Introduction
Thyroid carcinoma comprises 1–2% of all malignancies
in the USA (1). Epidemiological
studies have shown an increasing prevalence of differentiated
thyroid carcinoma (DTC); however the mortality rate has remained
stable, most likely due to the increased diagnostic scrutiny
(2). Over half of the mortalities
in the USA result from papillary carcinoma, the majority of which
are low-risk tumours (3). Among DTC
patients, ~80% exhibit no evidence of disease following the initial
treatment (4). However, the
recurrence rate is 10–30% (depending on the initial therapy), and
the cancer may reappear after several years (up to one-third of
cases recur after the first decade) (5,6),
indicating a requirement for prolonged follow-up.
According to the American Thyroid Association
recommendations (7), the
disease-free status comprises all of the following: i) No clinical
evidence of tumour; ii) no imaging evidence of tumour [i.e. no
uptake outside the thyroid bed on the initial post-treatment
whole-body scan (WBS) or, if uptake outside the thyroid bed is
present, no imaging evidence of tumour on a recent diagnostic scan
and neck ultrasound (US) is observed]; and iii) undetectable serum
thyroglobulin (Tg) levels during thyroid-stimulating hormone (TSH)
suppression and stimulation in the absence of interfering
antibodies.
The same guidelines also suggest that initial
follow-up for low-risk patients should be based predominantly on
TSH-suppressive serum Tg (supTg) and neck US, followed by
TSH-stimulated serum Tg (sTg) measurements if the supTg is
undetectable (7). However,
ultrasensitive methods for serum thyroglobulin determination may be
used to avoid TSH stimulation 9–12 months following surgery in
low-risk patients who have an undetectable serum thyroglobulin on
levothyroxine (LT4) treatment (8).
This was supported by Brassard et al (4), who published a prospective study of
715 DTC patients and concluded that in the majority of patients
(84%) who exhibited low supTg levels (<0.27 ng/ml), TSH
stimulation did not increase the negative predictive value of Tg
determination on the risk of recurrence (99% in the two
conditions). In this context, recent publications suggested that
negative sTg in the initial approach has such a high negative
predictive value, that future sTg are questionable (9,10), and
even the initial stimulation test may be replaced by a more
sensitive Tg assay (8). Another
predictive factor of persistent or recurrent DTC, with high
negative predictive value (NPV) and that has been studied in recent
years, is the Tg level at the time of recombinant human (rh)
TSH-aided ablation, which may be used as a prognostic marker
(11).
As the majority of the studies published thus far
have a short-term follow-up after the rhTSH stimulation test (≤7
years), and recurrence may occur in up to one-third of cases after
the first decade, the present study aimed to evaluate the accuracy
of rhTSH-stimulated Tg levels in patients with undetectable supTg
values (on LT4 therapy), to predict the remission after a follow-up
of 12.4 years.
Patients and methods
Patient selection
Between 1999 and 2002, the determination of Tg
levels following rhTSH stimulation testing was routinely performed
in low-risk DTC patients at the Department of Endocrinology at the
Portugese Institute of Oncology (Lisbon, Portugal). This study was
approved by the ethics committee of the Portugese Institute of
Oncology and written informed consent was obtained from all
patients.
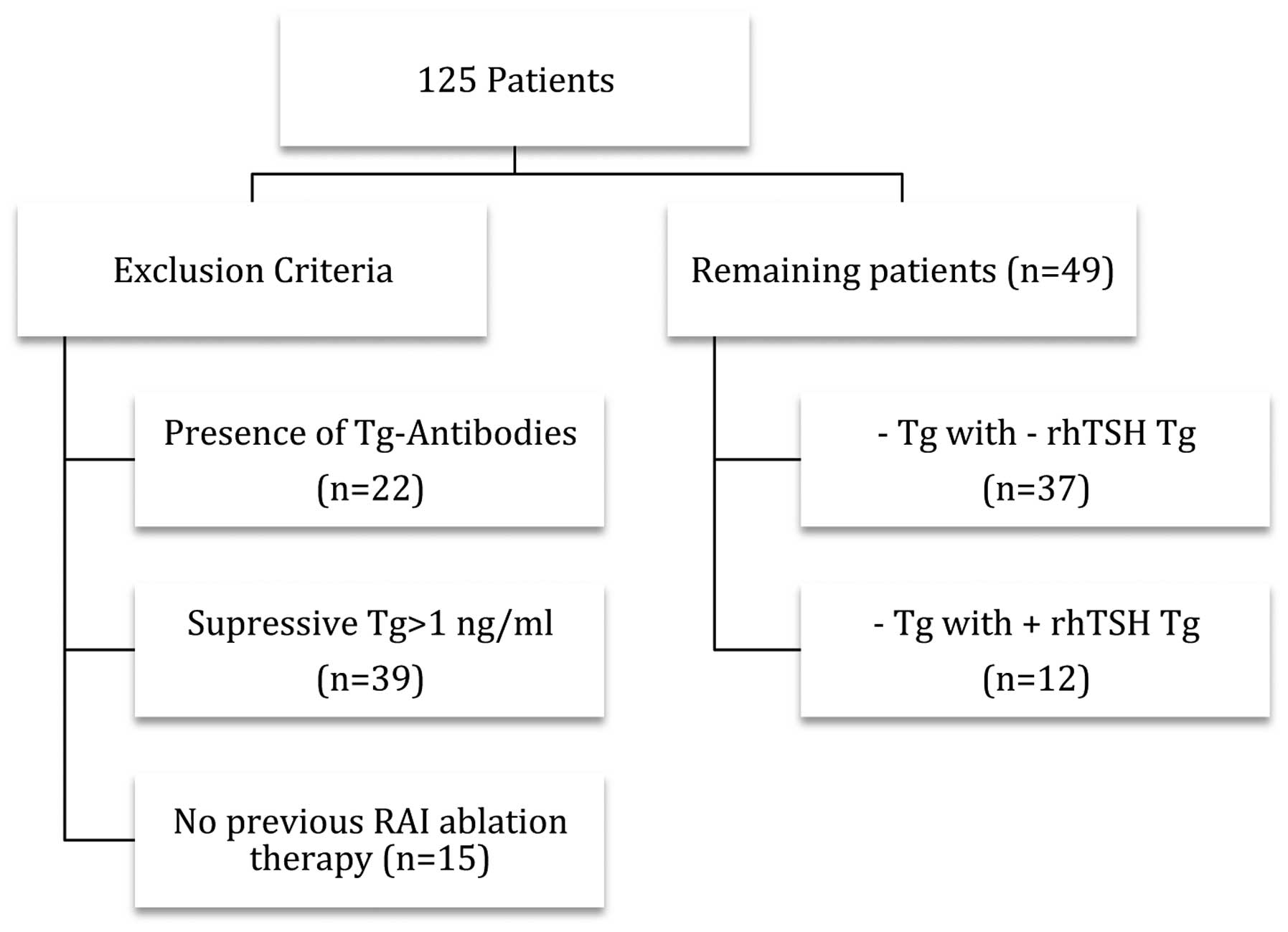
In total, 125 DTC patients were evaluated who
underwent a total or near total thyroidectomy, which was completed
by central neck dissection in 38% of cases, resulting in the
apparent complete resection of the neoplastic tissue. Overall, 88%
of these patients underwent radioactive iodine (RAI) ablation
therapy postoperatively. For all but one patient, no focus of
uptake outside the thyroid bed was detected on the WBS, performed 2
days following 131I therapy. Subsequently, all patients
were treated with suppressive LT4 doses. Tumour stages were
classified according to the TNM scoring system (12). Exclusion criteria for this study
were: i) presence of anti-Tg antibodies; ii) TSH-suppressive Tg
levels >1 ng/ml; and iii) absence of RAI ablation therapy
following surgery. Following the application of these criteria, 49
patients remained in the study (Fig.
1), who were divided in two groups according to: i) Positive
sTg levels >2 ng/ml and ii) negative sTg levels <2 ng/ml.
Procedures
Patients were given 0.9 mg rhTSH intramuscularly on
two consecutive days, followed by 4 mCi 131I after 24 h.
Additionally, the serum Tg measurement and diagnostic WBS (DxWBS)
were conducted on day 5 (72 h following the final rhTSH
injection).
DxWBS was classified as follows: i) negative when no
uptake was observed or in the case of neck iodine uptake consistent
with a normal thyroid remnant; or ii) suspicious in the case of
uptake outside the thyroid bed, suggesting underlying disease. The
Tg measurements were obtained using manual immunoradiometric Tg
assays, (SELco TG kits; Medipan GmbH, Selchow, Germany) with a
functional sensitivity of 1 ng/ml. Based on the functional
sensitivity of the assay, a concentration of 1 ng/ml was selected
as the cut-off value discriminating undetectable from detectable Tg
levels.
Follow-up
Follow-up assessments were conducted annually and
consisted of a clinical examination, serum supTg determination
under LT4 treatment and occasionally, neck US. Patients were
categorised as ‘no evidence of disease’ (NED) if the neck US was
negative and if all supTg levels were <1.0 ng/ml. Persistent
tumours were identified by fine needle aspiration cytology (when
the neck US was positive) or by 131I WBS showing
131I uptake outside the thyroid bed. Chest computed
tomography was performed when the neck US was positive or when the
supTg levels were >1.0 ng/ml with negative US.
Statistical analysis
Statistical analysis was performed using SPSS,
version 20 (IBM, Armonk, NY, USA). Comparisons of the categorical
data were performed using the χ2 test (Fisher’s exact).
P<0.05 was considered to indicate a statistically significant
difference. The results of the 49 patients were analysed for
estimation of the accuracy of the rhTSH stimulating Tg levels. A
2×2 analysis was constructed of NED (yes or no) and this was
compared to sTg levels (negative or positive with a cut-off value
of >2.0 ng/ml). The stimulated Tg accuracy was assessed through
sensitivity, specificity, positive predictive value (PPV) and NPV
determinations.
Results
Population data
The clinical characteristics of the study population
are reported in Table I. All
patients received at least one ablation dose of 131I,
nine patients (18.8%) received two doses, and nine received three
or more doses, with a mean dose of 160 mCi (range, 50–670 mCi). The
tumour stages are shown in Table I.
Distant metastases were observed in one patient when the initial
cancer diagnosis was determined; however, an excellent response to
therapy was exhibited, resulting in negative 131I WBS
and serum supTg levels <1.0 ng/ml, and the patient was
considered free of disease at the time of sTg evaluation. The time
between initial surgery and the rhTSH stimulation test ranged from
1–36 years [mean ±standard deviation (SD), 7.9± 7.6 years].
 | Table ICharacteristics of the 49 patients at
presentation. |
Table I
Characteristics of the 49 patients at
presentation.
| Patients (n=49) |
|---|
|
|
|---|
| Characteristics | n | % |
|---|
| Gender |
| Female | 41 | 83.7 |
| Male | 8 | 16.3 |
| Mean age, years | 60.6 |
| TNM classification
(12) |
| T1 | 4 | 8.2 |
| T2 | 10 | 20.4 |
| T3 | 9 | 18.4 |
| T4 | 7 | 14.3 |
| Tx | 19 | 38.8 |
| Lymph nodes |
| N0 | 21 | 42.9 |
| N1 | 22 | 44.9 |
| Nx | 6 | 12.2 |
| Distant
metastasis |
| M0 | 48 | 98.0 |
| M1 | 1 | 2.0 |
| Stage |
| I | 28 | 57.1 |
| II | 6 | 12.2 |
| III | 5 | 10.2 |
| IV | 1 | 2.0 |
| Unknown | 9 | 18.4 |
| Histology |
| Papillary | 44 | 89.8 |
| Follicular | 4 | 8.2 |
| Papillary +
follicular | 1 | 2.0 |
| Surgery type |
| Total
thyroidectomy | 24 | 49.0 |
| Near total
thyroidectomy | 6 | 12.2 |
| Total thyroidectomy
with central neck dissection | 19 | 38.8 |
Follow-up and recurrence
The mean (±SD) follow-up after the rhTSH stimulation
test was 12.4 years (± 0.7 years), with a minimum of 10 years and a
maximum of 13 years. In total, eight of the 49 patients were lost
to follow-up, seven patients exhibited no evidence of disease and
one exhibited recurrent disease at the time of the last appointment
(5 years following the rhTSH stimulation test). Following the rhTSH
stimulation test, 75.5% (37/49) of the patients with baseline Tg
levels <1.0 ng/ml exhibited no increase in sTg levels (<2
ng/ml). By contrast, 12 of the 49 patients (24.5%) exhibited
rhTSH-stimulated Tg levels >2 ng/ml. DxWBS following rhTSH
administration was negative in 23 patients (46.9%), revealed uptake
in the thyroid bed in seven patients (14.3%) and was not performed
in 19 patients (38.8%).
Stimulated Tg accuracy
In total, nine recurrences (18.4% of patients) were
identified during a mean of 12.4 years of follow-up after the rhTSH
stimulation test. Recurrence was detected after a median (±SD) of
5.9 years (± 2.0 years). These recurrences occurred in the thyroid
bed in two cases (22.2%), in the lateral neck lymph nodes in three
cases (30%), and in the lungs and bone in one case each (11.1%).
Additionally, two cases (22.2%) with persistent elevated supTg
levels were identified; however, no imaging or clinical evidence of
disease was observed. All recurrences occurred in patients with
papillary cancer and all of the patients exhibited lymph node
metastasis (N1) at presentation. Of all the 49 patients, 22 had
lymph node metastasis and 40.9% of these had a recurrence. The
individual relapse time is shown in Tables II and III.
 | Table IIRecurrent disease in negatively
stimulated thyroglobulin (<2 ng/ml) patients: False
negative. |
Table II
Recurrent disease in negatively
stimulated thyroglobulin (<2 ng/ml) patients: False
negative.
| Gender, agea (years) | Surgery | Histology | TNM | 131I total
dose, mCi | 131I
treatments, n | DxWBS | sTg, ng/dl | Recurrence | Time of recurrence
Cir/sTg, years |
|---|
| F, 31 | TT + CND | Papillary | T3mN1M0 | 130 | 2 | Neck uptake | <1 | AATg+
Lung | 21/7 |
| F, 39 | TT + CND | Papillary | T2mN1M0 | 220 | 2 | Negative | <1 | Tg ↑ thyroid bed | 10/6 |
| F, 49 | TT + CND | Papillary | TxN1M0 | 163 | 2 | Neck uptake | <1 | Tg ↑ Bone | 26/3 |
 | Table IIIRecurrent disease in positively
stimulated thyroglobulin (>2 ng/ml) patients: True positive. |
Table III
Recurrent disease in positively
stimulated thyroglobulin (>2 ng/ml) patients: True positive.
| Gender, agea (years) | Surgery | Histology | TNM | Stage | 131I
total dose, mCi | 131I
treatments, n | DxWBS | sTg, ng/dl | Recurrence | Time of recurrence
Cir/sTg, years |
|---|
| M, 43 | TT + CND | Papillary | TxN1M0 | I | 184 | 2 | Negative | 2.7 | Tg ↑ Lymph
nodes | 19/6 |
| F, 41 | TT + CND | Papillary | TxN1M0 | I | 50 | 1 | Neck uptake | 3.2 | Tg ↑ Lymph
nodes | 19/10 |
| M, 24 | TT + CND | Papillary | T2N1Mx | I | 278 | 3 | Negative | 2.6 | Tg ↑ Thyroid
bed | 13/5 |
| F, 22 | TT + CND | Papillary | T4N1M0 | I | 502 | 5 | Neck uptake | 35.9 | Tg ↑ No imag. | 8/5 |
| F, 32 | TT + CND | Papillary | T3N1M0 | I | 561 | 4 | Neck uptake | 9.1 | Tg ↑ Lymph
nodes | 7/4 |
| F, 40 | TT | Papillary | T2N1M0 | I | 70 | 1 | Neck uptake | 2.8 | Tg ↑ No image | 8/7 |
Among the 12 patients with sTg levels >2 ng/ml,
only six patients experienced a recurrence [true positive (TP)],
leading to a PPV of 50%. Among the other 37 patients who exhibited
negative sTg levels (<2 ng/ml), 34 patients did not experience
any recurrence (true negative (TN)], leading to an NPV of 91.9%.
Three false negatives and six false positives (Table IV) were also identified. Analysing
this data, a sensitivity of 66.6% and a specificity of 85.0% was
estimated for the sTg levels.
 | Table IVCrosstable between NED and sTg
level. |
Table IV
Crosstable between NED and sTg
level.
| NED | |
|---|
|
| |
|---|
| Yes | No | Total |
|---|
| sTg, ng/ml |
| <2 | 34 (TN) | 3 (FN) | 37 |
| >2 | 6 (FP) | 6 (TP) | 12 |
| Total | 40 | 9 | P=0.001 |
Discussion
The upgrading of Tg assays has been associated with
an improvement in diagnostic accuracy, leading to a lower
prevalence of tumour recurrence than in the past, among patients
declared free of disease (13). In
the current retrospective study, the aim was to evaluate the
accuracy of Tg-stimulated levels and its NPV and PPV in 49 patients
subjected to total thyroidectomy and RAI ablation therapy with a
long follow-up duration. A sensitivity of 66.6% and a specificity
of 85% were obtained, with a PPV of 50% and an NPV of 91.9%.
As described in a number of previous studies
(13–15), rhTSH sTg may have a low sensitivity
and low PPV. Results have been published indicating that ~20% of
patients who are clinically free of disease, with serum Tg levels
<1 ng/ml during suppression of TSH, are likely to have a serum
Tg levels >2 ng/ml after rhTSH or thyroid hormone withdrawal at
12 months following surgery and RAI. In these patients, only
one-third are likely to have an indication of persistent or
recurrent disease and increasing Tg levels (7). Due to this, sTg may result in
unnecessary additional testing and treatment in a number of
patients. In the current study, only half of the patients with sTg
levels >2 ng/ml exhibited evidence of disease in the follow-up.
Conversely, the NPV was high (91.9%), allowing the identification
of patients unlikely to experience disease recurrence and
permitting less aggressive management strategies. These results are
consistent with previously published results (Table V) (13).
 | Table VComparison between studies of
accuracy of sTg levels following recombinant human TSH
stimulation. |
Table V
Comparison between studies of
accuracy of sTg levels following recombinant human TSH
stimulation.
| First author (year)
[ref.] | Mean follow-up time
after sTg test, years | Time of sTg test,
years | sTg cut-off
(ng/ml) | Recurrence rate,
% | Sensitivity, % | Specificity, % | Positive predictive
value, % | Negative predictive
value, % |
|---|
| Present study | 12.4 | 7.9 | >2.0 | 18.4 | 66.6 | 85.0 | 50.0 | 91.9 |
| Brassard (2011)
[4] | 6.2 | 0.75–1.0 | >1.4 | 4.5 | 78.0 | 90.0 | 26.0 | 99 |
| Kloos (2010)
[13] | 7 | 5.5 | >2.5 | 17 | 80 | 97 | 84 | 95 |
| Robbins (2002)
[14] | 2 | <2 | >2.0 | 15.5 | 56.3 | 88.5 | 47.4 | 91.7 |
| Schlumberger (2007)
[18] | 2.33 | 0.75–1.0 | >0.9 | <3 | 68–76 | 81–91 | NR | NR |
In recent years, several studies have compared
ultrasensitive Tg assays with sTg, suggesting similar NPVs (99 vs.
100%) (16,17). In low-risk patients, it was
suggested that TSH stimulation tests may be avoided when sensitive
suppressive Tg levels are low (<0.27 ng/ml). In this study,
rhTSH stimulation improved the positive predictive value of serum
sTg determination only in patients with supTg levels >0.27
ng/ml, and the authors hypothesised that rhTSH must be restricted
to such patients (4,8). However, several studies using Tg
assays with lower functional sensitivities have demonstrated that
improved Tg sensitivity for disease detection is counterbalanced by
an increase in false positives (17,18).
When considering the DTC recurrence rate, long-term
follow-up may demonstrate higher rates of tumour recurrence
(13). This hypothesis was also
described by Mazzaferi and Kloos (6), who reported locoregional recurrences
and distant metastases (15 and 20%, respectively) more than two
decades following the initial therapy. The long follow-up period
(12.4 years) is a significant factor in the current report, and
allowed the confirmation that the recurrence rate remains high
(18.4%), even in a group of patients considered to be free of
disease. Comparing this with the results published to date
(Table V), a higher relapse rate
was observed in the current study, which is likely to be due to the
duration of follow-up, which was significantly longer than in the
compared studies. In the current study, almost all the patients
with recurrent disease were in TNM stage I (88.9%) predominantly
due to age (<45 years). The classification systems that use age
to stratify risk may be inaccurate in predicting recurrence-free
survival, as young patients have high recurrence rates (6). However, all patients with recurrences
exhibited lymph node metastases (N1) at initial surgery, which is a
well described risk factor for recurrent disease (6,19). In
the current study, the recurrence rate in patients with lymph node
metastases was 40.9%.
Pacini et al (20) suggested that neck US combined with
sTg has the highest diagnostic accuracy for the detection of
persistent disease, without the requirement for diagnostic WBS. In
the current study, although 38% of the relapsed patients had not
undergone DxWBS following rhTSH administration, a large number of
false negatives in DxWBS were identified, leading to a low
sensitivity and a low PPV of this diagnostic approach, confirming
the results from other groups (21).
In the majority of published studies, Tg stimulation
tests are performed within 1–2 years of surgery and
131I, considering that the majority of recurrences occur
early following the primary treatment. By contrast, the current
study includes patients whose primary treatment occurred within
varied and occasionally long periods prior to Tg stimulation tests
(median, 8.6 years; range, 1–36 years). Additionally, this study
confirmed that late recurrences may occur in differentiated thyroid
cancers, indicating that, even when varied and long periods of time
following primary treatment have elapsed, the prognostic ability of
the rhTSH stimulation test, particularly its NPV, is
maintained.
In conclusion, the accurate surveillance for
possible recurrence in patients considered to be free of disease is
the predominant goal of long-term follow-up. With new sensitive
serum Tg assays, rhTSH stimulation tests may not be routinely
necessary. The benefit is greater in patients who have undetectable
sTg levels (without serum Tg antibodies), allowing the
identification of the patients who are free of disease. In these
patients it is possible to ensure a more cost-effective and safe
follow-up, monitoring supTg levels and performing neck ultrasound
on an annual basis.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995 [see commetns].
Cancer. 83:2638–2648. 1998. View Article : Google Scholar
|
|
4
|
Brassard M, Borget I, Edet-Sanson A, et
al: Long-term follow-up of patients with papillary and follicular
thyroid cancer: a prospective study on 715 patients. J Clin
Endocrinol Metab. 96:1352–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazzaferri EL: An overview of the
management of papillary and follicular thyroid carcinoma. Thyroid.
9:421–427. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzaferri EL and Kloos RT: Clinical
review 128: Current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid N, Differentiated Thyroid Cancer.
Cooper DS, Doherty GM, Haugen BR, et al: Revised American Thyroid
Association management guidelines for patients with thyroid nodules
and differentiated thyroid cancer. Thyroid. 19:1167–1214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schlumberger M, Borget I, Nascimento C,
Brassard M and Leboulleux S: Treatment and follow-up of low-risk
patients with thyroid cancer. Nat Rev Endocrinol. 7:625–628. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castagna MG, Brilli L, Pilli T, et al:
Limited value of repeat recombinant human thyrotropin
(rhTSH)-stimulated thyroglobulin testing in differentiated thyroid
carcinoma patients with previous negative rhTSH-stimulated
thyroglobulin and undetectable basal serum thyroglobulin levels. J
Clin Endocrinol Metab. 93:76–81. 2008. View Article : Google Scholar
|
|
10
|
Kloos RT and Mazzaferri EL: A single
recombinant human thyrotropin-stimulated serum thyroglobulin
measurement predicts differentiated thyroid carcinoma metastases
three to five years later. J Clin Endocrinol Metab. 90:5047–5057.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melo M, Costa G, Ribeiro C, et al:
Stimulated thyroglobulin at recombinant human TSH-aided ablation
predicts disease-free status one year later. J Clin Endocrinol
Metab. 98:4364–4372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wittekind C, Compton CC, Greene FL and
Sobin LH: TNM residual tumor classification revisited. Cancer.
94:2511–2516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kloos RT: Thyroid cancer recurrence in
patients clinically free of disease with undetectable or very low
serum thyroglobulin values. J Clin Endocrinol Metab. 95:5241–5248.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robbins RJ, Chon JT, Fleisher M, Larson SM
and Tuttle RM: Is the serum thyroglobulin response to recombinant
human thyrotropin sufficient, by itself, to monitor for residual
thyroid carcinoma? J Clin Endocrinol Metab. 87:3242–3247. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mazzaferri EL and Kloos RT: Is diagnostic
iodine-131 scanning with recombinant human TSH useful in the
follow-up of differentiated thyroid cancer after thyroid ablation?
J Clin Endocrinol Metab. 87:1490–1498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giovanella L, Ceriani L, Ghelfo A, et al:
Thyroglobulin assay during thyroxine treatment in low-risk
differentiated thyroid cancer management: comparison with
recombinant human thyrotropin-stimulated assay and imaging
procedures. Clin Chem Lab Med. 44:648–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smallridge RC, Meek SE, Morgan MA, et al:
Monitoring thyroglobulin in a sensitive immunoassay has comparable
sensitivity to recombinant human tsh-stimulated thyroglobulin in
follow-up of thyroid cancer patients. J Clin Endocrinol Metab.
92:82–87. 2007. View Article : Google Scholar
|
|
18
|
Schlumberger M, Hitzel A, Toubert ME, et
al: Comparison of seven serum thyroglobulin assays in the follow-up
of papillary and follicular thyroid cancer patients. J Clin
Endocrinol Metab. 92:2487–2495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nascimento C, Borget I, Al Ghuzlan A, et
al: Persistent disease and recurrence in differentiated thyroid
cancer patients with undetectable postoperative stimulated
thyroglobulin level. Endocr Relat Cancer. 18:R29–R40. 2011.
|
|
20
|
Pacini F, Molinaro E, Castagna MG, et al:
Recombinant human thyrotropin-stimulated serum thyroglobulin
combined with neck ultrasonography has the highest sensitivity in
monitoring differentiated thyroid carcinoma. J Clin Endocrinol
Metab. 88:3668–3673. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torlontano M, Crocetti U, Augello G, et
al: Comparative evaluation of recombinant human
thyrotropin-stimulated thyroglobulin levels, 131I whole-body
scintigraphy, and neck ultrasonography in the follow-up of patients
with papillary thyroid microcarcinoma who have not undergone
radioiodine therapy. J Clin Endocrinol Metab. 91:60–63. 2006.
View Article : Google Scholar
|