Introduction
Currently, the preferred treatment strategy for
metastatic and recurrent cancer is chemotherapy (1–4), as
numerous types of metastatic and recurrent cancer appear to respond
well to this mode of therapy (1–6). In
addition, multidrug therapies result in improved responses compared
with single agent therapies.
The aim of the present study was to evaluate the
employment of irinotecan and oral uracil-tegafur (UFUR) (TEGAFIRI)
with leucovorin to treat metastatic and recurrent colorectal
cancer. To decrease bias, all of the patients in the present study
were analyzed by a single health worker. As previous case studies
reported variation in the dosage of irinotecan and the dosing
schedule oral UFUR with or without leucovorin (10–14), a
pilot study of recurrent or metastatic colorectal cancer patients
was conducted by Hsu (15) to
evaluate the response rates of different combination chemotherapy
regimens of TEGFIRI with or without leucovorin, and to determine
the optimal regimen dose. Thus, the present study employed the
optimal regimen dose in a larger patient population to clarify the
results of the pilot study.
Materials and methods
Treatment regimen
In the present study, 113 metastatic or recurrent
colorectal cancer patients from Mackay Memorial Hospital (Taipei,
Taiwan) were treated with a combination of irinotecan (Pfizer,
Inc., New York, NY, USA) and UFUR (TTY BioPharm, Co., Ltd., Taipei,
Taiwan) with or without leucovorin. Each UFUR capsule contained a
1:4 molar ratio of the 5-fluorouracil (5-FU) prodrug tegafur (100
mg) and the dihydropyrimidine dehydrogenase inhibitor uracil (224
mg). Each leucovorin tablet (TTY BioPharm, Co., Ltd.) contained 15
mg leucovorin. The present study was approved by the Institutional
Review Board of the National Health Bureau of Taiwan (Taipei,
Taiwan) and all of the patients provided written informed consent
prior to receiving chemotherapy treatment.
Patient selection
The patients selected to participate in the present
study were aged ≥18 years and exhibited histologically determined
colorectal cancer, characterized by a minimum of one measurable
lesion and an Eastern Cooperative Oncology Group performance status
of 0 or 1 (16). Prior to study
enrollment, the following inclusion criteria were determined: A
Karnofsky performance status of ≥80% (17), ≤2.0 mg/dl bilirubin, ≤1.5 mg/dl
creatinine, an absolute granulocyte count of ≥1500/μl, and a
platelet count of ≥100,000/μl. Patients who had not received
chemotherapy in the six months prior to the present study were
included; however, patients who had undergone chemotherapy for
metastatic colorectal cancer, who exhibited central nervous system
metastasis or had a life expectancy of less than three months were
excluded. All 113 patients were monitored from January 2006 until
December 2010 or mortality.
Patients eligible for the pilot study (15) received different regimens and doses
of TEGAFIRI with or without leucovorin. The patients were
randomized into three groups: Group I, 150 mg/m2
irinotecan every two weeks and UFUR for one week every two weeks;
group II, 100 mg/m2 irinotecan for two weeks followed by
a one week of rest, with continuous UFUR and leucovorin; and group
III, 150 mg/m2 irinotecan every two weeks with
continuous UFUR and leucovorin. The UFUR dose was standardized at
300 mg/m2/day and leucovorin was administered at 45–60
mg/m2/day. Although the intention was to include a
greater number of patients in the pilot study, the initial results
demonstrated that group I patients exhibited lower response rates
compared with the other two groups; therefore, it was unethical to
proceed with the group I regimen and the enrollment was terminated
after 33 patients had enrolled. From the pilot study it was
determined that that the group III regimen was optimal for the
treatment of metastatic or recurrent colorectal cancer; thus, this
regimen was administered to patients eligible for the present
study.
In the present study, all of the 113 enrolled
patients were treated with irinotecan at a standard dose of 150
mg/m2 every two weeks with continuous UFUR (300
mg/m2/day) and leucovorin (45–60 mg/m2/day).
The Dukes’ stage of colorectal cancer before recurrence was
determined and evaluated for each patient (18). In addition, prior to irinotecan
administration, patients were administered with 10 mg dexamethasone
intravenously, 3 mg granisetron or 8 mg ondansetron intravenously,
and 0.5 mg atropine subcutaneously. Supportive care included
loperamide for diarrhea, antiemetic agents and oral cephradine for
diarrhea lasting >48 h. Upon progression, patients were
administered with an oxaliplatin-based salvage regimen in addition
to UFUR and leucovorin (TEGAFOX); however, agents such as
bevacizumub and cetaximub were not routinely used due to a lack of
funding during the study period.
Patient monitoring and follow-up
In the pilot study, patients in each group were
evaluated by determining the serum carcinoembryonic antigen (CEA)
and performing a chest X-ray, abdominal ultrasound and computed
tomography scan of the chest or abdomen every three months. In
addition, the Response Evaluation Criteria In Solid Tumors (RECIST)
was used to assess the efficacy of each chemotherapy regimen by
categorizing the response into the following four grades:
Progression, stable disease, partial response and complete response
(19). Progressive disease was
defined as an increase in CEA levels, a ≥25% increase in the number
or size of the metastatic lesions or the development of new
lesions; a partial response was defined as a decrease in CEA levels
or a ≥25% decrease in the number or size of the metastatic lesions;
and a complete response was defined CEA levels with the normal
range or as the disappearance of metastatic lesions.
Dose modification
The severity of the adverse effects was evaluated
using the National Cancer Institute Toxicity Criteria (version 2.0)
(20). Upon the initial appearance
of grade II toxicity, no dose reduction was required. In addition,
upon the appearance of reactions, which were determined as unlikely
to become serious or life-threatening, no treatment interruption or
dose reduction was implemented. However, in cases of grade III or
greater toxicity, TEGAFIRI/leucovorin treatment was interrupted and
was not resumed until the toxicity had resolved or had improved to
grade I. When treatment was resumed, the dose of leucovorin was as
before; however the doses of irinotecan and UFUR were reduced as
follows: Irinotecan and UFUR doses were reduced by 25% in patients
who exhibited a second occurrence of grade II toxicity or any
occurrence of grade III toxicity; and irinotecan and UFUR were
reduced by 50% in patients who experienced a third occurrence of
grade II toxicity or a second occurrence of grade III toxicity.
Treatment was discontinued if, despite dose reduction, grade II
toxicity occurred for a fourth time, grade III toxicity occurred
for a third time or if grade IV toxicity occurred at all. If
granulocytes decreased to <500/mm3, grade III–IV
diarrhea developed or granulocytes decreased to
<1,000/mm3 with concomitant fever, irinotecan and
UFUR doses were reduced by 20% for one cycle.
Statistical methods
The baseline characteristics of the patients were
quantified using descriptive statistics (median, percentile, and
range) and the principle results were overall survival and
progression-free survival. Overall survival was defined as the
period of time from the commencement of irinotecan therapy to
mortality. Progression-free survival was defined as the period of
time from group randomization to disease progression, or mortality
from disease progression or an unknown cause. For multivariate
analysis, factors associated with the time to progression were
identified by performing Cox’s regression analysis with forward
stepwise conditional analysis. Furthermore, the progression-free
and overall survival curves were calculated according to the
Kaplan-Meier method and compared using a log-rank test. All
statistical analyses were performed using SPSS software version
17.0.1 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Pilot study
In the pilot study (15), no significant differences were
identified between the three groups in terms of dose intensity and
dose delivery. Groups II and III exhibited improved response rates
compared with group I (Table I).
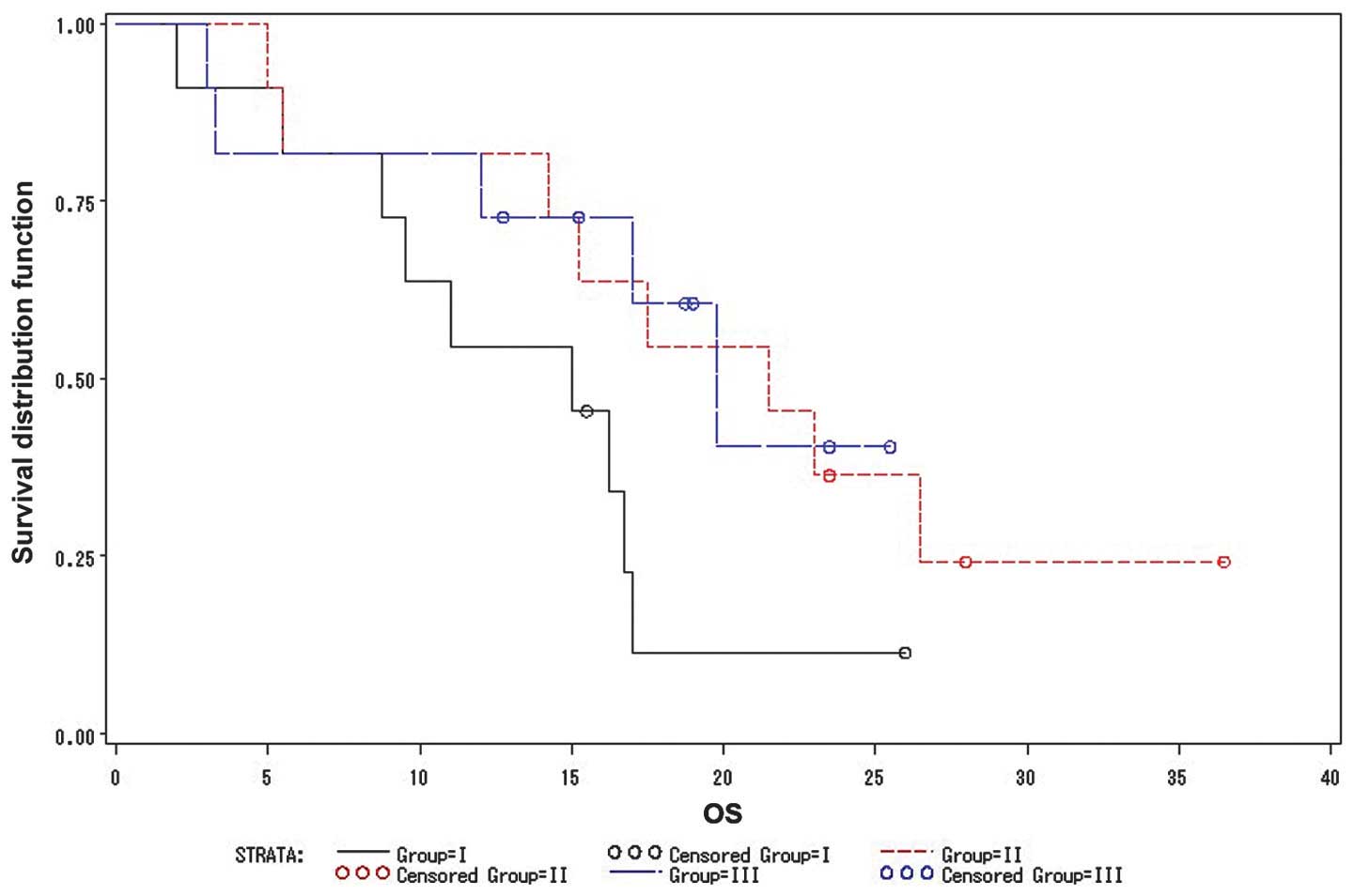
The overall survival times of groups I, II and III were 15, 21.5
and 19.8 months, respectively (Fig.
1). No statistically significant differences were identified
between the three groups in terms of overall survival; however,
group III patients exhibited fewer non-hematological side effects
and improved tolerance to the regimen compared with groups I and
II. Therefore, the group III regimen from the pilot study was
selected for use in the present study (n=113).
 | Table IResponse rate and outcome of the three
groups from the pilot study. Adapted from the pilot study by Hsu
(23). |
Table I
Response rate and outcome of the three
groups from the pilot study. Adapted from the pilot study by Hsu
(23).
| Group I (n=11) | Group II (n=11) | Group III (n=11) |
|---|
|
|
|
|
|---|
| Response | n | % | n | % | n | % |
|---|
| Complete
response | 0 | 0.0 | 2 | 18.2 | 2 | 18.2 |
| Partial response | 3 | 27.3 | 4 | 36.4 | 4 | 36.4 |
| Mortalities | 9 | 81.8 | 9 | 81.8 | 8 | 72.7 |
| Survival range,
months | 6–30 | 5–31 | 5–33 |
Present study
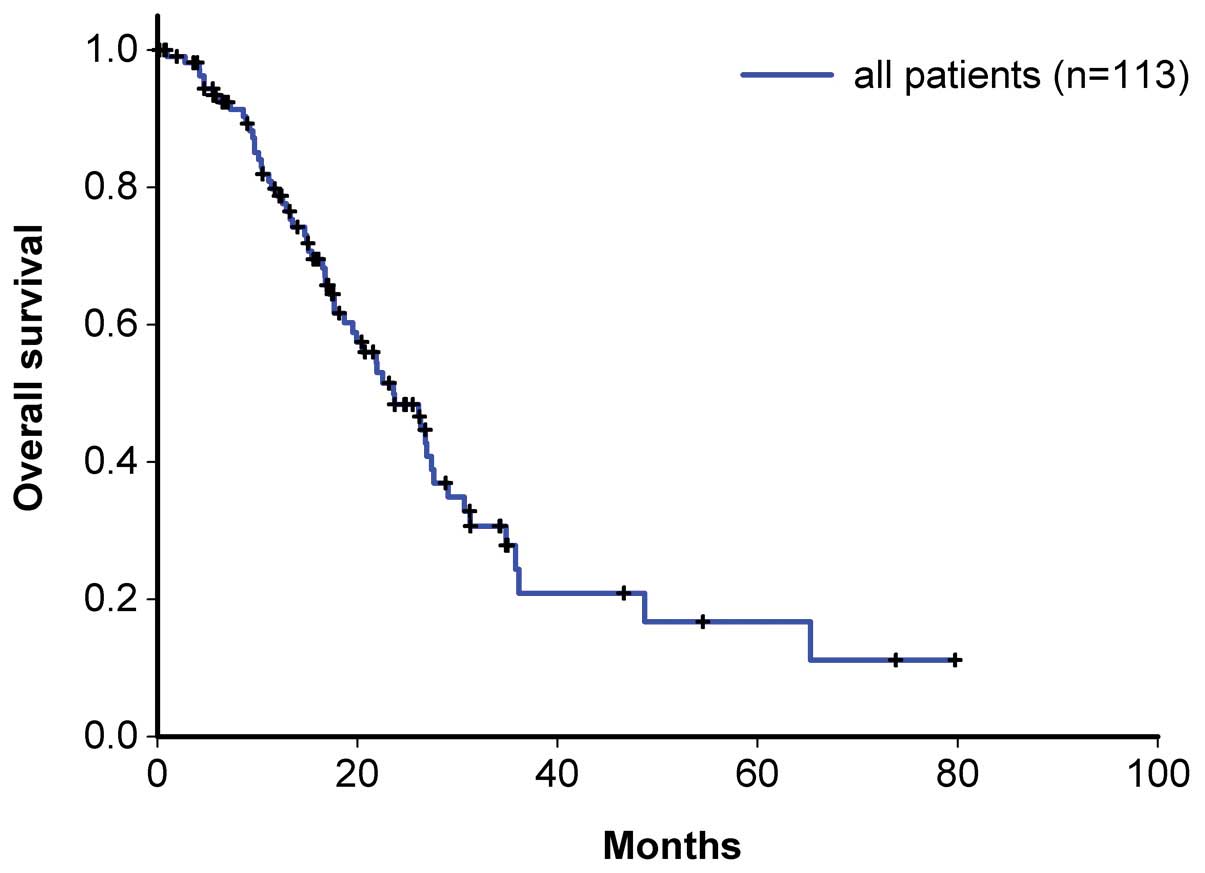
Table II indicates
the demographic data of the 113 patients enrolled in the present
study. The ratio of patients with initial Dukes’ stage B:C:D was
19:47:47. According to the RECIST criteria, the response rate of
the present study was 52.2%, which is similar to that of the pilot
study (54.5%). In addition, the incidence of diarrhea, alopecia and
hematologic toxicities (Table
III), as well as the necessity of delaying or decreasing the
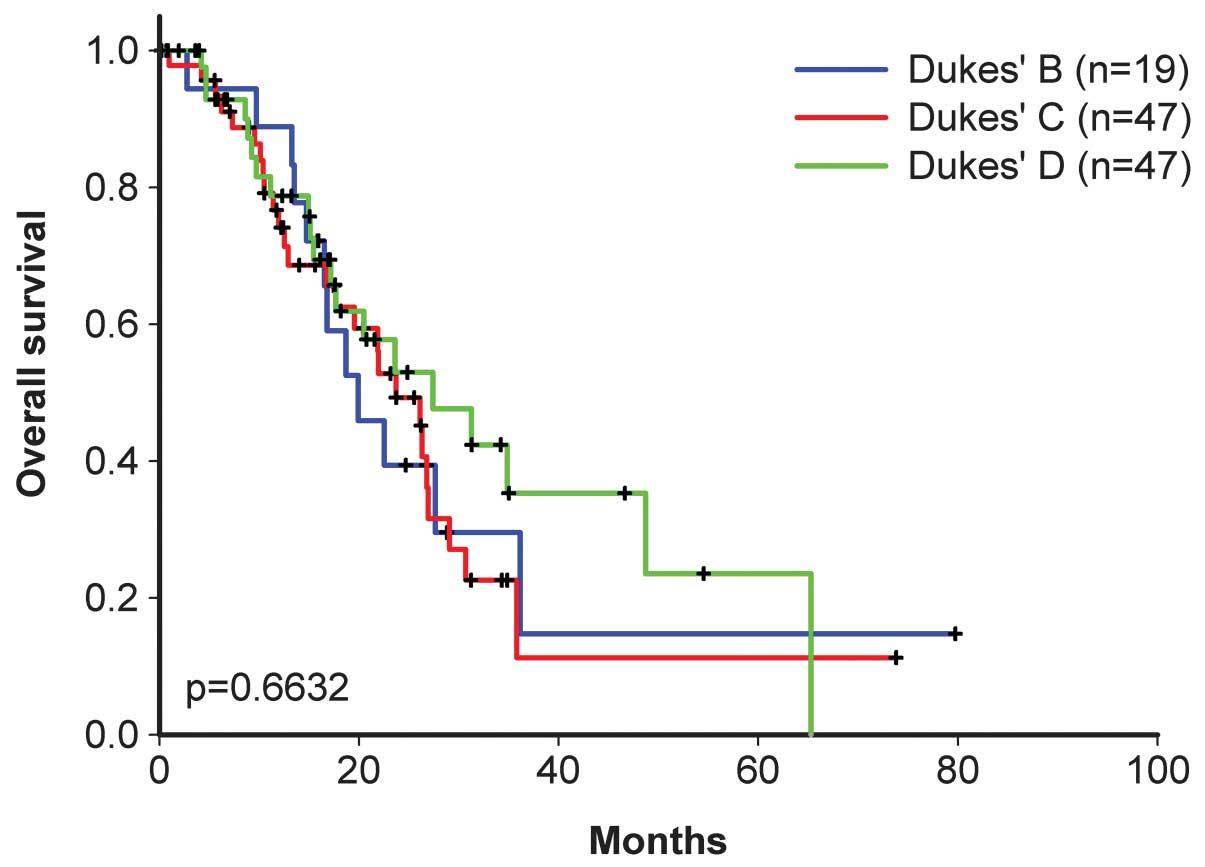
dosage were similar to those of the pilot study. For Dukes’ stages
B, C and D, the overall patient survival time was 19.9, 23.7 and
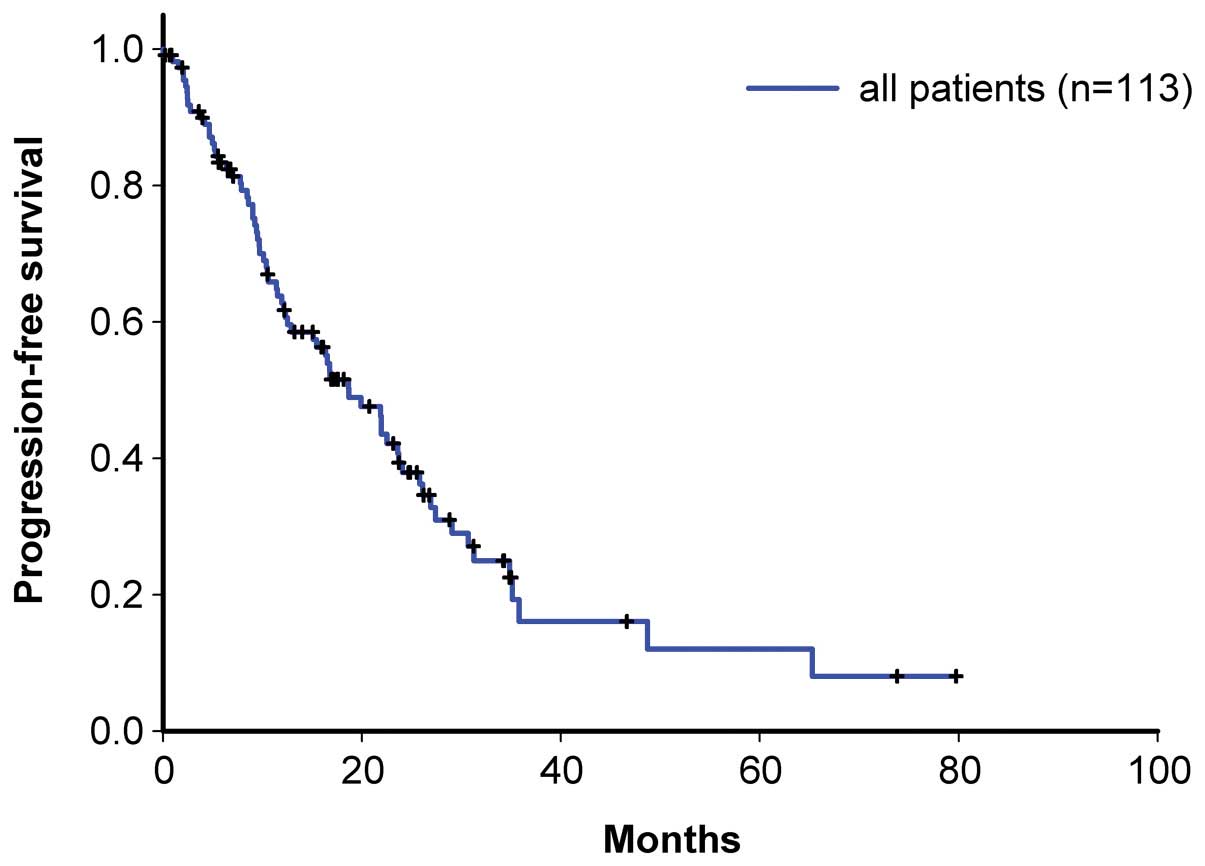
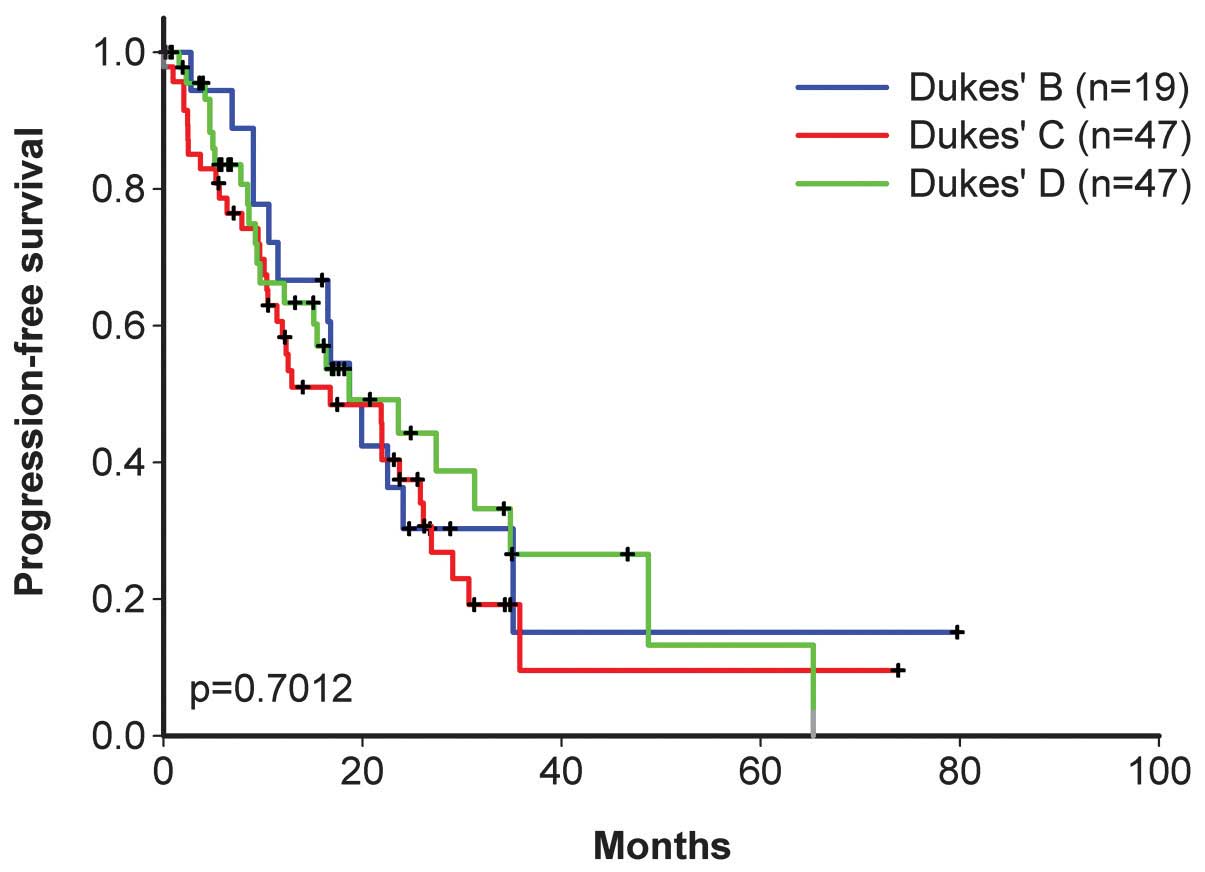
27.4 months, respectively (Figs. 2
and 3), and the progression-free
survival time was 18.7, 16.8 and 18.7 months, respectively
(Figs. 4 and 5).
 | Table IIPatient demographic data of the
present study. The median age of the patients was 61 years (range,
21–81 years). |
Table II
Patient demographic data of the
present study. The median age of the patients was 61 years (range,
21–81 years).
| Patients (n=113) |
|---|
|
|
|---|
| Characteristic | n | % |
|---|
| Gender |
| Male | 56 | 49.6 |
| Female | 57 | 50.4 |
| Site of the primary
tumor |
| Colon | 49 | 43.4 |
| Rectum | 63 | 55.8 |
| Two locations | 1 | 0.9 |
| Position of the
primary tumor |
| Cecum | 3 | 2.7 |
| Ascending colon | 12 | 10.6 |
| Transverse
colon | 5 | 4.4 |
| Descending
colon | 7 | 6.2 |
| Sigmoid colon | 21 | 18.6 |
| Anus | 63 | 55.8 |
| Two locations | 2 | 1.8 |
| Dukes’ stage of the
primary tumor |
| B | 19 | 16.8 |
| C | 47 | 41.6 |
| D | 47 | 41.6 |
 | Table IIIAdverse effects experienced by
patients in the present study exhibiting an overall toxicity grade
of III–IV. |
Table III
Adverse effects experienced by
patients in the present study exhibiting an overall toxicity grade
of III–IV.
| Patients (n=113) |
|---|
|
|
|---|
| Adverse effect | n | % |
|---|
|
Non-hematological |
| Diarrhea | 22 | 19.5 |
| Vomiting | 28 | 24.8 |
| Alopecia | 79 | 69.9 |
| Nausea | 45 | 39.8 |
| Hematological |
| Anemia | 34 | 30.1 |
| Neutropenia | 67 | 59.3 |
| Febrile
neutropenia | 5 | 4.4 |
|
Thrombocytopenia | 12 | 10.6 |
Discussion
As the worldwide incidence rate of colorectal cancer
increases, increasing numbers of patients are succumbing to the
disease (21,22). In 2012, there were approximately
694,000 mortalities as a result of colorectal cancer (23), and the American Cancer Society have
estimated that 136,830 novel cases of colorectal cancer will be
diagnosed and 50,310 mortalities as a result of the disease will
occur in 2014 (24). In Taiwan,
colorectal cancer is the third leading cause of cancer-related
mortality and the second most common cause of malignancy. The
principal treatment strategy for colorectal cancer is curative
resection; however, remission of metastatic colorectal cancer is
rarely achieved (25). Therefore,
chemotherapy is currently employed as the preferred treatment
strategy for metastatic disease (1–6).
A novel inhibitor of the DNA enzyme topoisomerase I,
irinotecan exerts cytotoxic activity by interrupting DNA
replication and transcription. In studies conducted in Western
countries, TEGAFIRI with leucovorin administration resulted in
response rates of 25–40% (4,6).
Furthermore, good response or survival rates have been observed in
a number of Taiwanese studies of first- and second-line irinotecan
therapy. However, the addition of 5-FU or its precursors plus
leucovorin were crucial for achieving satisfactory response
rates.
Tegafur is an oral fluoropyrimidine, which is
metabolized to 5-FU in vivo (26). In the management of metastatic
colorectal cancer, tegafur appears to be an active and minimally
toxic alternative to other types of fluoropyrimidine (27). Additionally, uracil is a naturally
occurring pyrimidine, which is able to incorporate into nucleic
acids (28). Together, these agents
may be administered as oral UFUR, which consists of tegafur
combined with uracil in 4:1 molar ratio. Preclinical studies have
demonstrated that this combination of tegafur and uracil is
associated with higher plasma levels of 5-FU compared with tegafur
treatment alone (29,30). Furthermore, this difference in 5-FU
plasma levels was associated with greater antitumor activity
(29,30). Two phase III studies comparing
UFUR/leucovorin with 5-FU/leucovorin demonstrated that the response
rate, time to progression and overall survival time were similar
between the two regimens, with an overall survival of 12–13 months.
However, diarrhea, nausea and vomiting, stomatitis and mucositis,
and myelosuppression occurred significantly less frequently in the
UFUR/leucovorin compared with the 5-FU/leucovorin group (27,31).
In a number of Japanese and Taiwanese studies, UFUR
was administered in favor of 5-FU as an agent in combination
chemotherapy; for example, in the FOLFIRI regimen, UFUR replaced
5-FU in combination with irinotecan (9,32,33).
In these previous studies, the dose of irinotecan (70, 80, 100,
150, 180, 220 or 350 mg/m2) and the interval between the
doses (for example, once weekly, once every two weeks and once
every three weeks) varied broadly.
Although leucovorin appears to enhance the antitumor
efficacy of 5-FU in the treatment of metastatic colorectal cancer,
the healthcare worker may select UFUR administration with or
without leucovorin. Thus, we aimed to determine the optimal dosing
schedule and dosage for the TEGAFIRI regimen in the metastatic
colorectal setting. Previous studies indicated that the TEGAFIRI
regimen was well-tolerated (11,14)
and, by modulating with leucovorin, the TEGAFIRI and TEGAFOX
regimens demonstrated comparable efficiency and safety (10,12,13).
The aim of the pilot study was to evaluate the
response rates of different regimens of combination chemotherapy
employing TEGAFIRI with or without leucovorin for patients with
recurrent or metastatic colorectal cancer. To decrease bias, all of
the patients in the present study were analyzed by a single health
worker. The results indicated that groups II and III exhibited
similar response rates and were preferable to the regimen employed
in group I. Furthermore, the response rates in groups II and III
were similar to those of previous studies, which employed the
FOLFIRI regimen with leucovorin (11,13,34).
Grade III/IV diarrhea, alopecia and hematologic side effects were
acceptable and similar in the three groups. Subsequently, the
present study evaluated the response of a larger cohort to 150
mg/m2 irinotecan every two weeks with continuous UFUR
and leucovorin without interruption, and demonstrated similar
side-effect and survival benefits, such that continuous UFUR and
leucovorin without interruption appeared to be essential for
improved patient survival.
This type of combination chemotherapy is
advantageous as it requires no hospital admission, has a shorter
injection time, does not require any additional apparatus for
injection, is well tolerated by the majority of patients, and
exhibits acceptable hematological and non-hematological side
effects. However, this regimen is associated with poor patient
compliance due to a number of reasons; for example, the irinotecan
injection is associated with nausea and vomiting, which may
interfere with the desire to self-administer oral agents, as well
as vomiting and diarrhea, which may decrease the actual intake of
oral agents.
Despite the possibility of the abovementioned
disadvantages occurring, the pilot and the present study indicated
similar response rates to the TEGAFIRI regimen compared with
previous reports in the literature. For example, TEGAFIRI results
in satisfactory response rates and patients report tolerable side
effects. Furthermore, continuous UFUR administration without
interruption appeared to result in improved outcomes compared with
the intermittent administration of UFUR, and leucovorin is an
essential component of the treatment regimen.
In conclusion, TEGAFIRI combination chemotherapy is
a satisfactory alternative therapy to the FOLFIRI regimen,
producing acceptable response rates for recurrent or metastatic
colorectal cancer patients. The present study recommends that
TEGAFIRI should be administered in combination with leucovorin and
oral UFUR administration should not be interrupted during
treatment.
References
|
1
|
Yeh KH, Cheng AL, Lin MT, et al: A phase
II study of weekly 24-hour infusion of high-dose 5-fluorouracil and
leucovorin (HDFL) in the treatment of recurrent or metastatic
colorectal cancers. Anticancer Res. 17:3867–3871. 1997.
|
|
2
|
Mermershtain W, Lavrenkov K and Cohen Y:
Phase II study of weekly high-dose fluorouracil in previously
treated patients with metastatic colorectal cancer. J Chemother.
12:183–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Pyrhönen S, James RD, et al:
Randomized trial of irinotecan plus supportive care versus
supportive care alone after fluorouracil failure for patient with
metastatic colorectal cancer. Lancet. 352:1413–1418. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicenter randomized trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothenberg ML, Cox JV, Devore RF, et al: A
multicenter phase II trial of weekly irinotecan (CPT-11) in
patients with previously treated colorectal carcinoma. Cancer.
85:786–795. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Pozzo C, Starkhammar H, et
al: A phase II study of irinotecan alternated with five days bolus
of 5-fluorouracil and leucovorin in first-line chemotherapy of
metastatic colorectal cancer. Ann Oncol. 9:1199–1204. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ota K, Taguchi T and Kimura K: Report on
nationwide-pooled data and cohort investigation in UFT phase II
study. Cancer Chemother Pharmacol. 22:333–338. 1988. View Article : Google Scholar
|
|
8
|
Malet-Martino M and Martino R: Clinical
studies of three oral prodrugs of 5-fluorouracil (capecitabine,
UFT, S-1): a review. Oncologist. 7:288–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kusunoki M, Yanagi H, Noda M, et al:
Results of pharmacokinetic modulating chemotherapy in combination
with hepatic arterial 5-fluorouracil infusion and oral UFT after
resection of hepatic colorectal metastases. Cancer. 89:1228–1235.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mackay HJ, Hill M, Twelves C, et al: A
phase I/II study of oral uracil/tegafur (UFT), leucovorin, and
irinotecan in patients with advanced colorectal cancer. Ann Oncol.
14:1264–1269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mibu R, Tanaka S, Futami K, et al: Phase
I/II study of irinotecan and UFT for advanced or metastatic
colorectal cancer. Anticancer Res. 27:2673–2678. 2007.PubMed/NCBI
|
|
12
|
Bajetta E, Bartolomeo MD, Buzzoni R, et
al: Uracil/ftorafur/leucovorin combined with irinotecan (TEGAFIRI)
or oxaliplatin (TEGAFOX) as first-line treatment for metastatic
colorectal cancer patient: results of randomized phase II study. Br
J Cancer. 96:439–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delord JP, Bennouna J, Artru P, et al:
Phase II study of UFT with leucovorin and irinotecan (TEGAFIRI):
first-line therapy for metastatic colorectal cancer. Br J Cancer.
97:297–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogata Y, Mori S, Ishibashi Y, et al:
Metronomic chemotherapy using weekly low-dosage CPT-11 and UFT as
postoperative adjuvant therapy in colorectal cancer at high risk to
recurrence. J Exp Clin Cancer Res. 26:475–482. 2007.
|
|
15
|
Hsu TC: Combination chemotherapy with
irinotecan and oral uracil-tegafur for recurrent or metastatic
colorectal cancer - results of different regimens. J Chinese Oncol
Soc. 25:254–261. 2009.(In Chinese).
|
|
16
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: reliability, validity, and
guidelines. J Clin Oncology. 2:187–193. 1984.
|
|
18
|
Dukes CE: The surgical pathology of rectal
cancer. J Clin Pathol. 2:95–98. 1949. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
20
|
Cancer Therapy Evaluation Program. Common
Toxicity Criteria, Version 2.0. April 30–1999, http://prevention.cancer.gov/files/clinical-trials/common-toxicity-criteria.pdf.
Accessed November 22, 2014
|
|
21
|
Jemal A, Tiwari RC, Murray T, et al:
American Cancer Society: Cancer statistics, 2004. CA Cancer J Clin.
54:8–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parkin DM, Pisani P and Ferlay J: Global
cancer statistics. CA Cancer J Clin. 49:33–64. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colorectal Cancer. Estimated Incidence,
Mortality and Prevalence Worldwide in 2012. GLOBOCAN. 2012,
http://globocan.iarc.fr/old/FactSheets/cancers/colorectal-new.asp.
Accessed November 22, 2014
|
|
24
|
Colorectal Cancer Facts & Figures
2014–2016. American Cancer Society; 2014, http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf.
Accessed November 22, 2014
|
|
25
|
DeVita VT Jr, Hellman S and Rosenberg SA:
Cancer Principles and Practice of Oncology. 5th edition.
Lippincott-Raven; Philadelphia, PA: pp. 437–452. 1997
|
|
26
|
Fujii S, Kitano S, Ikenaka K and Shirasaka
T: Studies on coadministration of uracil or cytosine on antitumor
activity of FT-207 or 5-FU derivatives. Jpn J Cancer Chemother.
6:377–384. 1979.
|
|
27
|
Douillard JY, Hoff P, Skillings JR, et al:
Multicenter phase III study of uracil/tegafur and oral leucovorin
versus fluorouracil and leucovorin in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 20:3605–3616.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoff PM, Lassere Y and Pazdur R:
Tegafur/uracil + calcium folinate in colorectal cancer: double
modulation of fluorouracil. Drugs. 58:77–83. 1999. View Article : Google Scholar
|
|
29
|
Köhne CH and Peters GJ: UFT: mechanism of
drug action. Oncology (Williston Park). 14(10 Suppl 9): 13–18.
2000.
|
|
30
|
Pazdur R, Hoff PM, Medgyesy D, et al: The
oral fluorouracil prodrugs. Oncology (Williston Park). 12(10 Suppl
7): 48–51. 1998.
|
|
31
|
Carmichael J, Popiela T, Radstone D, et
al: Randomized comparative study of tegafur/uracil and oral
leucovorin versus parenteral fluorouracil and leucovorin in
patients with previously untreated metastatic colorectal cancer. J
Clin Oncol. 20:3617–3627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kusunoki M, Yanagi H, Noda M and Yamamura
T: The usefulness of pharmacokinetic modulating chemotherapy (UFT
plus 5FU) in the treatment of unresectable colorectal carcinomas.
Oncol Rep. 6:547–552. 1999.PubMed/NCBI
|
|
33
|
Lin JK, Wang WS, Hsieh RK, et al: Phase II
study of oral tegafur-uracil and folinic acid as first-line therapy
for metastatic colorectal cancer: Taiwan experience. Jpn J Clin
Oncol. 30:510–514. 2000. View Article : Google Scholar
|
|
34
|
Hsiao SC, Lin JF, Chuang MT, Lee YA and Wu
DL: Retrospectively comparative evaluation of the first- and
second-line chemotherapy with campto and oxaliplatin combined with
oral tegafur/uracil (UFT)/leucovorin (LV) in patients with
metastatic colorectal cancer. Int Surg. 94:298–303. 2009.
|