Introduction
Thymoma is a type of benign or low-grade malignant
tumor, developed on the thymic epithelium, and the most frequent
anterior mediastinal neoplasm in adults; however, its overall
incidence is only 0.13 per 100,000 individuals/year according to
data from the Surveillance, Epidemiology and End Results database
(1–3). Due to the low incidence of this
neoplasm, its etiology and pathogenesis remain unknown. In
addition, epidemiological data have revealed that 20–25% of
patients with thymomas also suffer from myasthenia gravis (MG;
hereafter referred to as MG-associated thymoma), whereas 10–20% of
myasthenic patients suffer from thymomas (4). MG, is an autoimmune disease of unclear
etiology, results in even more complex etiology and pathogenesis of
MG-associated thymoma. The ratio of patients with thymoma and MG is
higher than that of any ofther autoimmune disease. Pivotal markers
and cross-linked molecules of thymoma and MG have been the aim of
several previous studies (5,6).
Thymus is a primary organ and the initial site for
the development of the T cell immunological function (7). The development of thymoma is
frequently accompanied by a rich infiltrate of T cells (3). When released into the circulation,
these abnormally conditioned T cells are hypothesized to be
responsible for the autoimmune conditions that often accompany
thymoma, particularly MG (3). MG is
a known prototypical CD4+ T cell-dependent autoimmune
disease. Various subsets of T helper (Th) cells, including Th1, Th2
and Th17, and regulatory T cells (Treg) have been suggested to be
involved in the pathogenesis of MG (8). In addition, the imbalance of different
Th cell subsets is important in the progression of the disease.
Thymoma and MG are multifactorial and noninherited diseases, with
an established genetic predisposition. Numerous studies have
demonstrated that certain human leukocyte antigen (HLA) and non-HLA
genes, including HLA-death receptor 3, PTPN22 and CTLA4, present an
evident association with MG (9).
However, a clear correlation of particular genes/molecules with
MG-associated thymoma cannot be gained from the limited present
research (10).
T cell immunoglobulin and mucin domain-3 (Tim-3) is
a subtype of the Tim protein family, which was initially identified
in 2002, and is selectively expressed on Th1 cells, but not on Th2
cells (11). Tim-3 has been
demonstrated to negatively regulate Th1 response and induce immune
tolerance through the Tim-1/galectin-9 signaling pathway in
autoimmune diseases (12).
Furthermore, suppression of Tim-3 expression has been revealed to
enhance the pathological severity of experimental autoimmune
encephalomyelitis (EAE) (13),
while Tim-3-deficient mice have been found to be refractory to the
induction of immune tolerance in EAE (14). Besides the important role of Tim-3
in autoimmune diseases, a recent study has indicated that Tim-3 is
also a molecular switch for tumor escape from innate immunity
(15). Previous studies have
further identified Tim-3 expression on exhausted T cells in human
tumors (16,17) and preclinical tumor models (18,19).
The expression of Tim-3 is significantly increased on T cells in
tumor-infiltrated tissues and on tumor-infiltrating lymphocytes in
peripheral lymphoid tissues or the blood of tumor patients,
indicating that Tim-3 may be upregulated in a tumor-derived
environment (17,18). Therefore, the present study
hypothesized that Tim-3 may be a potentially pivotal molecule in
autoimmune disease-associated tumors, such as MG-associated
thymoma.
Tim-3 polymorphism has been demonstrated to alter
the interaction between Tim-3 and its ligand, thereby affecting the
process that results in certain immune diseases (19,20)
and being actively involved in the pathogenesis of tumors (21). Considering the aforementioned
findings, Tim-3 appears to be an important regulatory molecule that
plays a critical role in MG-associated thymoma, which may be
triggered mainly by the deviation of Th cell subtypes. However, to
the best of our knowledge, Tim-3 polymorphism and its association
with MG-associated thymoma in the Han Chinese population of North
China have not been evaluated. The aim of the present study was to
investigate the polymorphism of the −574 locus in the promoter of
Tim-3 and its association with MG-associated thymoma in the Han
Chinese population of North China.
Materials and methods
Study approval
The experiments of this study were approved by the
Ethics Committee of the Tianjin Medical University General Hospital
(Tianjin, China). Written informed consent was obtained from all
the patients prior to participation in the study. All the subjects
were from the Han population of North China, since the study was
performed at Tianjin, China.
Patients and DNA samples
In total, 116 patients with thymoma and MG were
enrolled from the Department of Cardiothoracic Surgery of the
Tianjin Medical University General Hospital, comprising the study
group (MG-associated thymoma group). In addition, 124 patients with
thymoma, but without MG, were randomly selected from the Department
of Cardiothoracic Surgery of the Tianjin Medical University General
Hospital, forming the control group (non-MG-associated thymoma
group). All the subjects were subjected to a comprehensive
examination in order to obtain a definite diagnosis of thymoma and
MG and exclude other autoimmune diseases. For the diagnosis of
thymoma, the results of chest X-ray, computed tomography and
magnetic resonance imaging, as well as surgical and pathological
findings, were considered. The diagnostic criteria of MG included
myasthenic manifestation, neostigmine test, repetitive nerve
stimulation test and serum autoantibody detection. All the patients
included in this study were genetically unrelated individuals of
the Han Chinese population. Genomic DNA was extracted from the
white blood cells of each sample using a DNA isolation kit (GK1072,
Generay Biotech Co, Ltd., Shanghai, China), according to the
manufacturer’s instructions. The extracted genomic DNA was
dissolved in sterile double-distilled water. Subsequently, the
concentrations and absorbance ratios at 260 nm/280 nm (A260/A280)
of the DNA solutions were measured using a nucleic acid
spectrometer (ScanDrop 200; Analytik Jena AG, Jena, Germany). DNA
samples with A260/A280 ratios ranging between 1.7 and 2.0 were
selected as polymerase chain reaction (PCR) templates and stored at
−80°C.
-574 locus genotyping
Allele-specific PCR (AS-PCR) was used to investigate
the −574 locus and three primers were designed using Primer 5.0
software (Premier Biosoft, Palo Alto, CA, USA), according to the
flanking sequence (GenBank no. NM_032782). The primer sequences
were as follows: Forward 1 (F1), 5′-GGCTTATGCTGGGAGTTGCT-3′;
forward 2 (F2), 5′-GGCTTATGCTGGGAGTTGCG-3′; reverse for F1 and F2
(R), 5′-GGTGTCTGATTGCCAGTGATTC-3′. The F1 and R primers were used
to amplify T allele fragments, whereas F2 and R primers were used
to amplify G allele fragments. All the amplified fragments were 539
bp long and each sample was subjected to AS-PCR with F1/R and F2/R.
The reaction mixture was as follows: 2.5 μl 10× PCR buffer; 1.5 μl
deoxyribonucleotide triphosphate (2.5 mmol/l each); 1 μl forward
primer (5 μmol/l); 1 μl reverse primer (5 μmol/l); 1.0 μl DNA
template; and 0.5 μl Taq DNA polymerase (2.5 U/μl) (Beijing AuGCT
DNA-Syn Biotechnology Co., Ltd., Beijing, China). Double-distilled
water was added to obtain a final reaction volume of 25 μl and,
subsequently, touchdown PCR was performed to amplify the target
amplicons. The reaction mixture was heated in a Thermo Cycler
(Mastercycler 5333; Eppendorf AG, Hamburg, Germany) under the
following conditions: 95°C for 5 min; 94°C for 30 sec; progressive
lowering of the annealing temperature by 1°C every three 30 sec
annealing cycles (between 65°C and 56°C); extension at 65°C for 35
sec, followed by 30 cycles at 72°C for 35 sec; final extension at
72°C for 10 min. The amplified products were subjected to 1.8%
agarose gel electrophoresis and visualized using ethidium bromide
staining, followed by capturing images using FireReader software
(XS D5626M Auto + FC26WL + Uviband; UVItec Ltd., Cambridge,
UK).
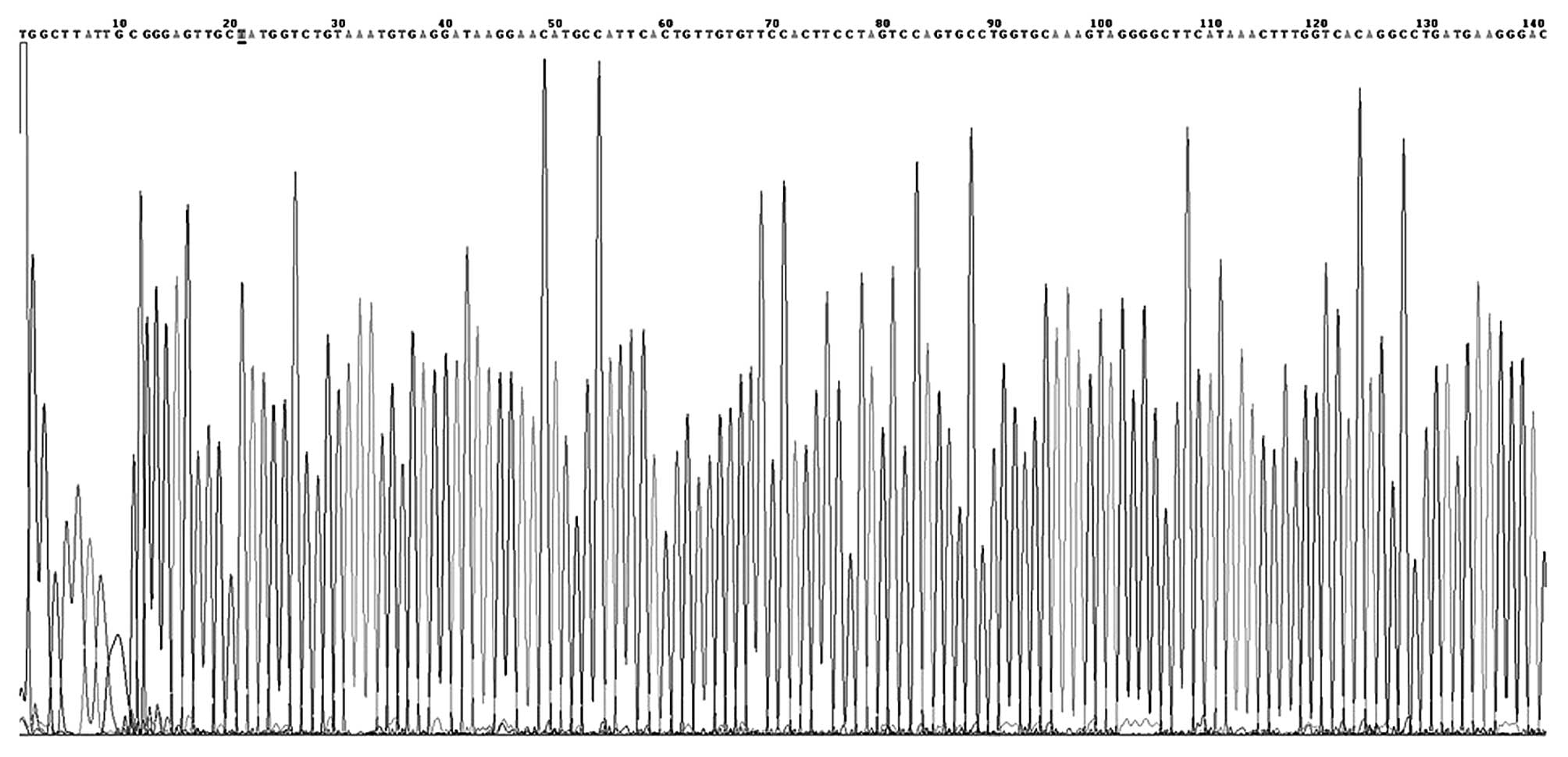
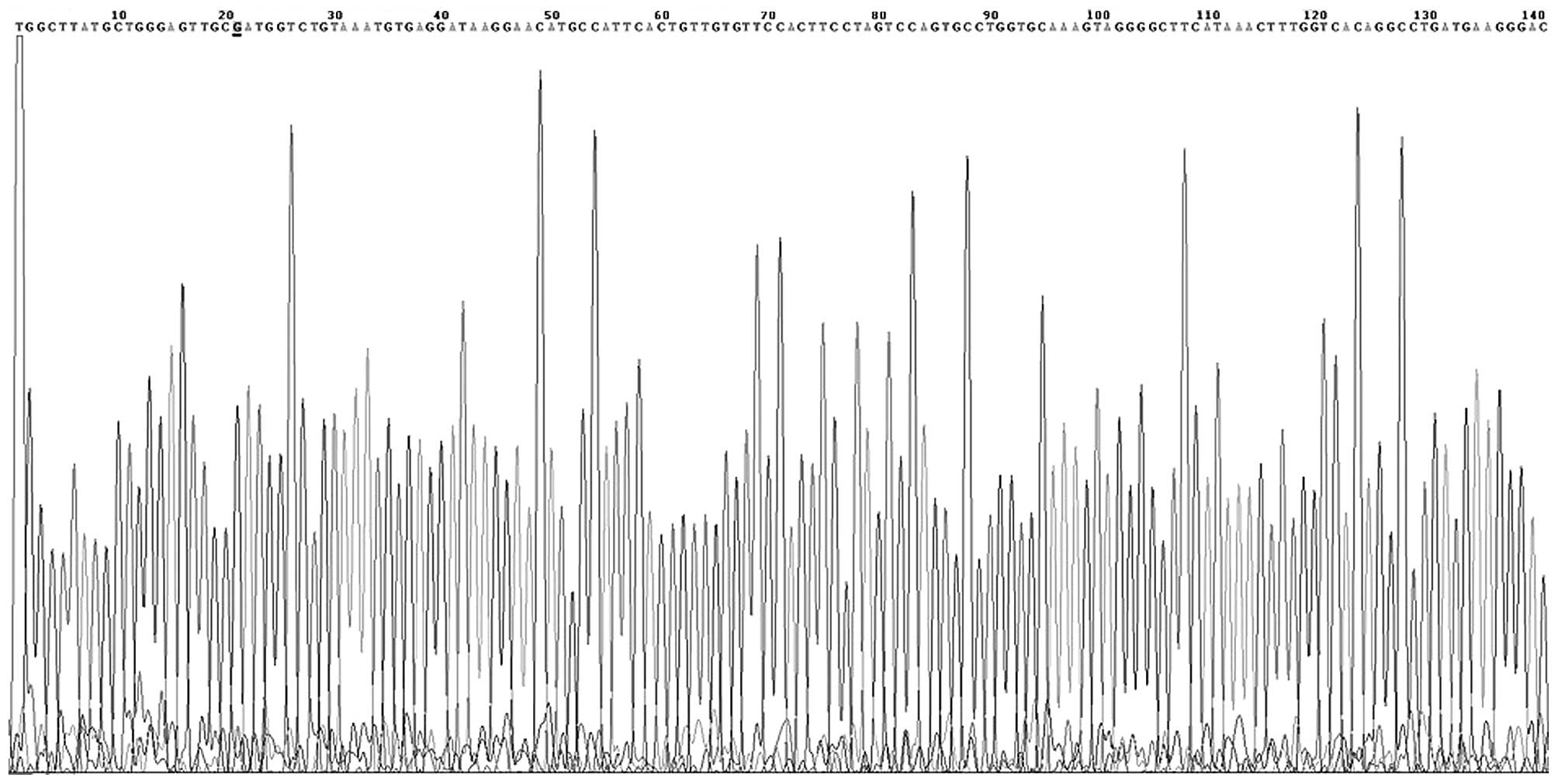
DNA sequencing
Several PCR products were randomly selected and
purified using a PCR purification kit (DK004-01B; NovoProtein
Biotechnology Co., Ltd., Shanghai, China). Sequencing of the
purified products was performed by Beijing AuGCT DNA-SYN
Biotechnology Co., Ltd. (Beijing, China).
Statistical analyses
Statistical analysis was performed using the SPSS
17.0 software package (SPSS, Inc., Chicago, IL, USA). χ2
test was performed to compare the allele frequencies of each group,
while the Hardy-Weinberg equilibrium test was conducted to
investigate the demographic representation of the study and control
groups. The relative risk was presented as the odds ratio (OR) and
its 95% confidential interval (CI). P<0.05 was considered to
indicate a statistically significant difference.
Results
General characteristics
The ages of the MG-associated thymoma patients were
18–62 years with a mean age of 45.6±4.5 years, while the ages of
the non-MG-associated thymoma patients were 20–63 years with a mean
age of 46.4±4.3 years. No statistically significant difference was
observed between the mean ages of the two patient groups
(P>0.05). In addition, the Hardy-Weinberg equilibrium test
results for the −574 locus revealed no statistically significant
differences between the study and control groups (P>0.05), which
indicated that the two groups were in Hardy-Weinberg equilibrium
and demographically representative.
-574 locus
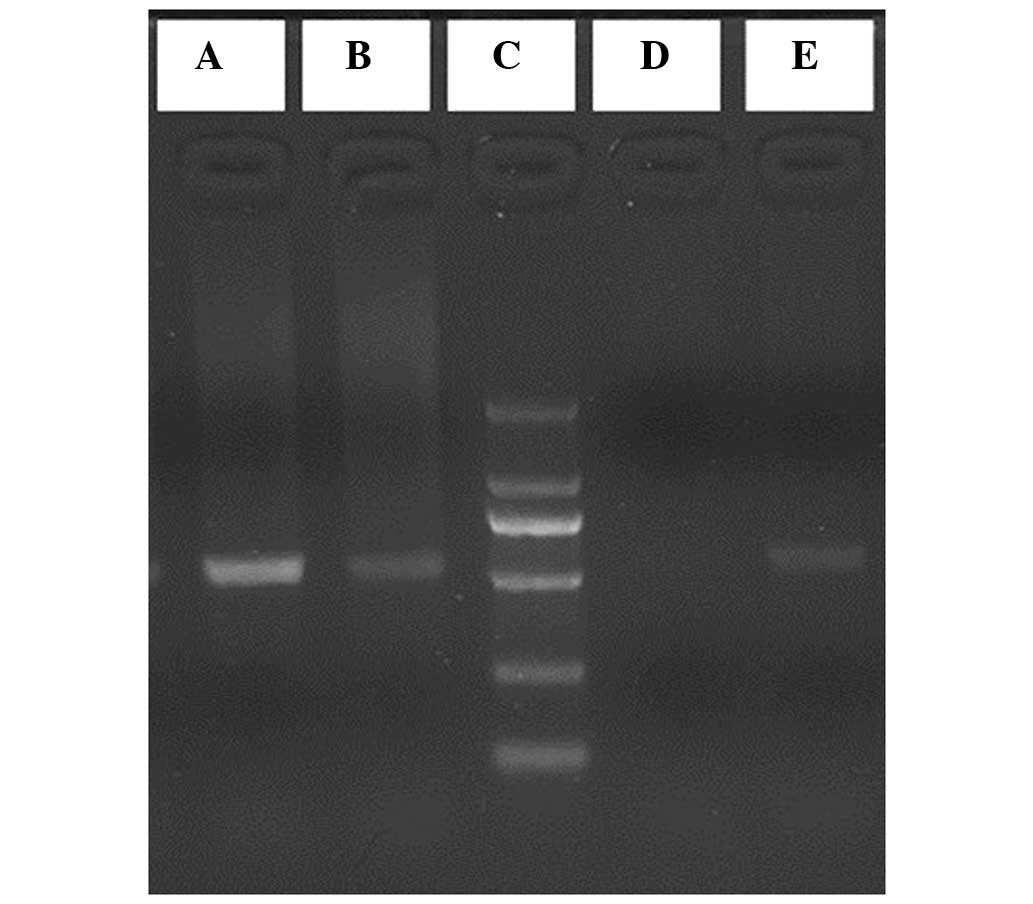
At the −574 locus, the G and T alleles were defined
as wild-type and mutant alleles, respectively. Following agarose
gel electrophoresis, the GG genotypes were defined as wild GG
homozygotes, the TT genotypes were defined as mutant TT homozygotes
and the GT genotypes were defined as GT heterozygotes (Fig. 1). In the present study, the GG
genotype was identified in 80 MG-associated thymoma patients, while
the GT genotype was identified in the remaining 36 MG-associated
thymoma patients. By contrast, the GG genotype was detected in 108
non-MG-associated thymoma patients, while the GT genotype was
detected in the remaining 16 patients of the group. The TT genotype
was not identified in the study or control patients (Figs. 2 and 3).
Genotypes and frequencies
At the −574 locus of Tim-3 in the MG-associated
group, the GG and (GT+TT) genotypes presented frequencies of 68.97
and 31.03%, respectively. By contrast, 87.10% of the control
subjects possessed the GG genotype and the remaining 12.90%
possessed the (GT+TT) genotype. A statistically significant
difference was observed between the two groups
(χ2=11.609, P=0.001). The frequency of the GT+TT
genotype on the −574 locus of the MG-associated thymoma group was
found to be statistically different compared with the control group
(P=0.001, OR=0.329; Table I). In
addition, the frequencies of T allele on the −574 loci of the two
groups were statistically different (P=0.001, OR=0.375; Table II).
 | Table IDistribution of -574 locus of Tim-3
genotypes of the studied population (%). |
Table I
Distribution of -574 locus of Tim-3
genotypes of the studied population (%).
| Genotype | Study group (n=116),
n (%) | Control group
(n=124), n (%) |
χ2-value | P-value | OR | 95% CI |
|---|
| GG | 80 (68.97) | 108 (87.10) | 11.609 | 0.001 | 0.329 | 0.171–0.634 |
| GT+TT | 36+0 (31.03) | 16+0 (12.90) | | | | |
 | Table IIDistribution of different alleles on
the -574 locus. |
Table II
Distribution of different alleles on
the -574 locus.
| Alleles | Study group (n=116),
n (%) | Control group
(n=124), n (%) |
χ2-value | P-value | OR | 95% CI |
|---|
| G | 196 (84.48) | 232 (93.55) | 10.198 | 0.001 | 0.375 | 0.202–0.697 |
| T | 36 (15.52) | 16 (6.45) | | | | |
Discussion
Tim-3, a surface molecule on T cells, is important
in immune regulatory functions and presents a strong correlation
with various tumor types. In humans, Tim-3 is located on chromosome
5q33.2 and comprises 301 amino acids, including elementary
structural domains, such as the signal domain, mucin-like
structural domain, transmembrane zone and cytomere domain of the
phosphorylation site (13–17,20).
In the immunoglobulin V structural domain, two antiparallel β
fragments and a metal ion ligand binding site function together as
the ligand-binding site of Tim-3 (14,23).
Evidence indicates that Tim-3 is a negative
regulator of T cell responses and is involved in the modulation of
autoimmune diseases (20).
Identification of galectin-9 as a ligand of Tim-3 may induce the
death of Th1 cells and downregulate Th1 responses (12,23). A
previous study demonstrated that in vivo treatment with
Tim-3 monoclonal antibodies during the induction of EAE, which is a
mouse model of multiple sclerosis (MS), accelerated disease
progression (11). In human
patients with MS, T cell clones derived from the cerebrospinal
fluid expressed lower levels of Tim-3 compared with those from
healthy control subjects (24). A
study on a mouse model of autoimmune diseases has indicated that
inhibition of the interaction between Tim-3 and its ligand may
dramatically aggravate the manifestation of autoimmune diseases
(25). Furthermore, the
distribution frequencies of +4259 T/G in Tim-3 in patients with
rheumatoid arthritis have been demonstrated to be statistically
different compared with healthy individuals in the Han and Hui
Chinese populations (26).
Therefore, the present study hypothesized that Tim-3 may be a
protective factor of autoimmune diseases (it may protect patients
from suffering from autoimmune diseases or decrease the possibility
of suffering from autoimmune diseases), while Tim-3 polymorphisms
may be closely associated with autoimmune diseases.
The role of Tim-3 in tumor tissues has been
investigated in numerous studies. Piao et al (27) identified that Tim-3 expression was
higher in prostate cancer tissues compared with the adjacent benign
tissues. In addition, Cao et al (28) revealed that Tim-3 expression in
cervical cancer promoted tumor metastasis. Furthermore, a number of
studies identified that the distribution frequencies of +4259 T/G
in Tim-3 in patients with pancreatic cancer or renal cell carcinoma
were statistically different compared with healthy individuals
(29,30). A recent study also demonstrated
that, following tumor-associated expression of the receptor Tim-3
by dendritic cells, Tim-3 inhibited the antitumor efficacy of DNA
vaccines and chemotherapy (31).
Therefore, Tim-3 may be a risk factor of tumorigenesis, while Tim-3
polymorphisms may be associated with cancer.
In the present study, polymorphisms at the −574
locus of Tim-3 were investigated. Statistical analysis revealed
that, in the MG-associated and non-MG-associated thymoma groups,
the mutant-type homozygote TT frequencies were zero. Thus, the
mutant-type homozygote TT and heterozygote GT phenotypes were
merged and reanalyzed using the χ2 test. The results
detected a statistically significant difference in the distribution
frequencies of the GT+TT genotype between the two groups (Table I). In addition, the distribution
frequencies of T allele on the −574 locus were significantly
different between the two groups (Table II). These findings indicated an
association between the −574 locus polymorphism and MG-associated
thymoma in the Han population of North China. Furthermore, the OR
values were found to be 0.329 and 0.375 for the distribution
frequencies of the GT+TT genotype and T allele, respectively, on
the −574 locus between the two groups. Based on the results of the
aforementioned studies, Tim-3 was hypothesized to be a protective
factor in autoimmune diseases and a risk factor of tumorigenesis;
however, to date, the association between tumorigenesis and
autoimmune diseases remains unclear. Thus, the present study
investigated the correlation between Tim-3 and MG-associated
thymoma, which is a tumor commonly associated with autoimmune
diseases. In conclusion, Tim-3 was found to be a protective factor
in MG-associated thymoma, indicating that thymoma is affected by
MG. Therefore, future research on thymoma should investigate the
possible association with MG.
Acknowledgements
This study was supported by a grant from the Tianjin
Medical University General Hospital. The authors would like to
thank Dr Wang Beilei (Endocrine Laboratory, Tianjin Medical
University, Tianjin, China) for her technical assistance.
Abbreviations:
|
AS-PCR
|
allele-specific polymerase chain
reaction
|
|
CI
|
confidence interval
|
|
EAE
|
experimental autoimmune
encephalomyelitis
|
|
MG
|
myasthenia gravis
|
|
MS
|
multiple sclerosis
|
|
OR
|
odds ratio
|
|
Tim
|
T-cell immunoglobulin and mucin
domain
|
References
|
1
|
Priola AM and Priola SM: Imaging of thymus
in myasthenia gravis: from thymic hyperplasia to thymic tumor. Clin
Radiol. 65:e230–e245. 2014. View Article : Google Scholar
|
|
2
|
Shapiro M and Korst RJ: Surgical
approaches for stage IVA thymic epithelial tumors. Front Oncol.
3:3322014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Engels EA: Epidemiology of thymoma and
associated malignancies. J Thorac Oncol. 5(10 Suppl 4): S260–S265.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lucchi M, Ricciard R, Melfi F, et al:
Association of thymoma and myasthenia gravis: oncological and
neurological results of the surgical treatment. Eur J Cardiothorac
Surg. 35:812–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu L, Zhang XJ, Ma S, et al: Different
characteristics of thymomas with and without myasthenia gravis. Ann
Surg Oncol. 19:94–98. 2012. View Article : Google Scholar
|
|
6
|
Zheng K, Xu G, Lu X, et al: Expression and
polymorphisms of T cell immunoglobulin domain and mucin domain
protein-1 in thymoma with or without myasthenia gravis. Oncol Lett.
8:317–322. 2014.PubMed/NCBI
|
|
7
|
Pearse G: Normal structure, function and
histology of the thymus. Toxicol Pathol. 34:504–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Wang W, Chen Y and Wei D: T helper
type 17 cells expand in patients with myasthenia-associated
thymoma. Scand J Immunol. 76:54–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zagoriti Z, Kambouris ME, Patrinos GP, et
al: Recent advance in genetic predisposition of myasthenia gravis.
Biomed Res Int. 2013:4040532013. View Article : Google Scholar
|
|
10
|
Zheng K, Zhang J, Guo Y and Zhang P:
Expression and clinical significance of protein tyrosine
phosphatase nonreceptor 22 in resected thymoma. Clin Lab.
59:1041–1044. 2013.PubMed/NCBI
|
|
11
|
Monney L, Sabatos CA, Gaglia JL, et al:
Th1-specific cell surface protein Tim-3 regulates macrophage
activation and severity of an autoimmune disease. Nature.
415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu C, Anderson AC, Schubart A, et al: The
Tim-3 ligand galectin-9 negatively regulates T helper type 1
immunity. Nat Immunol. 6:1245–1252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson AC and Anderson DE: TIM-3 in
autoimmunity. Curr Opin Immunol. 18:665–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabatos CA, Chakravarti S, Cha E, et al:
Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1
responses and induction of peripheral tolerance. Nat Immunol.
4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mattei F and Schiavoni G: TIM-3 as a
molecular switch for tumor escape from innate immunity. Front
Immunol. 3:4182013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fourcade J, Sun Z, Benallaoua M, et al:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma
patients. J Exp Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baitsch L, Baumgaertner P, Devêvre E, et
al: Exhaustion of tumor-specific CD8+ T cells in
metastases from melanoma patients. J Clin Inverst. 121:2350–2360.
2011. View
Article : Google Scholar
|
|
18
|
Sakuishi K, Apetoh L, Sullivan JM, et al:
Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and
restore anti-tumor immunity. J Exp Med. 207:2187–2194. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Q, Munger ME, Veenstra RG, et al:
Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell
exhaustion phenotype in mice with disseminated acute myelogenous
leukemia. Blood. 117:4501–4510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mclntire JJ, Umetsu SE, Macaubas C, et al:
Immunology: hepatitis A virus link to atopic disease. Nature.
425:5762003. View
Article : Google Scholar
|
|
21
|
Shen Y, Wang C, Hong D, et al: The
relationship between polymorphisms in the promoter region of Tim-3
and unexplained recurrent spontaneous abortion in Han Chinese
women. Reprod Biol Endocrinol. 11:1042013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shang Y, Li Z, Li H, et al: TIM-3
expression in human osteosarcoma: Correlation with the expression
of epithelial-mesenchymal transition-specific biomarkers. Oncol
Lett. 6:490–494. 2013.PubMed/NCBI
|
|
23
|
Freeman GJ, Casasnovas JM, Umetsu DT and
DeKruyff RH: TIM genes: a family of cell surface phosphatidylserine
receptors that regulate innate and adaptive immunity. Immunol Rev.
235:172–189. 2010.PubMed/NCBI
|
|
24
|
Koguchi K, Anderson DE, Yang L, et al:
Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp
Med. 203:1413–1418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meyers JH, Sabatos CA, Chakravarti S and
Kuchroo VK: The TIM gene family regulates autoimmune and allergic
diseases. Trends Mol Med. 11:362–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Yang Y, Liu X and Wang Y: The
-1541C>T and +4259G>T of TIM-3 polymorphisms are associated
with rheumatoid arthritis susceptibility in a Chinese Hui
population. Int J Immunogenet. 38:513–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piao YR, Piao LZ, Zhu LH, et al:
Prognostic value of T cell immunoglobulin mucin-3 in prostate
cancer. Asian Pac J Cancer Prev. 14:3897–3901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Y, Zhou X, Huang X, et al: Tim-3
expression in cervical cancer promotes tumor metastasis. PLoS One.
8:e538342013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai C, Wang L, Wu Z, et al: T-cell
immunoglobulin- and mucin-domain-containing molecule 3 gene
polymorphisms and renal cell carcinoma. DNA Cell Biol.
31:1285–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong D, Zhou Y, Chen W, et al: T cell
immunoglobulin- and mucin-domain-containing molecule 3 gene
polymorphisms and susceptibility to pancreatic cancer. Mol Biol
Rep. 39:9941–9946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang D and Lotze MT: Tumor immunity times
out: TIM-3 and HMGB1. Nat Immunol. 13:808–810. 2012. View Article : Google Scholar : PubMed/NCBI
|