Introduction
Glioma is among the most common human malignancies
in the brain. Although the five-year survival rate of patients with
glioma has been improved in recent years due to the combination of
surgery, radiotherapy and chemotherapy, the prognosis of patients
with invasive glioma remains poor (1,2). It
has been demonstrated that dysfunction of oncogenes or tumor
suppressors is closely associated with the development and
progression of glioma (2).
Accordingly, developing novel molecular targets may be promising
for the development of therapeutic strategies for invasive
glioma.
MicroRNAs (miRNAs), a class of non-coding RNAs 18–25
nucleotides in length, can induce mRNA degradation or suppress
protein translation by binding to the seed sequences in the
3′-untranslational region (UTR) of mRNAs. By negatively regulating
the protein expression of their targets, microRNAs exert adverse
effects on cell survival, proliferation and motility (3). Dysfunctions of miRNAs, which act as
oncogenes or tumor suppressors, have been demonstrated to be
associated with human malignancies (4). Furthermore, deregulations of numerous
miRNAs have been revealed to contribute to the development and
progression of invasive glioma, including miRNA-20a (miR-20a),
miR-106a, miR-145, miR-494 and miR-124 (5–8).
MiR-27b has been reported to be associated with glioma (9). However, the detailed role of miR-27b
in the regulation of invasive glioma remains largely unknown.
Sprouty homolog 2 (Spry2), a member of the sprouty
family, contains a carboxyl-terminal cysteine-rich domain essential
for the inhibition of receptor tyrosine kinase signaling (10) Spry2 can function as a regulator of
mitogen-activated protein kinase signaling, which plays a crucial
role in the regulation of cancer cell invasion (11,12).
The protein level of Spry2 has previously been reported to be
significantly decreased in invasive glioma tissues, suggesting that
Spry2 may participate in the regulation of glioma invasion
(13). However, the underlying
molecular mechanism remains unclear.
The present study aimed to explore the roles of
miR-27b and Spry2 in the regulation of glioma cell invasion. In
addition, the underlying molecular mechanism of these roles was
investigated.
Materials and methods
Tissue specimen collection
The present study was approved by the Ethical
Committee of Nanhai Hospital of Southern Medical University
(Foshan, China). In total, 30 glioma tissue and matched adjacent
normal tissue samples were obtained from the Department of
Neurosurgery of Nanhai Hospital of Southern Medical University. For
each patient, informed consent was obtained. Subsequent to surgical
removal, the tissue samples were frozen in liquid nitrogen until
usage.
Cell culture
The human glioma U87, U251 and SHG44 cell lines were
purchased from the Cell Bank of Southern Medical University
(Guangzhou, China). The human astrocyte HA cell line was purchased
from ScienCell Research Laboratories (Carlsbad, CA, USA). The cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10%
fetal bovine serum (FBS) at 37°C in a 5 % CO2
atmosphere.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted using TRIzol reagent (Life
Technologies, Carlsbad, CA, USA). An miRNA reverse transcription
kit (Life Technologies) was used to convert RNA into cDNA,
according to the manufacturer’s instructions. qPCR was then
performed using an miRNA Q-PCR Detection kit (GeneCopoeia,
Rockville, MD, USA) on the ABI 7500 thermocycler (Applied
Biosystems Life Technologies, Foster City, CA, US). The U6 gene was
used as an internal reference. Relative expression was analyzed by
the 2−ΔΔCt method.
Western blotting
Tissues and cells were lysed in cold
radioimmunoprecipitation assay lysis buffer. The proteins were
separated with 10% SDS-PAGE, and transferred onto a polyvinylidene
difluoride (PVDF) membrane. The PVDF membrane was incubated
overnight with phosphate-buffered saline containing 5% milk at 4°C.
Following incubation, the PVDF membrane was incubated with
monoclonal mouse anti-human Spry2 (1:200; ab60719) and monoclonal
mouse anti-human GAPDH primary antibodies (1:200; ab125247; Abcam,
Cambridge, UK) at room temperature for 3 h, and then incubated with
polyclonal rabbit anti-mouse secondary antibodies (1:5,000;
ab175743; Abcam) at room temperature for 1 h. An
electrochemiluminescence kit (Pierce Chemical, Rockford, IL, USA)
was then used to perform chemiluminescent detection. The relative
protein expression was analyzed by Image-Pro plus software 6.0
(Media Cybernetics, Inc., Rockville, MD, USA) and was presented as
the density ratio versus GAPDH.
Transfection
The cells were cultured to 70% confluence, and
resuspended in serum-free medium. Lipofectamine 2000 (Life
Technologies) was used to perform transfection according to the
manufacturer’s instructions. Briefly, miR-27b inhibitor, Spry2
plasmid or Spry2 siRNA (all from Niunbio Company, Changsha, China)
were diluted (1:50) with serum-free medium. Lipofectamine 2000 was
also diluted (1:50) with serum-free medium. The diluted
Lipofectamine 2000 was added to the diluted miR-27b inhibitor,
Spry2 plasmid or Spry2 siRNA, incubated for 20 min at room
temperature and then added to the cell suspension. The cells were
then incubated at 37°C in 5% CO2 for 6 h. Following
incubation, the medium in each well was replaced by normal
serum-containing medium and cultured for 24 h prior to the
subsequent assays. Bioinformatic analysis was performed to
predicate the target association between miR-27b and Spry2.
Dual luciferase reporter assay
A Directed Mutagenesis kit (Stratagene Inc., La
Jolla, CA, USA) was used to generate a mutant type 3′-UTR of Spry2.
The wild or mutant type 3′-UTR of Spry2 was inserted into the
psiCHECK™2 vector (Promega, Madison, WI, USA). The U251 cells were
transfected with the psiCHECK2-Spry2-3′-UTR or psiCHECK2-mutant
Spry2 -3′-UTR vector, with or without 100 nM miR-27b mimics.
Subsequently, the U251 cells were incubated at 37°C with 5 %
CO2 for 48 h. The luciferase activities were then
examined on a LD400 luminometer (Beckman Coulter, Brea, CA, USA).
Renilla luciferase activity was normalized to firefly luciferase
activity.
Transwell invasion assay
A cell suspension containing 5×105
cells/ml was prepared in serum-free media for the Transwell
invasion assay (Corning Inc., Corning, NY, USA). Then, 500 μl of
DMEM supplemented with 10% FBS was added into the lower chamber and
300 μl of the cell suspension was added to the upper chamber. The
cells were then incubated at 37°C with 5 % CO2 for 24 h.
Following incubation, the cells on the upper surface were removed
using a cotton-tipped swab, while the cells on the lower surface
were stained for 30 min. Under the microscope, the cell number was
counted in at least five randomly selected fields.
Statistical analysis
All data were expressed as the mean ± standard
deviation. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
used to perform the statistical analysis. Differences were analyzed
using one-way analysis of variance and P<0.05 was considered to
indicate a statistically significant difference.
Results
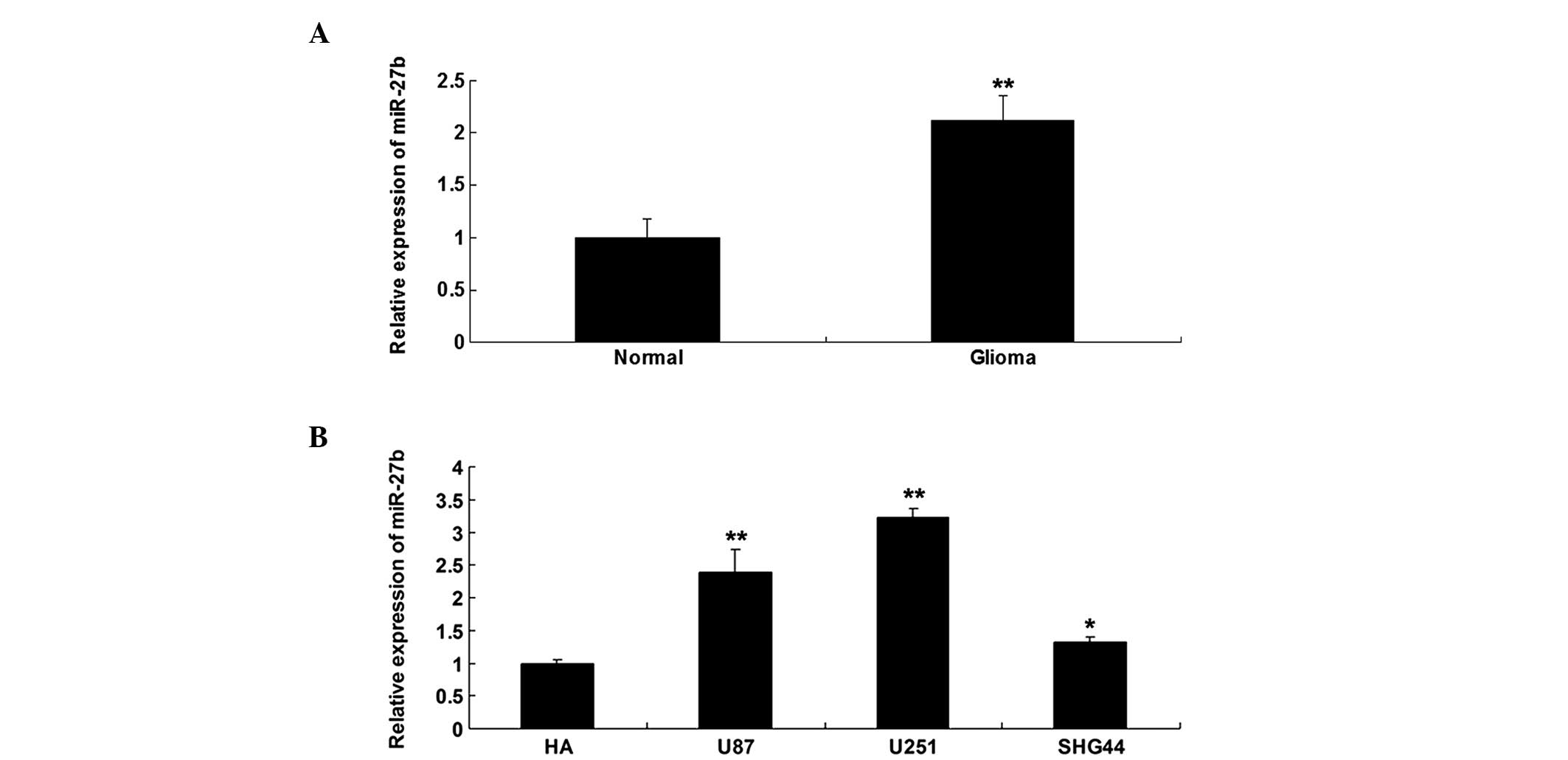
MiR-27b was upregulated in glioma tissues
and cell lines
The expression level of miR-27b was determined using
RT-qPCR in glioma tissues and their matched normal adjacent
tissues. It was found that miR-27b was significantly upregulated in
glioma tissues, when compared with normal adjacent tissues
(Fig. 1A). The expression level of
miR-27b was also determined in three common glioma cell lines, U87,
U251 and SHG44, and in the normal human astrocyte HA cell line.
Compared with normal human HA astrocytes, the glioma cells
demonstrated notable upregulation of miR-27b, particularly in U251
cells (Fig. 1B). Accordingly, U251
cells were used in the subsequent experiments.
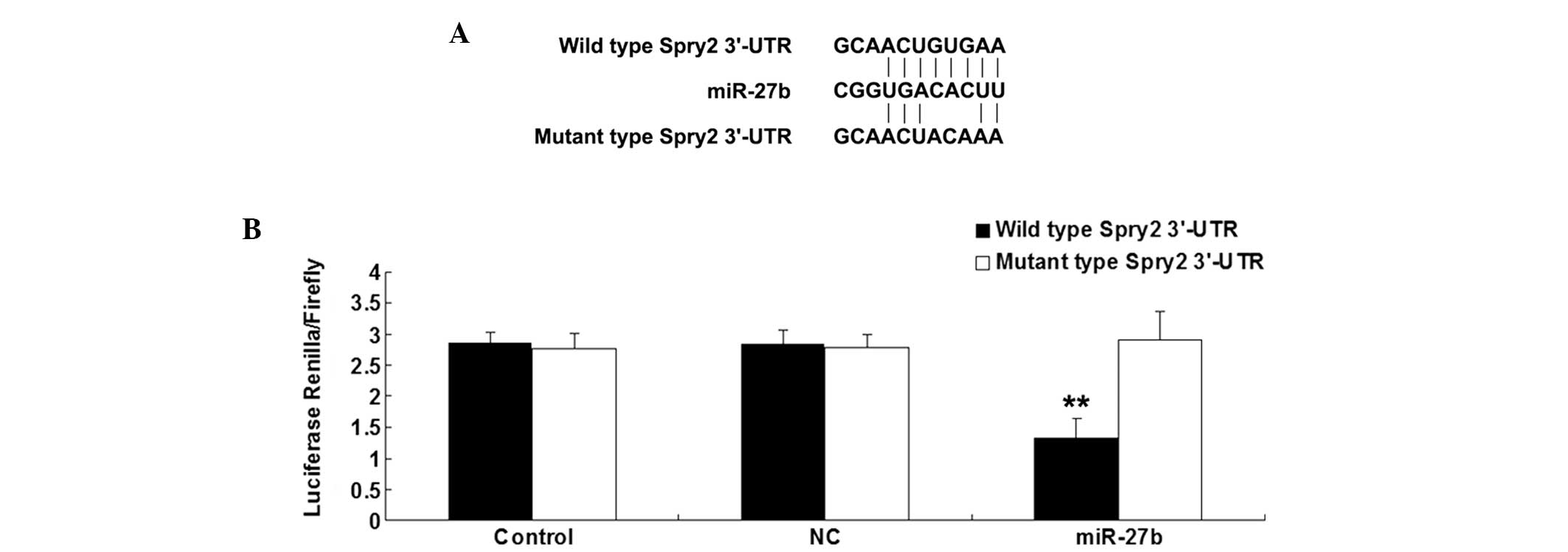
Spry2 was identified as a direct target
of miR-27b in U251 cells
Bioinformatic analysis was performed to predicate
the target association between miR-27b and Spry2. The putative seed
sequences for miR-27b in the 3′UTR of Spry2 were evolutionarily
conserved. As shown in Fig. 2A, the
wild and mutant types of the Spry2 3′-UTR were then generated.
Subsequently, U251 cells were transfected with
psiCHECK2-Spry2-3′-UTR or psiCHECK2-mutant Spry2 -3′-UTR vector,
with or without miR-27b mimics. Following incubation for 48 h, the
luciferase activity was examined. The present data revealed that
the luciferase activity was notably reduced only in U251 cells
co-transfected with the wild type 3′-UTR of Spry2 and miR-27b
mimics, suggesting that miR-27b could directly bind to the 3′UTR of
Spry2 mRNA in U251 cells (Fig.
2B).
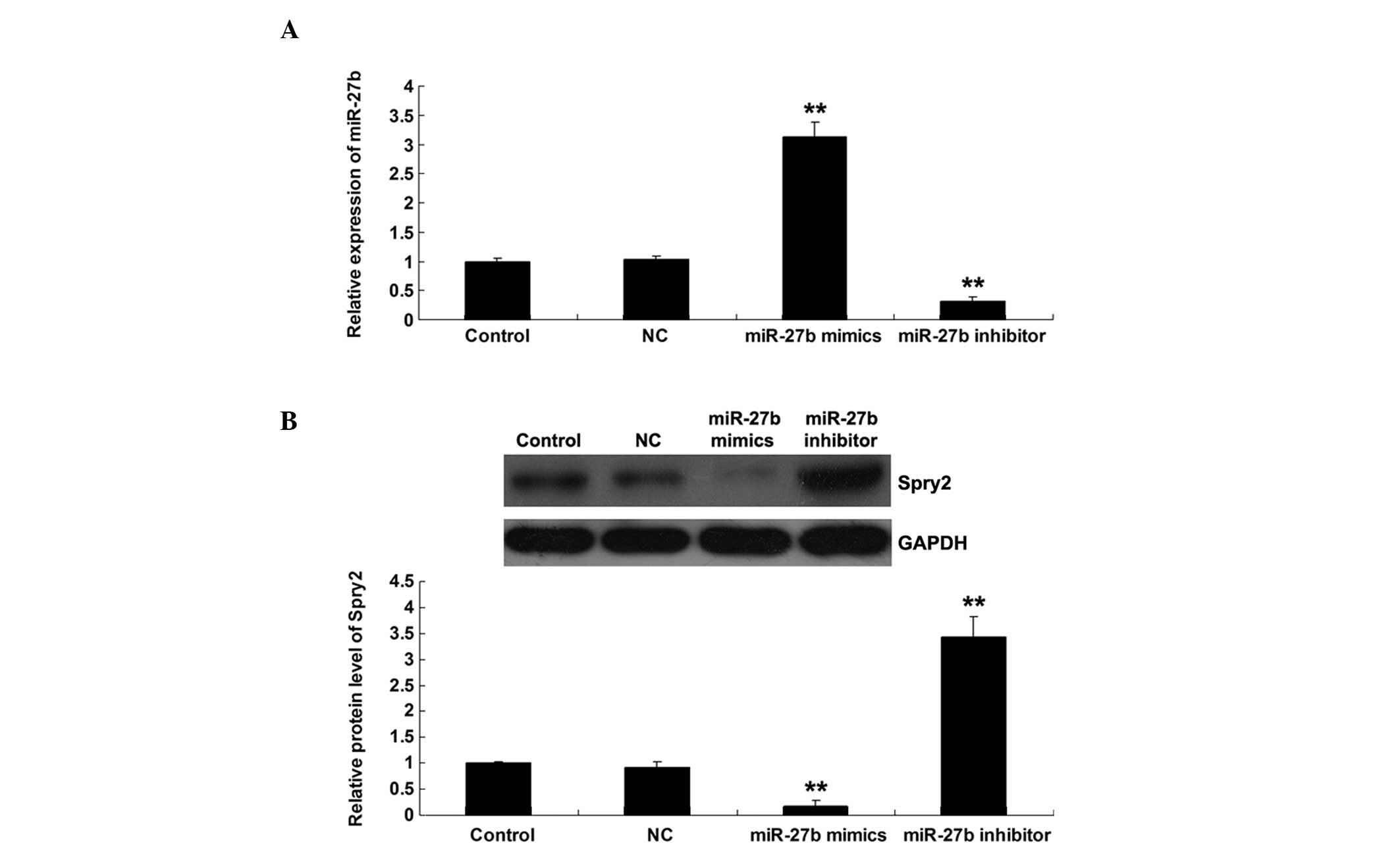
MiR-27b negatively regulated Spry2
expression at a post-transcriptional level in U251 cells
U251 cells were transfected with scramble miRNA
control (NC), miR-27b mimics or the miR-27b inhibitor.
Subsequently, the expression level of miR-27b in each group was
examined. As shown in Fig. 3A, the
miR-27b level was significantly upregulated following transfection
with miR-27b mimics, while notably reduced subsequent to
transfection with the miR-27b inhibitor. To explore the regulatory
association between miR-27b and Spry2, the protein level of Spry2
was determined in U251 cells transfected with the miR-27b mimics or
inhibitor. As shown in Fig. 3B,
upregulation of miR-27b significantly inhibited the protein
expression of Spry2 in U251 cells. By contrast, inhibition of
miR-27b resulted in an increased protein expression of Spry2 in
U251 cells. Based on these data, it was suggested that miR-27b
negatively regulated Spry2 expression at a post-transcriptional
level in glioma U251 cells.
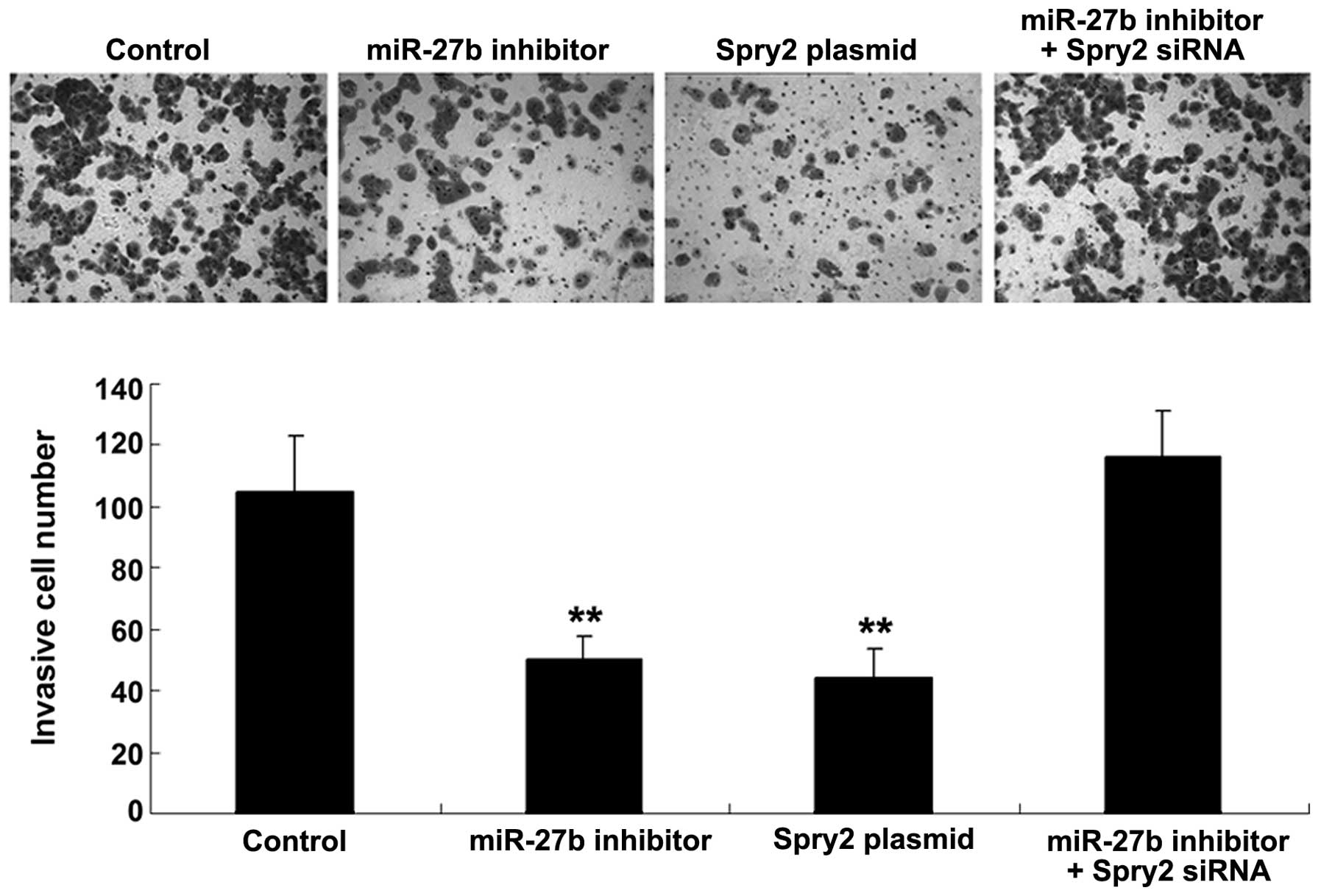
MiR-27b promoted U251 cell invasion by
targeting Spry2
As Spry2 has previously been suggested to be
associated with invasive glioma (9)
and miR-251 negatively regulated the Spry2 expression in U251
cells, it was speculated that miR-27b may also participate in the
regulation of glioma U251 cell invasion. To verify this
speculation, U251 cells were transfected with the miR-27b inhibitor
or Spry2 plasmid, or the cells were co-transfected with the miR-27b
inhibitor and Spry2 siRNA. It was then demonstrated that the
inhibition of miR-27b activation and overexpression of Spry2
significantly suppressed U251 cell invasion (Fig. 4). However, the inhibitory effect of
miR-27b inhibition on U251 cell invasion was attenuated by
siRNA-induced Spry2 downregulation (Fig. 4). The present data suggested that
miR-27b may promote the regulation of U251 cell invasion via direct
targeting of Spry2.
Discussion
Dysfunction of miR-27b and Spry2 has been found to
be involved in the development and progression of glioma (9,13).
However, to the best of our knowledge, the roles of and association
between miR-27b and Spry2 have never been studied in glioma. In the
present study, it was revealed that the expression level of miR-27b
was markedly increased in glioma tissues and the U87, U251 and
SHG44 glioma cell lines compared with normal brain tissue and
astrocytes. In addition, the present study identified Spry2 as a
direct target of miR-27b, and demonstrated that the protein
expression of Spry2 was negatively regulated by miR-27b in glioma
U251 cells. Furthermore, inhibition of miR-27b and upregulation of
Spry2 could suppress glioma cell invasion, while downregulation of
Spry2 reversed the suppressive effect of miR-27b inhibition on
glioma cell invasion. Based on these data, it can be suggested that
miR-27b may promote glioma cell invasion through direct inhibition
of Spry2 expression.
miRNAs have been found to regulate various
biological processes, and deregulation of miRNAs participates in
the development and progression of human malignancies (14). The roles of numerous miRNAs in
glioma have been widely investigated, including miR-20a, miR-106a,
miR-145, miR-494 and miR-124 (5–8).
MiR-27b has been demonstrated to be involved in several cancers.
Wan et al revealed that miR-27b was notably decreased in
non-small cell lung cancer (NSCLC) tissues and cell lines, and that
overexpression of miR-27b significantly suppressed NSCLC cell
proliferation and invasion (15),
indicating that miR-27b acts as a tumor suppressor in NSCLC. The
majority of studies have demonstrated that miR-27b plays an
inhibitory role in the development and progression of human
malignancies, including colon and prostate cancer and neuroblastoma
(16–18). However, a few studies have also
suggested that miR-27b may act as a tumor promoter. Jin et
al revealed that miR-27b was highly upregulated in human breast
cancer, and that knockdown of miR-27b substantially repressed
breast cancer growth (19).
Chen et al previously suggested that miR-27b
acts as an oncogene in glioma. This study found that miR-27b was
upregulated in glioma tissues and cells (9), which is consistent with the present
findings. However, the effect of miR-27b on glioma cell invasion
and the involved mechanism remains largely unknown. In the present
study, it was found that miR-27b played a promoting role in the
regulation of glioma U251 cell invasion, and further molecular
mechanism investigation suggested that the promotion of U251 cell
invasion by miR-27b occurred partially by direct inhibition of
Spry2.
As a negative regulator of receptor tyrosine
kinase-mediated signaling, Spry2 has been found to play a role in
various cancers (13,20,21).
Spry2 mainly acts as a tumor suppressor. Rathmanner et al
revealed that Spry2 inhibited cell proliferation and migration in
osteosarcoma cells (21). Li et
al reported that Spry2 was downregulated in renal cell
carcinoma (RCC) tissues compared with adjacent normal tissues, and
that Spry2 could inhibit RCC cell proliferation and invasion
(20). Spry2 has previously been
reported to be significantly downregulated in invasive glioma
tissues, suggesting that Spry2 may participate in the regulation of
glioma invasion (13). In the
present study, it was revealed that overexpression of Spry2
significantly inhibited the invasion of glioma U251 cells. These
findings suggest that Spry2 inhibits the regulation of glioma cell
invasion. In addition, miR-27b was shown to regulate the protein
expression of Spry2 by directly targeting the 3′UTR of Spry2 mRNA
in glioma U251 cells. miR-27b has previously been reported to
directly target Spry2 in zebrafish (22). However, the existence of this
targeting association between miR-27b and Spry2 has never been
reported in humans. Furthermore, by the gain of function assay, it
was found that miR-27b inhibition led to a significant inhibition
of U251 cell invasion, similar to the effect of Spry2
overexpression. Additionally, inhibition of Spry2 reversed the
suppressive effect of miR-27b downregulation on glioma U251 cell
invasion. These findings further confirmed that miR-27b plays a
role in the regulation of glioma cell invasion through direct
targeting of Spry2.
In conclusion, the present study suggests that
upregulation of miR-27b in glioma may promote glioma cell invasion
by inhibiting the expression of its target, Spry2. Therefore,
miR-27b may serve as a promising target for the prevention and
treatment of glioma invasion.
References
|
1
|
Watts C, Price SJ and Santarius T: Current
concepts in the surgical management of glioma patients. Clin Oncol
(R Coll Radiol). 26:385–394. 2014. View Article : Google Scholar
|
|
2
|
Haapasalo J, Hyartt A, Salmi M, et al:
Diagnosis and prognosis of gliomas - current prospects of molecular
diagnostics. Duodecim. 130:893–901. 2014.(In Finnish).
|
|
3
|
Peng Y, Yu S, Li H, et al: MicroRNAs:
emerging roles in adipogenesis and obesity. Cell Signal.
26:1888–1896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo S, Miracco C, Pirtoli L and
Comincini S: Emerging roles of microRNA in modulating cell-death
processes in malignant glioma. J Cell Physiol. 229:277–286. 2014.
View Article : Google Scholar
|
|
5
|
Wang Z, Wang B, Shi Y, et al: Oncogenic
miR-20a and miR-106a enhance the invasiveness of human glioma stem
cells by directly targeting TIMP-2. Oncogene. Apr 7–2014.(Epub
ahead of print). View Article : Google Scholar
|
|
6
|
Wan X, Cheng Q, Peng R, et al: ROCK1, a
novel target of miR-145, promotes glioma cell invasion. Mol Med
Rep. 9:1877–1882. 2014.PubMed/NCBI
|
|
7
|
Kwak SY, Yang JS, Kim BY, Bae IH and Han
YH: Ionizing radiation-inducible miR-494 promotes glioma cell
invasion through EGFR stabilization by targeting p190B rhoGAP.
Biochim Biophys Acta. 1843:508–516. 2014. View Article : Google Scholar
|
|
8
|
An L, Liu Y, Wu A and Guan Y: microRNA-124
inhibits migration and invasion by down-regulating ROCK1 in glioma.
PLoS One. 8:e694782013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Li H, Han L, et al: Expression and
function of miR-27b in human glioma. Oncol Rep. 26:1617–1621.
2011.PubMed/NCBI
|
|
10
|
Cabrita MA and Christofori G: Sprouty
proteins, masterminds of receptor tyrosine kinase signaling.
Angiogenesis. 11:53–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mei Y, Bian C, Li J, et al: miR-21
modulates the ERK-MAPK signaling pathway by regulating SPRY2
expression during human mesenchymal stem cell differentiation. J
Cell Biochem. 114:1374–1384. 2013. View Article : Google Scholar
|
|
12
|
Wang C, Delogu S, Ho C, et al:
Inactivation of Spry2 accelerates AKT-driven hepatocarcinogenesis
via activation of MAPK and PKM2 pathways. J Hepatol. 57:577–583.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwak HJ, Kim YJ, Chun KR, et al:
Downregulation of Spry2 by miR-21 triggers malignancy in human
gliomas. Oncogene. 30:2433–2442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tay FC, Lim JK, Zhu H, Hin LC and Wang S:
Using artificial microRNA sponges to achieve microRNA
loss-of-function in cancer cells. Adv Drug Deliv Rev. May
22–2014.(Epub ahead of print). PubMed/NCBI
|
|
15
|
Wan L, Zhang L, Fan K and Wang J: MiR-27b
targets LIMK1 to inhibit growth and invasion of NSCLC cells. Mol
Cell Biochem. 390:85–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye J, Wu X, Wu D, et al: miRNA-27b targets
vascular endothelial growth factor C to inhibit tumor progression
and angiogenesis in colorectal cancer. PLoS One. 8:e606872013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishteiwy RA, Ward TM, Dykxhoorn DM and
Burnstein KL: The microRNA -23b/-27b cluster suppresses the
metastatic phenotype of castration-resistant prostate cancer cells.
PLoS One. 7:e521062012. View Article : Google Scholar
|
|
18
|
Lee JJ, Drakaki A, Iliopoulos D and Struhl
K: MiR-27b targets PPARgamma to inhibit growth, tumor progression
and the inflammatory response in neuroblastoma cells. Oncogene.
31:3818–3825. 2012. View Article : Google Scholar :
|
|
19
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Prooncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer
Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li P, Tao L, Yang J, et al: Sprouty2 is
associated with prognosis and suppresses cell proliferation and
invasion in renal cell carcinoma. Urology. 82:253.e1–e7. 2013.
View Article : Google Scholar
|
|
21
|
Rathmanner N, Haigl B, Vanas V, Doriguzzi
A, Gsur A and Sutterlüty-Fall H: Sprouty2 but not Sprouty4 is a
potent inhibitor of cell proliferation and migration of
osteosarcoma cells. FEBS Lett. 587:2597–2605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Biyashev D, Veliceasa D, Topczewski J, et
al: miR-27b controls venous specification and tip cell fate. Blood.
119:2679–2687. 2012. View Article : Google Scholar :
|