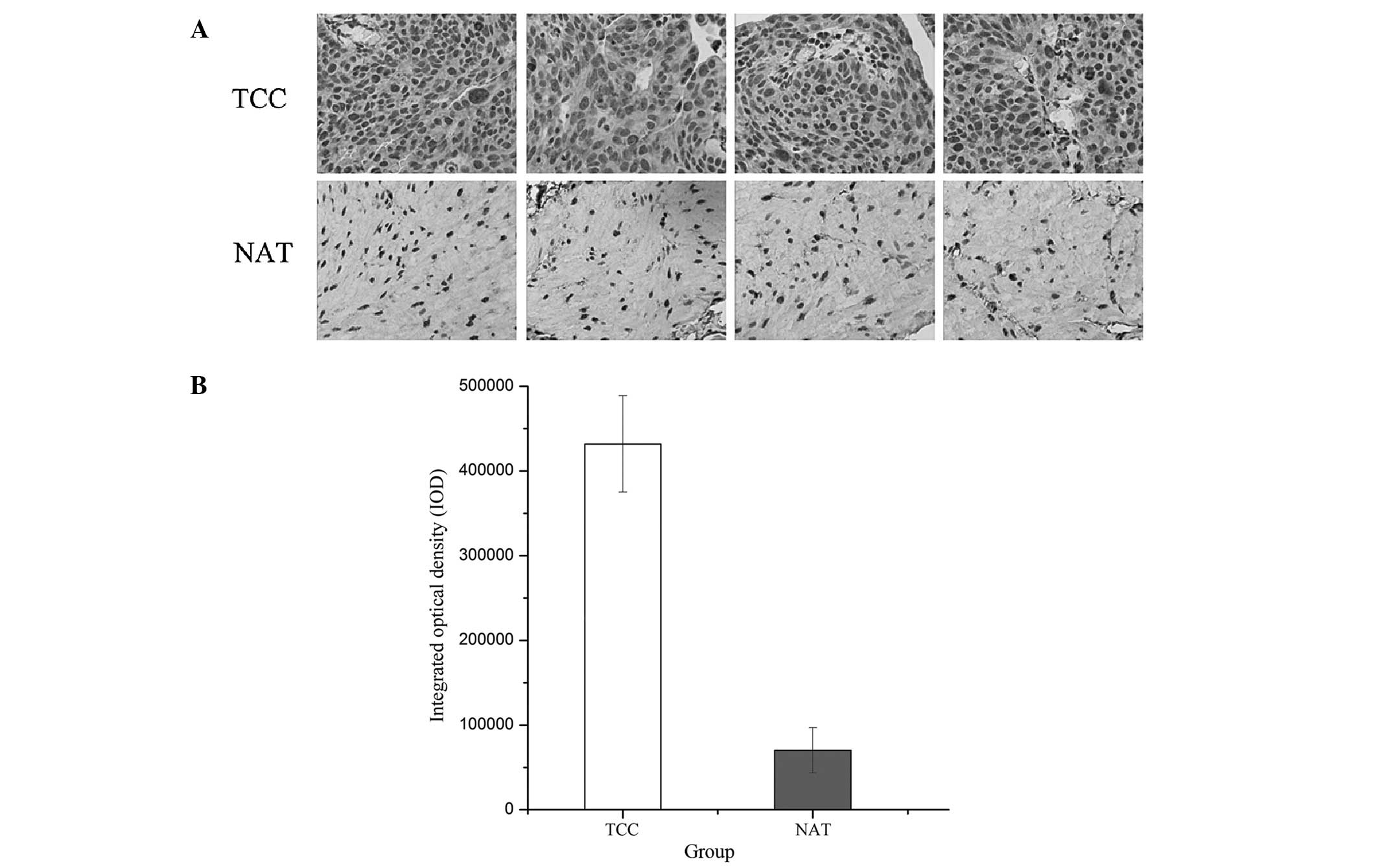
The protein level of B7-H3 in TCCs and NATs was
compared using immunohistochemistry (Fig. 2A). For quantitative analysis of
immunostaining intensity, the IOD was calculated using Image-Pro
Plus 6.0 software (Media Cybernetics; Fig. 2B). The results indicated that the
B7-H3 protein level was significantly upregulated in the TCC
samples compared with the paired NAT samples. Therefore,
dysregulation of B7-H3 in TCCs may be important during the
progression of bladder cancer.
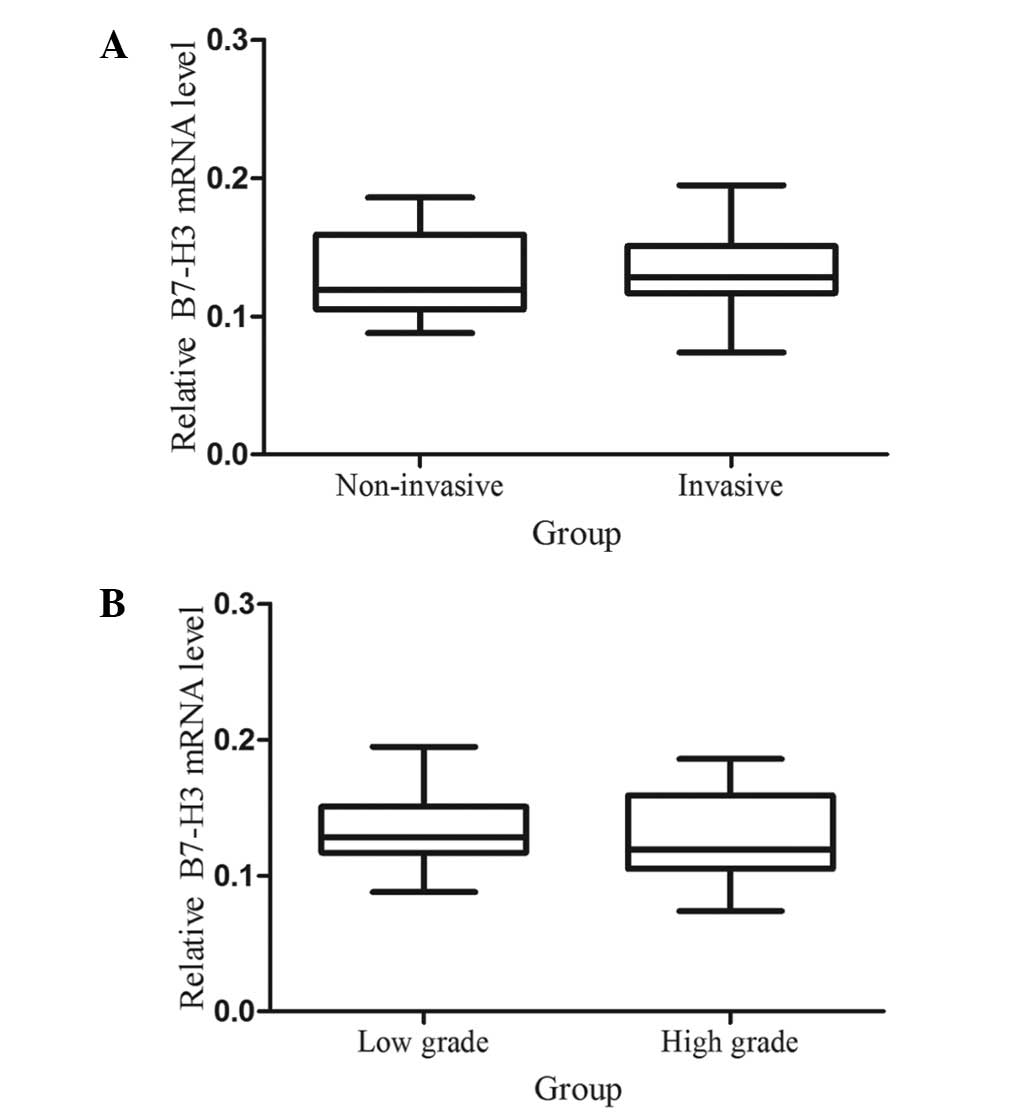
Association between B7-H3 mRNA expression
level, and the tumor stage and grade of TCC patients
Previous studies have demonstrated that the
expression of B7-H3 is associated with aggressive behavior in
prostate cancer and clear cell renal cell carcinoma (15,16).
Therefore, the present study investigated whether the mRNA
expression level of B7-H3 is associated with the tumor stage and
grade in bladder cancer patients. Statistical analysis indicated no
significant association between the B7-H3 mRNA expression level,
and the tumor stage (Fig. 3A) and
grade (Fig. 3B).
Discussion
In the present study, the mRNA and protein
expression levels of B7-H3 were evaluated in TCC samples. In a
significant fraction of bladder cancer patients, the mRNA and
protein expression levels of B7-H3 were upregulated. To the best of
our knowledge, this is the first report demonstrating alterations
in the expression of B7-H3 in TCC.
Human B7-H3 is a member of the B7 family and shares
20–27% amino acid identity with other B7 proteins (17). B7-H3 exists in two forms which
appear to be functionally identical; mouse B7-H3 contains
extracellular IgV-IgC domains, whereas tandemly duplicated
(IgV-IgC)n domains are present in human B7-H3 due to
exon duplication (18). B7-H3 was
initially detected in interferon-γ (IFN-γ)-treated dendritic cells,
and has since been identified to be inducible in monocytes, T
cells, B cells and natural killer cells (19). Detailed analysis of the expression
of B7-H3 in various tissues has revealed its immunobiological
significance. At the transcriptional level, B7-H3 is expressed in
the majority of organs (17,20),
however, at the protein level, B7-H3 is expressed in human breast,
bladder, liver, lung, lymphoid organs, placenta, prostate and
testis (10). Furthermore, B7-H3
has been observed to be upregulated in prostate cancer (15), non-small cell lung cancer (21), gastric (22) and ovarian cancer (23). However, the specific cell type of
B7-H3-positive cells in each of these organs has yet to be
determined. The differential mRNA and protein expression patterns
indicate that B7-H3 is post-transcriptionally regulated, although,
the molecular mechanisms regulating B7-H3 expression remain
unclear.
At present, there is no consensus regarding the
physiologic or pathophysiologic roles of B7-H3, as immune
stimulatory and inhibitory effects have been described for this
ligand (24). The study that
initially identified human B7-H3 demonstrated that it exhibits a
co-stimulatory effect on T-cells (20) and a further in vitro study
demonstrated that B7-H3 increased the proliferation of CD4 and CD8
T-cell populations and selectively stimulated IFN-γ production
(25). In addition, B7-H3′s
co-stimulatory effect on T-cell function is evidenced by reduced
rates of acute and chronic cardiac allograft rejection in B7-H3
knockout mice (26). Furthermore,
upon transient transfection of B7-H3 into melanoma cells, the
induction of human primary CD8 cytotoxic T-cells was enhanced.
Subsequently, mouse cancer models demonstrated that ectopic
expression of B7-H3 results in the activation of tumor-specific
cytotoxic T-cells that aid in slowing tumor growth or, in certain
cases, completely eradicating the tumor. Furthermore, mice
implanted with a B7-H3-transfected colon cell line exhibited
significantly prolonged survival when compared with controls
(27,28).
By contrast, various studies demonstrated B7-H3
acting as a T-cell co-inhibitor. In the majority of studies
conducted thus far, human and mouse B7-H3 inhibit CD4 T-cell
activation, and reduce the production of effector cytokines, such
as IFN-γ and interleukin-4 (19,30,31). Inhibition of CD4 T-cell
activation may be controlled via the
N-[4-(5-nitro-2-furyl)-2-thiazolyl]acetamide, NF-KB and AP-1
signaling pathways, which T-cell receptors utilize to regulate gene
transcription (10). In an
independent study, the B7-H3 protein was upregulated in human
malignant tumor cells, indicating an associated with increased
disease severity (12). Prostate
cancer patients exhibiting B7-H3 overexpression were at an
increased risk of clinical cancer recurrence, metastatic
development prior to surgery and cancer-related mortality.
Furthermore, B7-H3 was upregulated in ovarian tumor vessels, which
are associated with poor clinical outcome (15). These findings indicate that tumors
may exploit B7-H3 as an immune evasion pathway via the
downregulation of T-cell-mediated antitumor immunity. Thus, it was
proposed that tumor-associated B7-H3 could be utilized as a novel
therapeutic strategy as a targeted agent or for the enhancement of
antitumor immunity (12). In the
present study, the expression levels of B7-H3 were upregulated in
bladder cancer, indicating that B7-H3 may act as a co-inhibitor in
TCC. Although the present study identified that B7-H3 expression
levels were higher in invasive and high grade TCCs, compared with
non-invasive and low grade TCCs, the association of B7-H3
expression with these clinicopathological features was not
statistically significant. The small sample size (n=17) may have
contributed to the lack of significant results. Thus, further
studies with larger sample sizes are required to clarify the
present data.
In addition, further investigations are required to
determine an arbitrary cutoff value for B7-H3 expression levels in
the prognosis of bladder, to improve its clinical significance. In
addition, further investigation may explain the mechanisms of B7-H3
upregulation and reveal a novel predictor of human bladder
cancer.
In conclusion, our data demonstrate that B7-H3 is
significantly upregulated in TCC samples compared with the paired
NAT samples, indicating that higher expression of B7-H3 may be
important during the progression of bladder cancer.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards BK, Ward E, Kohler BA, et al:
Annual report to the nation on the status of cancer, 1975–2006,
featuring colorectal cancer trends and impact of interventions
(risk factors, screening, and treatment) to reduce future rates.
Cancer. 116:544–573. 2010. View Article : Google Scholar
|
|
3
|
Kirkali Z, Chan T, Manoharan M, et al:
Bladder cancer: epidemiology, staging and grading, and diagnosis.
Urology. 66(Suppl 1): 4–34. 2005. View Article : Google Scholar
|
|
4
|
Wu D, Tao J, Ding J, et al:
Interleukin-11, an interleukin-6-like cytokine, is a promising
predictor for bladder cancer prognosis. Mol Med Rep. 7:684–688.
2013.
|
|
5
|
Hirata H, Ueno K, Shahryari V, et al:
Oncogenic miRNA-182–5p targets Smad4 and RECK in human bladder
cancer. PloS One. 7:e510562012. View Article : Google Scholar
|
|
6
|
Pasin E, Josephson DY, Mitra AP, et al:
Superficial bladder cancer: an update on etiology, molecular
development, classification, and natural history. Rev Urol.
10:31–43. 2008.PubMed/NCBI
|
|
7
|
Sylvester RJ, Oosterlinck W and van der
Meijden AP: A single immediate postoperative instillation of
chemotherapy decreases the risk of recurrence in patients with
stage Ta T1 bladder cancer: a meta-analysis of published results of
randomized clinical trials. J Urol. 171:2186–2190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Said N, Sanchez-Carbayo M, Smith SC and
Theodorescu D: RhoGDI2 suppresses lung metastasis in mice by
reducing tumor versican expression and macrophage infiltration. J
Clin Invest. 122:1503–1518. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zang X, Sullivan PS, Soslow RA, Waitz R,
Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, et al:
Tumor associated endothelial expression of B7-H3 predicts survival
in ovarian carcinomas. Mod Pathol. 23:1104–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hofmeyer KA, Ray A and Zang X: The
contrasting role of B7-H3. Proc Nat Acad Sci USA. 105:10277–10278.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: a widely expressed B7 family member that
inhibits T cell activation. Proc Nat Acad Sci USA. 100:10388–10392.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zang X and Allison JP: The B7 family and
cancer therapy: costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okazaki T and Honjo T: The PD-1-PD-L
pathway in immunological tolerance. Trends Immunol. 27:195–201.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barber DL, Wherry EJ, Masopust D, Zhu B,
Allison JP, Sharpe AH, Freeman GJ and Ahmed R: Restoring function
in exhausted CD8 T cells during chronic viral infection. Nature.
439:682–687. 2006. View Article : Google Scholar
|
|
15
|
Zang X, Thompson RH, Al-Ahmadie HA, Serio
AM, Reuter VE, Eastham JA, Scardino PT, Sharma P and Allison JP:
B7-H3 and B7× are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Nat Acad Sci
USA. 104:19458–19463. 2007. View Article : Google Scholar
|
|
16
|
Crispen PL, Sheinin Y, Roth TJ, Lohse CM,
Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich
BC, et al: Tumor cell and tumor vasculature expression of B7-H3
predict survival in clear cell renal cell carcinoma. Clin Cancer
Res. 14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun M, Richards S, Prasad DV, Mai XM,
Rudensky A and Dong C: Characterization of mouse and human B7-H3
genes. J Immunol. 168:6294–6297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ling V, Wu PW, Spaulding V, Kieleczawa J,
Luxenberg D, Carreno BM and Collins M: Duplication of primate and
rodent B7-H3 immunoglobulin V- and C-like domains: divergent
history of functional redundancy and exon loss. Genomics.
82:365–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waschbisch A, Wintterle S, Lochmuller H,
Walter MC, Wischhusen J, Kieseier BC and Wiendl H: Human muscle
cells express the costimulatory molecule B7-H3, which modulates
muscle-immune interactions. Arthritis Rheum. 58:3600–3608. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chapoval AI, Ni J, Lau JS, Wilcox RA,
Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K and Chen L:
B7-H3: a costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arigami T, Uenosono Y, Hirata M, Yanagita
S, Ishigami S and Natsugoe S: B7-H3 expression in gastric cancer: a
novel molecular blood marker for detecting circulating tumor cells.
Cancer Sci. 102:1019–1024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fauci JM, Straughn JM Jr, Ferrone S and
Buchsbaum DJ: A review of B7-H3 and B7-H4 immune molecules and
their role in ovarian cancer. Gynecol Oncol. 127:420–425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM,
Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML,
et al: B7-H3 ligand expression by prostate cancer: a novel marker
of prognosis and potential target for therapy. Cancer Res.
67:7893–7900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo L, Chapoval AI, Flies DB, Zhu G,
Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, et al: B7-H3
enhances tumor immunity in vivo by costimulating rapid clonal
expansion of antigen-specific CD8+ cytolytic T cells. J
Immunol. 173:5445–5450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Fraser CC, Kikly K, Wells AD, Han
R, Coyle AJ, Chen L and Hancock WW: B7-H3 promotes acute and
chronic allograft rejection. Eur J Immunol. 35:428–438. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo L, Qiao H, Meng F, Dong X, Zhou B,
Jiang H, Kanwar JR, Krissansen GW and Sun X: Arsenic trioxide
synergizes with B7H3-mediated immunotherapy to eradicate
hepatocellular carcinomas. Int J Cancer. 118:1823–1830. 2006.
View Article : Google Scholar
|
|
28
|
Lupu CM, Eisenbach C, Kuefner MA, Schmidt
J, Lupu AD, Stremmel W and Encke J: An orthotopic colon cancer
model for studying the B7-H3 antitumor effect in vivo. J
Gastrointest Surg. 10:635–645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suh WK, Gajewska BU, Okada H, Gronski MA,
Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et
al: The B7 family member B7-H3 preferentially down-regulates T
helper type 1-mediated immune responses. Nat Immunol. 4:899–906.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prasad DV, Nguyen T, Li Z, Yang Y, Duong
J, Wang Y and Dong C: Murine B7-H3 is a negative regulator of T
cells. J Immunol. 173:2500–2506. 2004. View Article : Google Scholar : PubMed/NCBI
|