Introduction
Based on the GLOBOCAN 2008 estimates (1), primary lung cancer accounts for 17% of
newly diagnosed cancer cases and 23% of total cancer-related
mortalities, and the lung is also the leading global cancer site in
males. In females, lung cancer is the fourth most frequently
diagnosed cancer and the second most common cause of cancer-related
mortality worldwide. At the time of diagnosis, approximately half
of patients have metastatic disease, with the reported
post-diagnosis survival rates being 20% at one year and 1% at five
years (2,3). The common metastatic sites of primary
lung cancer are the liver, bones, adrenal glands and central
nervous system, while gastrointestinal metastasis rarely occurs.
Gastric metastasis is uncommon, and autopsy results have reported
the incidence to range between 0.2 and 1.7% in different studies
(4,5). Only sporadic cases of gastric
metastasis have been published in past decades. At present, little
is known about its clinicopathological features and prognosis, and
gastric metastasis remains a challenging clinical problem.
The present study reports a case of primary lung
cancer metastasizing to the stomach and provides a systematic
review of the previously reported cases to study the
clinicopathological features and outcome of this rare entity.
Written informed consent was obtained from the patient.
Case report
Patient characteristics and case
presentation
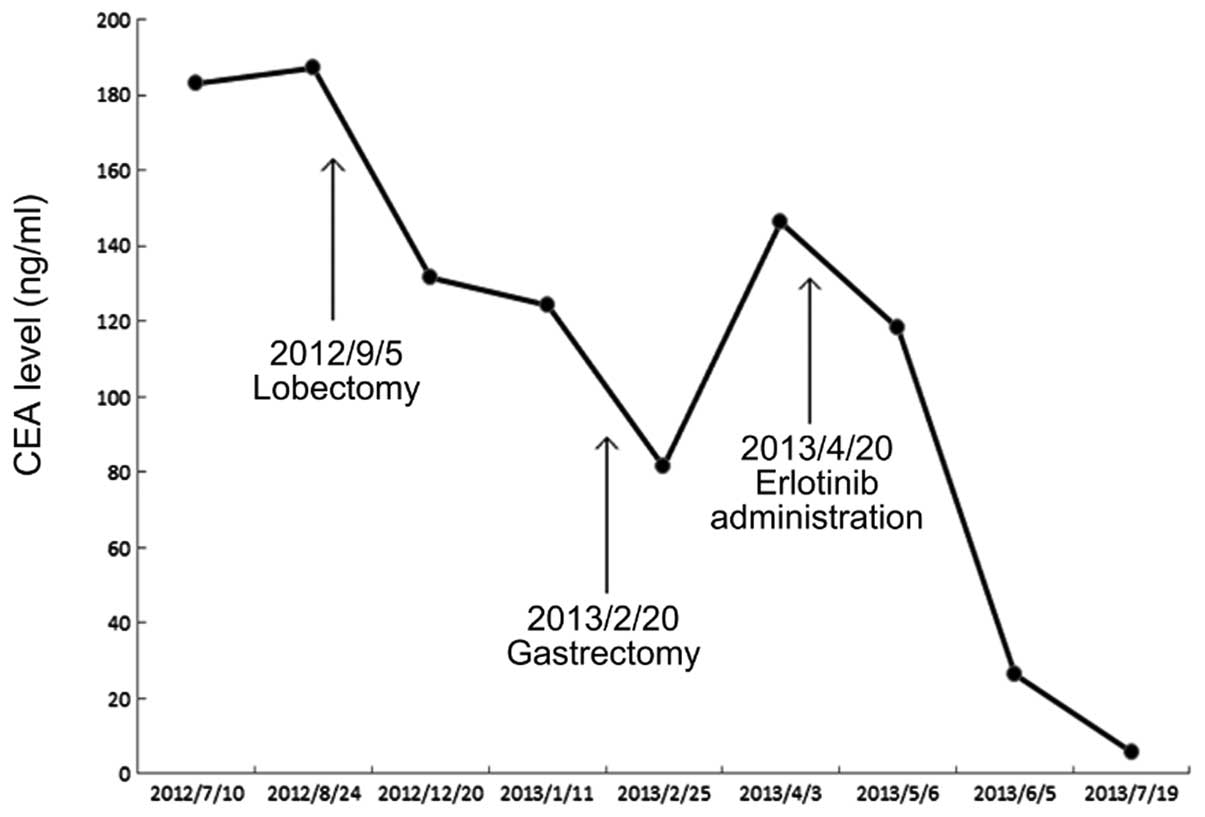
On a routine heath check-up in July 2012, a raised
carcinoembryonic antigen (CEA) value of 183.2 ng/ml (normal value,
0–5 ng/ml; Fig. 1) was found in an
asymptomatic, 61-year-old female who was a non-smoker and a
non-drinker. A prominent submucosal lesion, ~0.7×0.8 cm in size,
was detected in the fundus of the stomach through gastroscopic
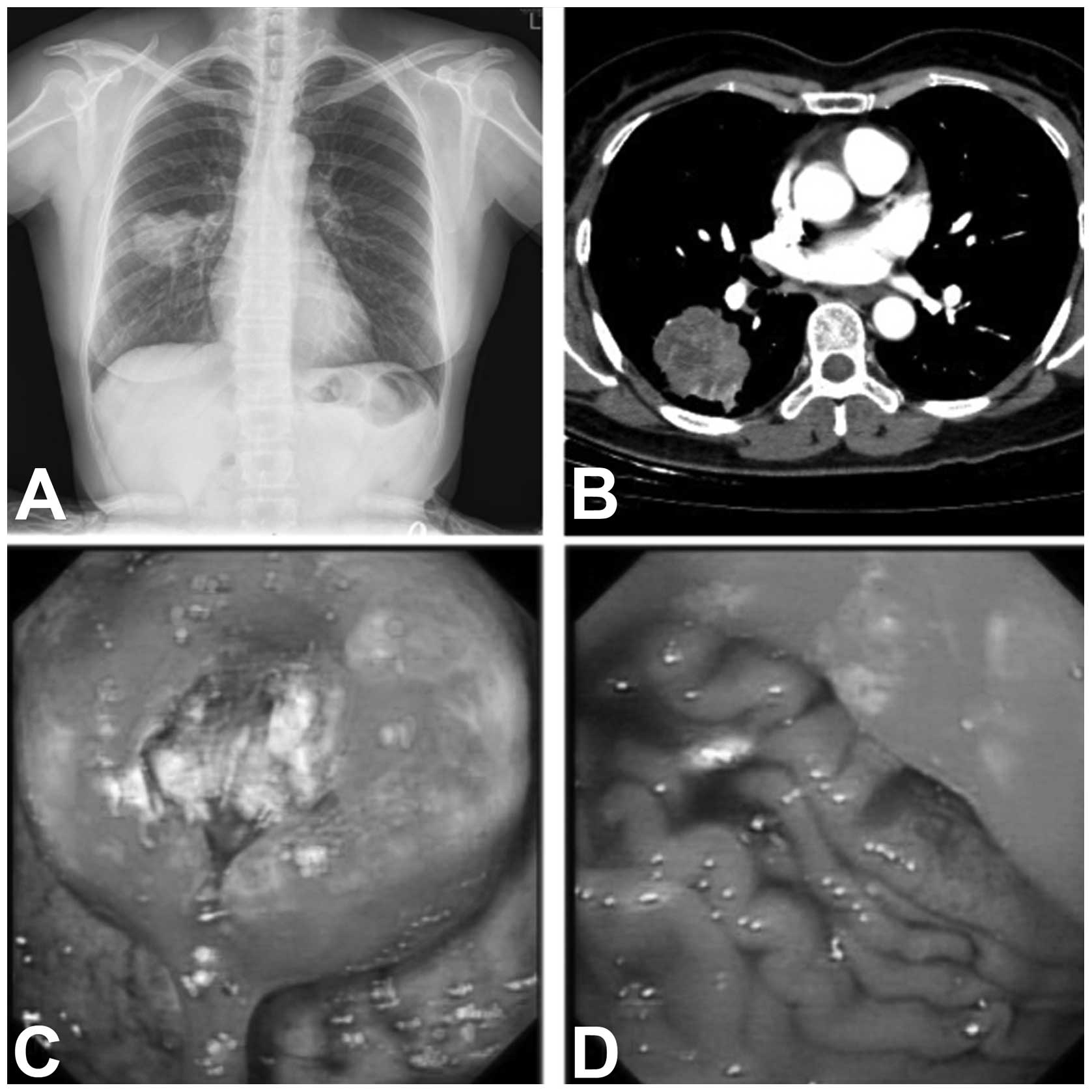
examination. An abnormal chest X-ray shadow in the right lower lobe
was later detected (Fig. 2A). A
computed tomography (CT) scan of the chest and abdomen revealed an
irregular mass without any abdominal abnormality (Fig. 2B). A right lower lobectomy, with
complete mediastinal lymph node dissection was performed.
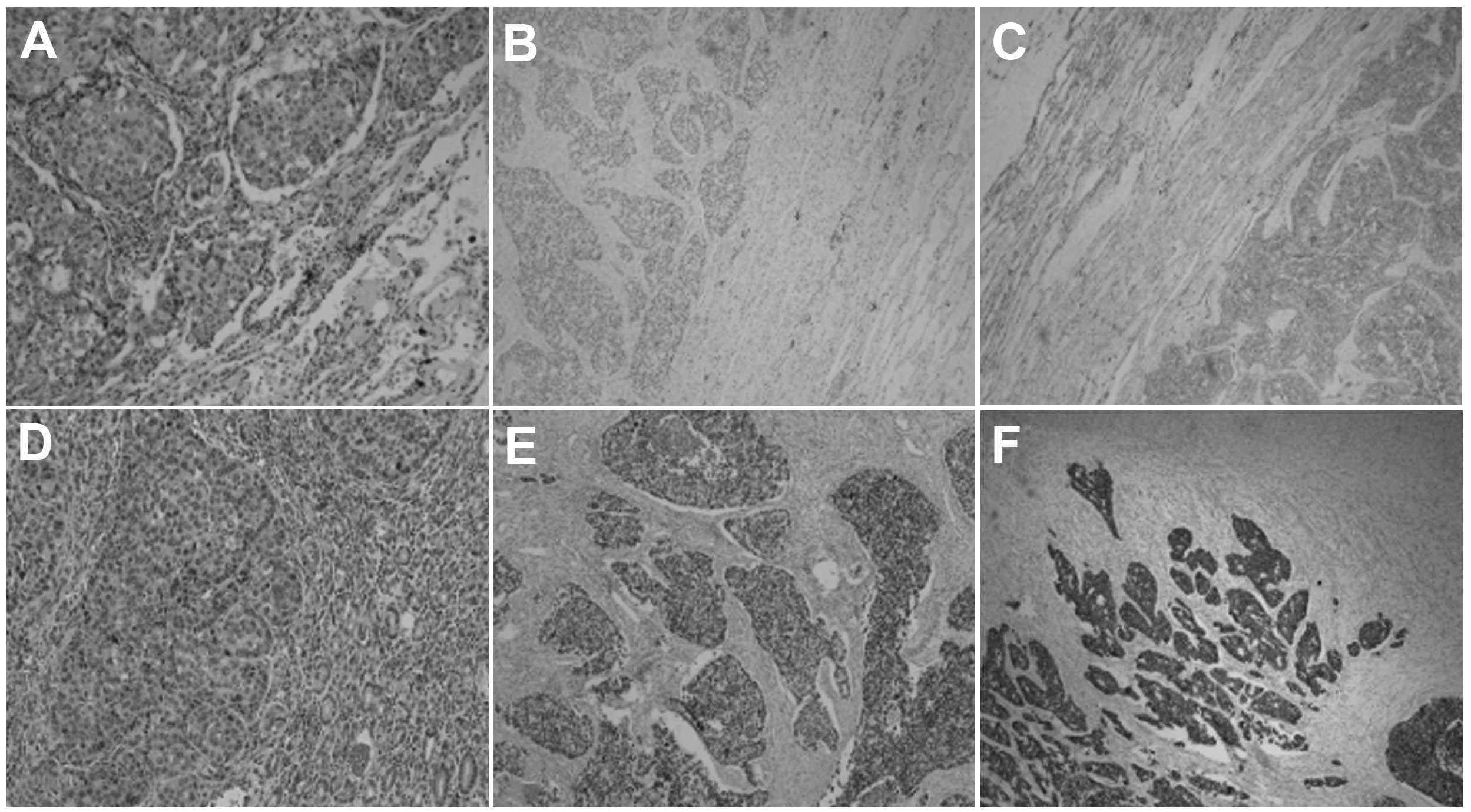
Pathological examination of the surgical specimen revealed a
poorly-differentiated, stage IB (T2aN0M0), adenocarcinoma (Fig. 3A). Immunohistochemically, the tumor
cells were positive for thyroid transcription factor (TTF)-1 and
cytokeratin (CK)-7 (Fig. 3B and
C).
Four months later, during follow-up, the patient
complained of epigastric discomfort without dysphagia or melena.
Laboratory examinations indicated a raised CEA level of 124.2
ng/ml. The positron emission tomography (PET)-CT scan revealed a
thickening of the cardia wall, with increased fluorodeoxyglucose
activity (maximum standardized uptake value, 19.4) that was
consistent with malignancy. Further gastroscopy revealed a mass
with a deep ulcer (Fig. 2C and D);
endoscopic ultrasonography (EUS) was not performed due to fear of
perforation. The biopsy confirmed the mass to be a
poorly-differentiated adenocarcinoma. The patient underwent a
partial gastrectomy, and the histology of the excised tissue was
found to be the same as that from the biopsy (Fig. 3D). The diagnosis of gastric
metastasis from primary lung cancer was confirmed
immunohistochemically by positive staining for TTF-1 and CK-7
(Fig. 3E and F), and negative
staining for CDX2 and Villin. The EGFR gene of the gastric
metastasis harbored a 19th exon mutation, identified by the
Amplified Refractory Mutation System method, which detects single
base pair mutations in a background of wild-type DNA (6), while the primary lung tumor showed a
wild-type EGFR sequence. Erlotinib treatment (150 mg, once a day)
was commenced in April 2013. The CEA level decreased to 5.9 ng/ml
in July 2013, and the patient was alive and ambulatory at the time
of writing this study.
Systematic review
A systematic review of the cases reported in the
literature was conducted to examine the nature of gastric
metastasis from primary lung cancer. The Medline database was
searched for literature published between 1966 and 31 December,
2012. The search strategy was (‘lung cancer’ OR ‘lung neoplasms’
[MeSH Terms]) AND (‘stomach’ OR ‘gastric’ [All Fields]) AND
(‘metastasis’ [All Fields]), filtering for case reports that were
in English and focused on humans. All potentially eligible studies
were retrieved and their references were carefully scanned to
identify other eligible studies.
The systematic review included studies that
fulfilled all of the following criteria: i) A focus on gastric
metastasis from primary lung cancer; ii) a diagnosis verified by
pathological examination; and iii) a previously unreported patient
group. Criteria for excluding articles for further review were: i)
Gastrointestinal metastasis without involvement of the stomach; ii)
provision of insufficient clinicopathological data, such as the
complaint and pathological type; and iii) autopsy studies.
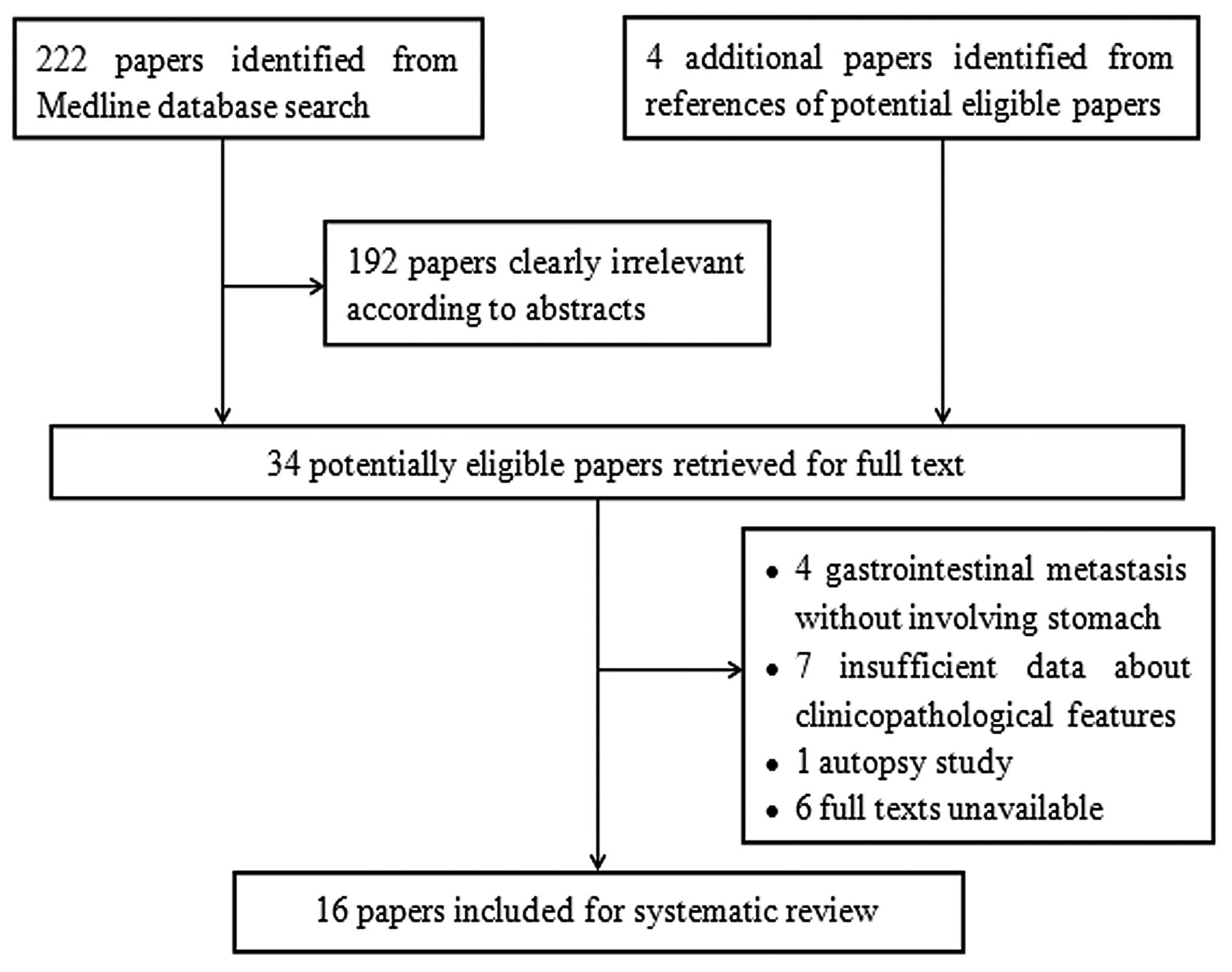
A total of 222 articles were retrieved by a
literature search of the Medline database, using the aforementioned
search strategy. As indicated in the search flow diagram (Fig. 4), a total of 16 studies were finally
included. These studies were comprised of the case reports of 22
patients. Table I summarizes the
patients, the tumor characteristics, the therapies implemented and
the survival times recorded.
 | Table IClinicopathological features and
outcome of the 22 cases. |
Table I
Clinicopathological features and
outcome of the 22 cases.
| Patient | First author, year
(ref.) | Gender | Age, years | Smoking | Main Complaint | Time span,
months | Endoscopic
features | Gastric metastatic
site | Histology | Primary lung
site | Treatment | Survival, months |
|---|
| 1 | Sileri, 2012
(6) | Male | 68 | Y | Epigastric pain with
nausea and anorexia | 48.0 | Neoplasm, originating
from muscular layer | Pylorus | AC | RUL | Subtotal
gastrectomy | >15 |
| 2 | Lee, 2010 (7) | Male | 77 | NR | None | 0.0 | Ulcer with raised
margin | Pylorus | AC | RUL | Lobectomy + subtotal
gastrectomy | NR |
| 3 | Ozdilekcan, 2010
(8) | Male | 46 | Y | Disphagia and
epigastric pain | 0.5 | Giant ulcer | Body | SCC | RUL | CRT | 1.00 |
| 4 | Okazaki, 2010
(15) | Male | 68 | Y | Epigastric pain | 0.0 | Erosive tumor | Body | AC | RLL | CT | 12.00 |
| 5 | Kanthan et al,
2009 (19) | Male | 75 | N | Epigastric and
right upper quadrant pain | 0.0 | Polyps | NR | AC | Right | NR | NR |
| 6 | Aokage et
al, 2008 (21) | Male | 69 | NR | General fatigue and
anemia | 5.0 | Hemorrhagic
tumor | Body | Pleomorphic
carcinoma | RUL | Distal
gastrectomy | 60.00 |
| 7 | | Male | 62 | NR | None | 0.0 | NR | Fundus | Pleomorphic | LUL | Partial gastrectomy
and splenectomy | 48.00 |
| 8 | Wu et al
(20) | Male | 73 | NR | Melena | 108.0 | NR | Cardia | SCC | NR | Conservation | 1.00 |
| 9 | | Male | 82 | NR | Melena | 5.0 | NR | Pylorus | AC | NR | Conservation | 1.00 |
| 10 | | Male | 70 | NR | Epigastric
pain | 5.0 | NR | Body | AC | NR | Conservation | 10.0 |
| 11 | Yang et al,
2006 (2) | Male | 71 | Y | Melena | 0.0 | NR | NR | SCC | LUL | | 4.50 |
| 12 | | Male | 65 | Y | Melena | 0.0 | NR | NR | SCC | RML | | 3.00 |
| 13 | | Male | 62 | Y | Melena | 1.7 | NR | NR | AC | RUL | | 12.40 |
| 14 | Casella et
al, 2006 (16) | Male | 63 | Y | Fever and
epigastric pain | 0.0 | A raised area
depressed on the tip | Pylorus | Small cell
cancer | LUL | Supportive
care | 1.00 |
| 15 | Altintas, 2006
(9) | Male | 55 | NR and melena | Hematemesis | 11.0 lesions | Two
volcano-like | Body | AC | NR | CRT | 0.75 |
| 16 | Alpar, 2006
(10) | Male | 66 | Y | Epigastric pain and
vomiting | 3.0 | Erosive and
atrophic pangastritis | NR | SCC | NR | None | 2.00 |
| 17 | Hamatake, 2001
(11) | Male | 65 | Y | Acute
hematemesis | 3.0 | Bleeding gastric
ulcer | Body | SCC | LLL | Total
gastrectomy | 4.00 |
| 18 | Kim et al,
1993 (17) | Male | 66 | NR | Epigastric pain and
weakness | 0.0 | Multiple submucosal
lesion with umbilications | Body and
fundus | Small cell
cancer | LUL | NR | NR |
| 19 | | Male | 68 | Y | None | 0.0 | Fungating mass | Body | SCC | LUL | NR | NR |
| 20 | Fukuda et
al, 1992 (12) | Female | 79 | NR | Epigastric
pain | 0.0 | Submucosal tumor
with central ulceration | Fundus | AC | RLL | NR | 12.00 |
| 21 | Maeda et al,
1992 (14) | Female | 60 | NR | Nausea and
vomiting | 3.0 | Multiple submucosal
tumors | NR | Small cell
cancer | RLL | Conservation | NR |
| 22 | Fletcher, 1980
(18) | Male | 70 | Y | Epigastric and
substernal pain | 3.5 prior | Raised mucosa with
ulceration | Body | SCC | LLL | Ulcer excision and
truncal vagotomy | 2.00 |
As detailed in Table
I, it was determined that the average age at presentation was
67.3 years (range, 46–82 years). There were 20 males (90.9%) and
two females (9.1%). Overall, 11 patients (50%) were cigarette
smokers, one (4.5%) had never smoked and the smoking status of 10
patients (45.5%) was not reported.
The presenting symptoms were mainly abdominal in
nature (18 patients, 81.8%), including epigastric pain, melena,
hematemesis and vomiting. Some patients also presented with fever,
anorexia, anemia or substernal pain. There were 10 patients whose
primary cancer and gastric metastases were confirmed during the
same series of work-up. For the 11 patients with gastric metastases
confirmed after lung cancer, the median time span between the
diagnosis of lung cancer and the detection gastric metastasis was 5
months. It is noteworthy that one patient had gastric ulcer
detected 14 weeks before lung cancer was detected.
Endoscopically, two main types of gastric lesions
were described: The nodular or fungating mass and the volcano-like
or umbilicated ulcer with raised margins, a number of them
hemorrhagic. The body of the stomach was the most common site of
metastasis in 62.5% of the 16 patients in which information
regarding the site of metastasis was available.
Gastric metastases were mostly from primary lung
adenocarcinoma (40.9%) followed by squamous-cell carcinoma (36.4%),
small cell lung cancer (13.6%) and pleomorphic carcinoma
(9.1%).
Nine patients (40.9%) developed gastric metastasis
as a single-site metastasis at the time of diagnosis and had no
other clinically detectable metastatic lesion, whereas 10 patients
(45.5%) also demonstrated other common metastatic sites of lung
cancer, including the bones, brain and liver. Common treatment
regimens for gastric metastases include surgery, chemotherapy or
chemoradiotherapy, and supportive care. Six of the nine patients
with single gastric metastasis received surgical treatment, ranging
from a total, subtotal or partial gastrectomy to excision of the
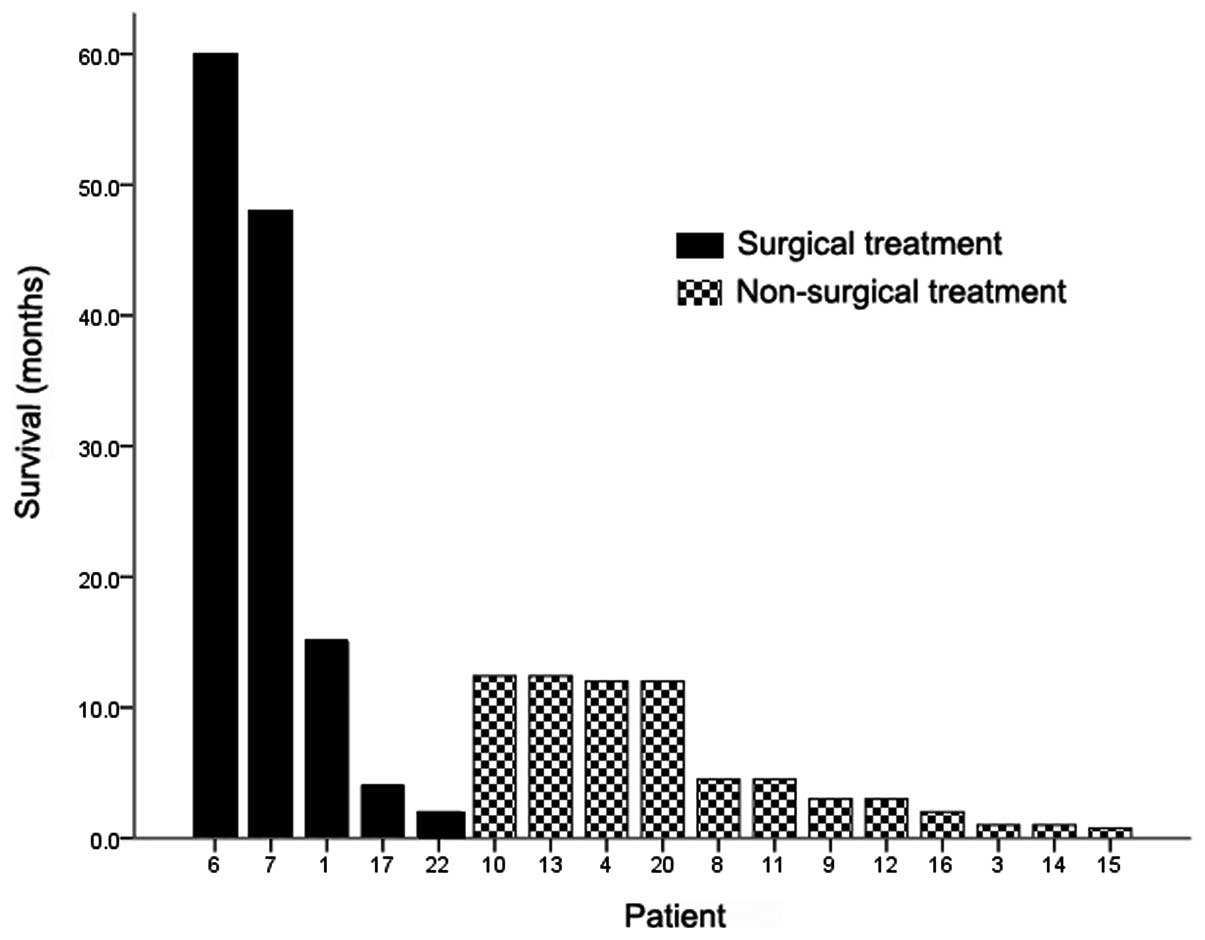
ulcer margin. The median survival of the 17 patients whose outcomes
were available was four months, and the one-year post-metastasis
survival rate was 35.3%. Three of the five patients who were
treated surgically for solitary gastric metastasis survived for
more than one year following confirmation of the metastasis
(Fig. 5).
Discussion
The identification of gastric metastasis from lung
cancer in a female patient who had never smoked was rare and
incidental in the present study. There is a high rate of
discrepancy between the clinical and autopsy diagnoses of gastric
metastasis from primary lung cancer, as the majority of the cases
are detected during autopsy (20).
Therefore, it may be estimated that a high number of gastric
metastases remain asymptomatic and clinically undetectable.
Among the symptomatic cases in the present
literature review, epigastric pain was the most common chief
complaint, followed by upper gastrointestinal bleeding (melena and
hematemesis), nausea/vomiting and general weakness or fatigue.
Gastric perforation due to metastasis was rare. These symptoms are
not specific and are usually regarded as side-effects of
chemotherapy or as symptoms of the involvement of the central
nervous system. This fact makes gastric metastasis occasionally
difficult to confirm (14). With
the rapid development of comprehensive treatment and supportive
care for lung cancer, and the subsequent survival benefit, an
increasing number of rare types of metastatic disease from primary
lung cancer are likely to be encountered (2), and clinicians therefore ought to be
aware of the possibility of their occurrence. Patients with
unspecific gastrointestinal symptoms following chemotherapy or
during the follow-up should be carefully monitored, and gastroscopy
should be performed when necessary.
Detection of a gastric abnormality is usually
incidental during the follow-up or the staging procedures of
primary lung cancer, and occasionally, detection could be even
earlier than that for lung cancer. Taking the present case and five
other cases (15–19) as examples, the primary lung lesions
were recorded as being found on chest X-ray following referral to
hospital for a gastric abnormality. There is a risk of misdiagnosis
associated with gastric metastases, since they can manifest prior
to the identification of the primary malignancy and may be
misdiagnosed as primary gastric cancer, so that consequently, the
true primary malignancy is not recognized (19).
Numerous gastric tumors consist of mucosal and
submucosal elements. Metastatic lesions in certain patients can
invade the submucosa or muscular layer, rather than the mucosa
(6). Besides the clinical
manifestations, the morphology of gastric metastasis observed on
gastroscopy could mimic that of other gastric tumors. There are no
typical appearances that define metastatic disease. Therefore, EUS
should be considered to be a powerful diagnostic tool in gastric
lesions, as it can determine the depth of invasion of the gastric
wall (22).
A nodular or fungating mass and a volcano-like or
umbilicated ulcer are the two types of endoscopic appearance of a
gastric metastatic malignancy. The lesion in the current case
presented as a submucosal nodule on the first gastroscopy, which
evolved to form a deep ulcer six months later upon reexamination by
gastroscopy. This phenomenon suggests that gastric metastases may
exhibit different appearances in connection with the stage of the
disease. In early stages, they could appear as nodules. Following
considerable growth, the metastases invade the mucosa and
ulceration develops. These lesions have previously been reported to
be usually located on the fundus (25), while results from the present study
and a study by Wu et al (20) have suggested that the body of the
stomach is the most common site of metastasis.
In the present literature review, when gastric
metastasis was diagnosed, the metastasis was associated with other
organs in more than half of the lung cancer patients. The prognosis
of patients with gastric metastasis following complete non-small
cell lung carcinoma (NSCLC) resection is generally poor and only
approximately one in three patients survive longer than one year.
The one-year post-metastasis survival rate in the present
literature review was close to that of patients with extrathoracic
recurrence following complete NSCLC resection, with a reported
one-year post recurrence survival rate of 26% (24).
Comprehensive and personalized treatment should be
the treatment strategy for gastric metastases from primary lung
cancer. Systematic chemotherapy with or without radiotherapy would
be the first option for selected patients, and molecular targeted
therapy may also be a reasonable choice if the patient was found to
possess an EGFR mutation or to be EML4-ALK-positive. Patients with
a poor performance status should be provided with supportive
treatment to improve the quality of life.
Generally, a distant metastatic lesion that has
originated from lung cancer is a contraindication for surgical
therapy. However, resection of a solitary metastatic lesion in the
brain or adrenal gland is becoming the standard of care that has
exhibited a survival benefit. In NSCLC patients with a single
metastasis other than metastasis in the brain or adrenal gland,
Salah et al (25) found that
metastasectomy significantly prolonged the five-year overall
survival rate. Additionally, a previous study reported long-term
survival following resection of solitary gastric metastases. Aokage
et al (21) observed two
patients with solitary gastric metastases from pulmonary
pleomorphic carcinoma who survived for four years and five years
after surgery, respectively. According to the present review data,
patients receiving surgical treatment for solitary gastric
metastases tended to survive longer than others. Since literature
data on the surgical treatment of single metastasis is scant, and
more cases are necessary to evaluate the effectiveness of the
surgical treatment for gastric metastasis from lung cancer. In
addition, surgical intervention is usually indicated when gastric
metastasis leads to continuous bleeding or perforation.
Locally advanced (stage III) or metastatic NSCLC
patients with activating mutations in the EGFR gene have exhibited
a dramatic response to EGFR tyrosine kinase inhibitors (EGFR-TKI),
such as gefitinib and erlotinib, since these activating mutations,
including exon 19 deletions and the L858R point mutation in exon
21, are recognized as markers of EGFR-TKI therapy sensitivity in
NSCLC (26). Activating EGFR
mutations predominate in never-smokers, females and tumors with
adenocarcinoma histology (27). For
the present patient, an EGFR gene mutation in exon 19 was detected
in the gastric metastasis, and therefore, erlotinib was
administered. The primary lung adenocarcinoma, however, harbored a
wild-type EGFR sequence. This mismatch is not novel; previous
studies have revealed discordances in EGFR status between the
primary tumor and the corresponding metastases in approximately
one-third of cases (28–30). Han et al also proved that a
significant portion of lung adenocarcinoma exhibits discordances in
EGFR mutation between primary tumors and the corresponding
metastases (31). Gow et al
(28) indicated that, in the
majority of discordant cases, the primary tumor possessed wild-type
EGFR while the corresponding metastasis possessed the EGFR
mutation. This suggests that the molecular properties of EGFR are
not stable and are likely to change during the process of lung
cancer metastasis (29,32). However, the prognostic role of EGFR
in metastatic gastric cancer has yet to be established. To the best
of our knowledge, identification of the EGFR gene mutation in the
gastric metastasis of a primary lung cancer patient with wild-type
EGFR has not been reported to date. The gradually decreasing CEA
level following erlotinib administration indicates that erlotinib
is beneficial for this type of patient.
In selecting lung cancer patients with solitary
gastric metastasis for specific targeted therapies by EGFR
analysis, special attention should be given to the metastatic
lesions rather than their corresponding primary tumors. EGFR-TKI
therapy may be a reasonable treatment for NSCLC patients harboring
an activating EGFR mutation in the metastatic lesion.
Primary lung cancer metastasizing to the stomach is
rare, however, clinicians should be aware of the possibility of its
occurrence. Comprehensive and personalized treatment may be
beneficial to such affected patients. EGFR TKI therapy may be the
treatment of choice for NSCLC patients harboring an activating EGFR
mutation in the metastatic lesion.
Acknowledgements
The authors would like to thank the authors of the
studies included in the present study.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang CJ, Hwang JJ, Kang WY, et al:
Gastro-intestinal metastasis of primary lung carcinoma: clinical
presentations and outcome. Lung Cancer. 54:319–323. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McNeill PM, Wagman LD and Neifeld JP:
Small bowel metastases from primary carcinoma of the lung. Cancer.
59:1486–1489. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antler AS, Ough Y, Pitchumoni CS, Davidian
M and Thelmo W: Gastrointestinal metastases from malignant tumors
of the lung. Cancer. 49:170–172. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sileri P, D’Ugo S, Del Vecchio Blanco G,
et al: Solitary metachronous gastric metastasis from pulmonary
adenocarcinoma: Report of a case. Int J Surg Case Rep. 3:385–388.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee MH, Kim SR, Soh JS, Chung MJ and Lee
YC: A solitary gastric metastasis from pulmonary adenocarcinoma: a
case report. Thorax. 65:661–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozdilekcan C, Songür N, Memiş L, et al:
Lung cancer associated with a single simultaneous solitary
metastatic lesion in stomach: a case report with the review of
literature. Tuberk Toraks. 58:78–84. 2010.PubMed/NCBI
|
|
9
|
Altintas E, Sezgin O, Uyar B and Polat A:
Acute upper gastrointestinal bleeding due to metastatic lung
cancer: an unusual case. Yonsei Med J. 47:276–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alpar S, Kurt OK, Ucar N, Orsel O, Aydog G
and Kurt B: A case of squamous cell lung carcinoma with gastric
metastasis. South Med J. 99:1313–1314. 2006. View Article : Google Scholar
|
|
11
|
Hamatake M, Ishida T, Yamazaki K, et al:
Lung cancer with p53 expression and a solitary metastasis to the
stomach: a case report. Ann Thorac Cardiovasc Surg. 7:162–165.
2001.PubMed/NCBI
|
|
12
|
Fukuda T, Ohnishi Y, Katagiri J, Ohnuki K
and Tachikawa S: A case of pulmonary adenocarcinoma with
sarcomatous elements initially manifested as a submucosal tumor of
the stomach. Acta Pathol Jpn. 42:454–459. 1992.PubMed/NCBI
|
|
13
|
Zhang XC, Wu YL, Wang J, et al: A
prospective comparison study on EGFR mutations by direct sequencing
and ARMS in completely resected Chinese non-small cell lung cancer
with adenocarcinoma histology (ICAN). J Clin Oncol. 31(suppl):
abstr 1547. 2013.
|
|
14
|
Maeda J, Miyake M, Tokita K, et al: Small
cell lung cancer with extensive cutaneous and gastric metastases.
Intern Med. 31:1325–1328. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okazaki R, Ohtani H, Takeda K, et al:
Gastric metastasis by primary lung adenocarcinoma. World J
Gastrointest Oncol. 2:395–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casella G, Di Bella C, Cambareri AR, et
al: Gastric metastasis by lung small cell carcinoma. World J
Gastroenterol. 12:4096–4097. 2006.PubMed/NCBI
|
|
17
|
Kim HS, Jang WI, Hong HS, et al:
Metastatic involvement of the stomach secondary to lung carcinoma.
J Korean Med Sci. 8:24–29. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fletcher MS: Gastric perforation secondary
to metastatic carcinoma of the lung: a case report. Cancer.
46:1879–1882. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanthan R, Sharanowski K, Senger JL, et
al: Uncommon mucosal metastases to the stomach. World J Surg Oncol.
7:622009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu MH, Lin MT and Lee PH:
Clinicopathological study of gastric metastases. World J Surg.
31:132–136. 2007. View Article : Google Scholar
|
|
21
|
Aokage K, Yoshida J, Ishii G, et al:
Long-term survival in two cases of resected gastric metastasis of
pulmonary pleomorphic carcinoma. J Thorac Oncol. 3:796–799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okai T, Minamoto T, Ohtsubo K, et al:
Endosonographic evaluation of c-kit-positive gastrointestinal
stromal tumor. Abdom Imaging. 28:301–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oda I, Kondo H, Yamao T, et al: Metastatic
tumors to the stomach: analysis of 54 patients diagnosed at
endoscopy and 347 autopsy cases. Endoscopy. 33:507–510. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugimura H, Nichols FC, Yang P, et al:
Survival after recurrent nonsmall-cell lung cancer after complete
pulmonary resection. Ann Thorac Surg. 83:409–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salah S, Tanvetyanon T and Abbasi S:
Metastatectomy for extra-cranial extra-adrenal non-small cell lung
cancer solitary metastases: systematic review and analysis of
reported cases. Lung Cancer. 75:9–14. 2012. View Article : Google Scholar
|
|
26
|
Jänne PA, Engelman JA and Johnson BE:
Epidermal growth factor receptor mutations in non-small-cell lung
cancer: implications for treatment and tumor biology. J Clin Oncol.
23:3227–3234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jackman DM, Miller VA, Cioffredi LA, et
al: Impact of epidermal growth factor receptor and KRAS mutations
on clinical outcomes in previously untreated non-small cell lung
cancer patients: results of an online tumor registry of clinical
trials. Clin Cancer Res. 15:5267–5273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gow CH, Chang YL, Hsu YC, et al:
Comparison of epidermal growth factor receptor mutations between
primary and corresponding metastatic tumors in tyrosine kinase
inhibitor-naive non-small-cell lung cancer. Ann Oncol. 20:696–702.
2009. View Article : Google Scholar
|
|
29
|
Gomez-Roca C, Raynaud CM, Penault-Llorca
F, et al: Differential expression of biomarkers in primary
non-small cell lung cancer and metastatic sites. J Thorac Oncol.
4:1212–1220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Italiano A, Vandenbos FB, Otto J, et al:
Comparison of the epidermal growth factor receptor gene and protein
in primary non-small-cell-lung cancer and metastatic sites:
implications for treatment with EGFR-inhibitors. Ann Oncol.
17:981–985. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han HS, Eom DW, Kim JH, et al: EGFR
mutation status in primary lung adenocarcinomas and corresponding
metastatic lesions: discordance in pleural metastases. Clin Lung
Cancer. 12:380–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daniele L, Cassoni P, Bacillo E, et al:
Epidermal growth factor receptor gene in primary tumor and
metastatic sites from non-small cell lung cancer. J Thorac Oncol.
4:684–688. 2009. View Article : Google Scholar : PubMed/NCBI
|