Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cause of cancer and is also the third leading cause of
cancer-associated mortality worldwide, with ~700,000 mortalities
reported annually (1,2). The incidence of HCC has demonstrated a
notable increase worldwide, particularly in developing countries
within Asia and sub-Saharan Africa, where hepatitis B and C viral
infections are endemic, and in regions where food contaminated with
Aflatoxin B1 is consumed (3). HCC
is usually diagnosed at an advanced, incurable and metastatic stage
due to the usual absence of specific symptoms and history of
cirrhosis. Thus, the five-year overall survival rate is <10%;
the effects of surgical and chemo-radiation therapies are poor for
advanced and metastatic stages of HCC (4).
HCC is a solid tumor with numerous blood vessels,
and the most important characteristic of HCC is the uncontrollable
cell proliferation, which leads to a novel hypoxic microenvironment
with increased oxygen consumption. Therefore, it is important to
establish a vascular and nutrient supply system to meet the oxygen
demands (5,6). Hypoxia is one of the fundamental
biological phenomena that are strongly associated with the
development and aggressiveness of a wide variety of solid tumors,
including HCC. It has been reported that hypoxia inducible factor-1
(HIF-1α), the key to mediating hypoxia-responsive genes, and
insulin-like growth factor I (IGF-1), which is secreted in the
liver, may potentially be synergistic in regulating the vascular
endothelial growth factor (VEGF) expression in type 2 diabetes
(7). The present study focused on
whether VEGF expression in HCC is also regulated by HIF-1α and
IGF-1.
In the current study, a model of hypoxia was created
using cobalt chloride (CoCl2) and an MTT assay was used
to observe the influence of hypoxia on the proliferation of HepG2
cells. The effect of hypoxia on HepG2 cell invasion and
angiogenesis was examined, as well as the expression and
correlation of HIF-1α, IGF-1 and VEGF under hypoxic conditions.
Materials and methods
Drug and reagents
Dulbecco’s modified Eagle’s medium (DMEM),
CoCl2 dissolved in DMEM,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit, RIPA buffer, goat anti-rabbit fluorescein isothiocyanate
immunoglobulin (Ig)G and tetramethylrhodamine isothiocyanate IgG
antibodies were purchased from Sigma-Aldrich (St Louis, MO, USA).
Goat anti-rabbit HIF-1α, IGF-1R and VEGF monoclonal antibodies were
purchased from Cell Signaling Technology (Beverly, MA, USA). Goat
anti-rabbit horseradish peroxidase (HRP) conjugated secondary and
β-actin monoclonal antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). TRIzol® reagent
was purchased from Invitrogen (Carlsbad, CA, USA). Revertaid First
Strand cDNA Synthesis Kit was purchased from Thermo Fisher
Scientific (Waltham, MA, USA).
Cell culture
The human hepatocellular carcinoma cell HepG2 was
obtained from the American Type Culture Collection. HepG2 cells
were maintained in DMEM medium supplemented with 10% fetal bovine
serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin.
The HepG2 cells were maintained under the standard culture and
normoxic conditions of 21% O2 and 5% CO2, at
37°C.
Establishing the hypoxia model and
groups
The HepG2 cells were plated in six-well plates in
medium containing 10% FCS and grown to 70–80% confluency.
Subsequently, the cells were washed extensively with serum-free
DMEM medium and incubated with CoCl2 DMEM medium. The
cells were divided into two groups. Since the results from the
concentration-based experiment would affect the concentration used
in the time-based experiment, according to previous studies and our
preliminary experiment, the first group was treated with 0, 50,
100, 200, 400, 800 or 2,000 μmol/l CoCl2, and the cells
were then maintained under standard culture conditions for 12 h
(8–10). The second group was treated with 200
μmol/l CoCl2, but was incubated for 0, 4, 8, 12, 24 or
48 h.
Cell survival assay
An MTT assay was conducted to investigate the effect
of the growth and proliferation of HepG2 cells under
CoCl2-induced hypoxic conditions. Overall, 5,000 cells
were seeded into each well of a 96-well plate and were incubated
under normoxic conditions overnight. The cells were then treated
with 0, 50, 100, 200, 400, 800 or 2,000 μmol/l CoCl2 and
incubated under standard culture conditions for 12 h. Cell
proliferation was determined using an MTT assay (Sigma-Aldrich)
according to the manufacturer’s instructions. The optical density
(OD) was determined at 570 nm on SpectraMax M2 (Molecular Devices
LLC, Sunnyvale, CA, USA). The experiment was repeated in
triplicate.
Quantitative PCR analysis to assess the
mRNA levels of HIF-1α, IGF-1R and VEGF
Total RNA was isolated from cells using
TRIzol® Reagent. Quantitative PCR analysis of HIF-1α,
IGF-1R and VEGF mRNA levels was performed using the One-step RT-PCR
kit from Thermo Fisher Scientific, according to the manufacturer’s
instructions. The following primers were designed for quantitative
PCR: HIF-1α forward, 5′-ACTAAAGGACAAGTCACCACAGGA-3′ and reverse,
5′-TGCTGAATAATACCACTCACAACG-3′; IGF-1R forward,
5′-CTCAGTTAATCGTGAAGTGGAACC-3′ and reverse
5′-GCAGTAATTGTGCCGGTAAAGG-3′; VEGF forward,
5′-GAGGGCAGAATCATCACGAAGT-3′ and reverse,
5′-TCCTATGTGCTGGCCTTGGTGA-3′; and β-actin forward,
5′-CACACAGGAGAGGTGATAGCAAGT-3′ and reverse,
5′-GACCAAAAGCCTTCATACATCTCA-3′. All primers were synthesized by
Shanghai Sangon Biological Engineering Technology and Services Co.,
Ltd. (Shanghai, China). The thermocycling conditions were as
follows: 95°C for 5 min; 72°C for 10 min; and 40 cycles at 95°C for
10 sec and 50°C for 30 sec for HIF-1α and IGF-1R, or 60°C for 30
sec for VEGF. The relative HIF-1α, IGF-1R and VEGF mRNA levels were
normalized to β-actin. The experiment was repeated in
triplicate.
Western blot analysis to assess the
protein levels of HIF-1α, IGF-1R and VEGF
Total protein was isolated from cells using RIPA
buffer reagent. The cells were then incubated at 4°C for 1 h. The
lysates were ultrasonicated and centrifuged at 12,000 × g for 10
min. The supernatants were collected and stored at −70°C. Protein
concentrations were determined using the bicinchoninic acid method.
Protein (50–100 μg) was separated on a 10% polyacrylamide-SDS gel
and electroblotted onto a nitrocellulose membrane. Subsequent to
being blocked with Tris-buffered saline/5% non-fat dry milk for 2
h, the membranes were incubated overnight at 4°C with antibodies
against HIF-1α (cat. no. 3176; dilution, 1:1,000), IGF-1R (cat. no.
9750; dilution, 1:1,000) and VEGF (cat. no. 2463; dilution,
1:1,000) (all rabbit polyclonal IgG; Cell Signaling Technology),
followed by incubation with a horseradish peroxidase-conjugated
secondary antibody (cat. no. sc-2048; dilution, 1:1,000; rabbit
polyclonal IgG; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 45 min at room temperature. The signals were visualized by the
enhanced chemiluminescence detection system [Life Science
(Research, Education, Process Separations, Food Science), Bio-Rad
Laboratories (Shanghai) Co., Ltd., Shanghai, China]. As a loading
control, the blots were reprobed using a specific antibody against
human β-actin (cat. no. sc-7210; dilution, 1:4,000; rabbit
polyclonal IgG provided at 200 μg/ml; Santa Cruz Biotechnology,
Inc.).
ELISA assay to assess the production of
VEGF
The concentration of VEGF protein in the conditioned
medium subsequnet to treatment with the various crocetin
concentrations and treatment times was determined using the human
VEGF ELISA development kit (R&D Systems, Inc., Minneapolis, MN,
USA), according to the manufacturer’s instructions. The results
were normalized to the cell number (2×105 cells/well)
and the experiment was repeated in triplicate.
Confocal immunofluorescence microscopy
assay
The cells were plated onto glass culture slides
coated with fibronectin or bovine serum albumin (BSA) and processed
by immunofluorescence. The cells were fixed, permeabilized and
blocked with BSA. The cells were incubated with HIF-1α and IGF-1R
antibodies overnight at 4°C, washed and were incubated with
fluorochrome-conjugated secondary antibodies. The cells were
incubated with rhodamine phalloidin to stain the stress fibers. The
nuclei were visualized by staining with Hoechst 33258. Images were
captured using fluorescence confocal microscopy [VF1000, Olympus
(China) Co., Ltd., Beijing, China].
Statistical analysis
The data are presented as the mean ± standard
deviation for three separate experiments. One-way analysis of
variance and the Bonferroni correction were employed for
statistical analysis using SPSS 13.0 software for Windows (SPSS,
Inc., Chicago, IL, USA). Pearson analysis was used for correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
The effect of hypoxia on HepG2 cells
In order to investigate the effect of hypoxia on
HepG2 cells, an MTT assay was conducted using the human HepG2
cells. The results revealed that there was no significant
difference in cell survival between the cells treated with 50, 100
and 200 μmol/l of CoCl2. However, when the cells were
treated with a higher concentration or were incubated for a longer
time, there was a significant difference between the cells treated
with 400 μmol/l and those treated with 2,000 μmol/l
CoCl2, and there was also a significant difference
between the cells treated with 0 μmol/l and those treated with 800
μmol CoCl2 at the same incubation times (Fig. 1).
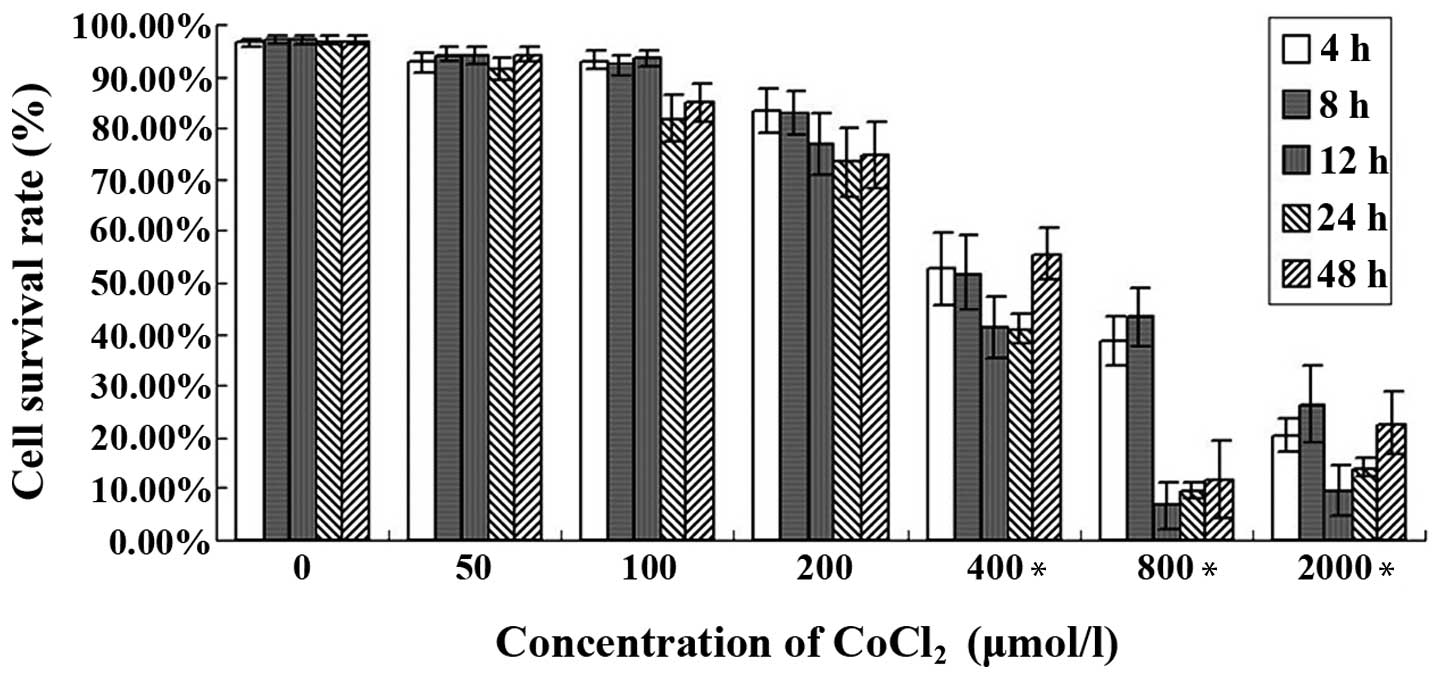 | Figure 1Effect of the CoCl2-induced
hypoxia by on HepG2 cell viability was determined by an MTT assay.
The HepG2 cells were treated with 0, 50, 100, 200, 400, 800 or
2,000 μmol/l CoCl2 and incubated at the standard culture
conditions for 0, 4, 8, 12, 24 or 48 h. The cell survival rate (%)
was correlated with the dose and time. *P<0.05 compared with
control group (0 μmol/l). CoCl2, cobalt chloride. |
Effect of CoCl2-induced
hypoxia on HIF-1α, IGF-1R and VEGF mRNA expression
The quantitative RT-PCR assay was conducted to
examine the effect of CoCl2-induced hypoxia on HIF-1α,
IGF-1R and VEGF mRNA expression in human HepG2 cells. The cells
were treated with 0, 50, 100, 200, 400 or 800 μmol/l of
CoCl2 for 12 h. As shown in Fig. 2A, there was no apparent change in
the HIF-1α mRNA levels in HepG2 cells. A significant difference in
the expression of IGF-1R and VEGF mRNA between the cells treated
with 200 and 400 μmol/l CoCl2 and the control group
(Table I). The expression of HIF-1α
mRNA was positively correlated with the expression of VEGF mRNA
(r=0. 77; P<0.05) in a dose-dependent manner under hypoxic
conditions.
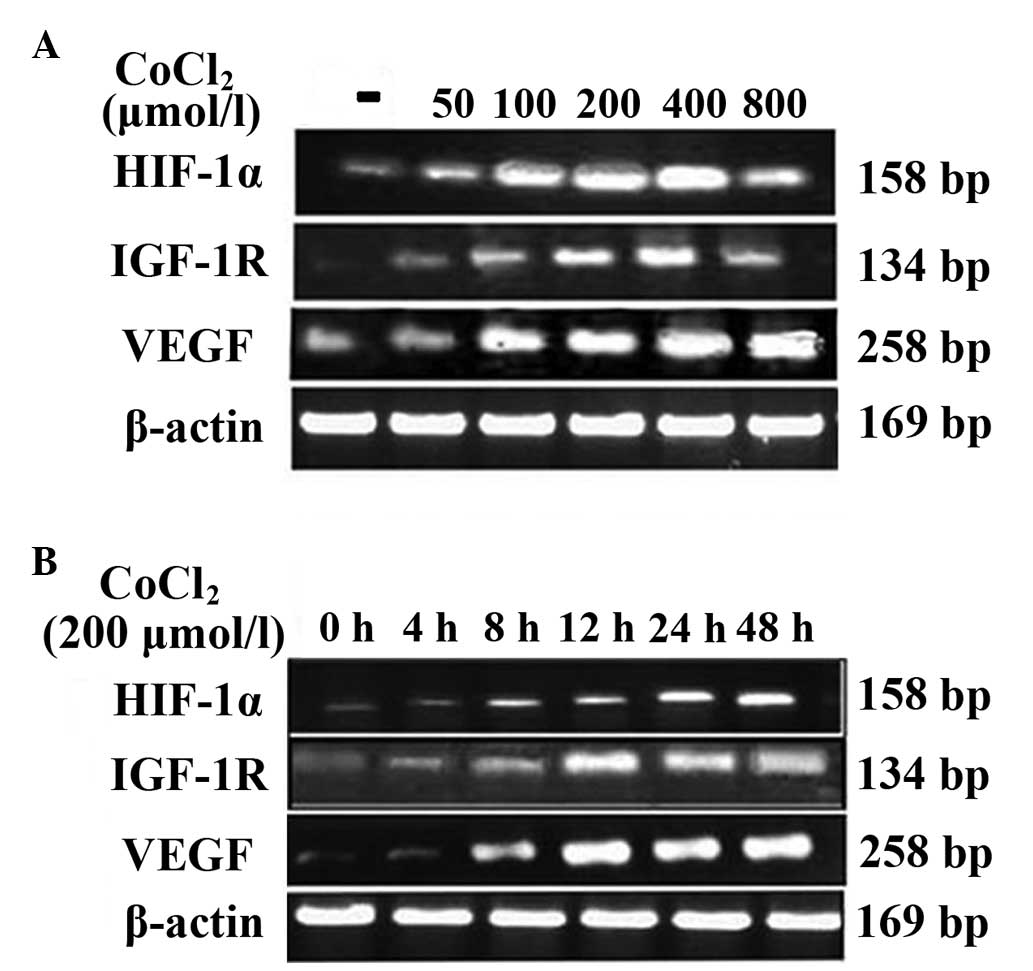 | Figure 2Effect of CoCl2- induced
hypoxia on the expression of HIF-1α, IGF-1R and VEGF mRNA. (A) The
HepG2 cells were treated with 0, 50, 100, 200, 400 or 800 μmol/l
CoCl2 for 12 h. (B) The HepG2 cells were treated for 0,
4, 8, 12, 24 or 48 h with 200 μmol/l CoCl2. A
significant difference in HIF-1α, IGF-1R and VEGF protein
expression was observed when cells were treated with 200 and 400
μmol/l CoCl2, and for 12, 24 and 48 h, compared with the
control (P<0.05). CoCl2, cobalt chloride; HIF-1α,
hypoxia inducible factor-1α; ICF-1R, insulin-like growth factor-1
receptor; VEGF, vascular endothelial growth factor. |
 | Table IThe correlation in the does dependence
between hypoxia induced and gene expression. |
Table I
The correlation in the does dependence
between hypoxia induced and gene expression.
| CoCl2,
μmol/l |
|---|
|
|
|---|
| Protein | 0 | 50 | 100 | 200 | 400 | 800 |
|---|
| HIF-1α | 0.41+0.10 | 0.65+0.06 | 0.85+0.04 | 0.88+0.04a | 1.33+0.05a | 0.78+0.08 |
| IGF-1R | 0.06±0.01 | 0.15±0.06 | 0.18±0.03 | 0.29±0.08a | 0.38±0.11a | 0.13±0.08 |
| VEGF | 0.87±0.08 | 1.48±0.85 | 1.82±0.67 | 2.33±0.85a | 2.98±1.08a | 1.76±0.19 |
The cells were also treated for 0, 4, 8, 12, 24 or
48 h with 200 μmol/l CoCl2. As shown in Fig. 2B, The expression of HIF-1α, IGF-1R
and VEGF mRNA in the cells treated for 4 and 8 h was not
significantly different compared with the expression in the control
group, while there was a significant difference between the cells
treated for 12, 24 and 48 h and the control group (Table II). It was also found that the
expression of HIF-1α mRNA was positively correlated with the
expression of VEGF mRNA (r=0.85, P<0.05), dependent on the
incubtation time with CoCl2.
 | Table IIThe correlation in the time dependence
between hypoxia induced and gene expression. |
Table II
The correlation in the time dependence
between hypoxia induced and gene expression.
| Time, h |
|---|
|
|
|---|
| Protein | 0 | 4 | 8 | 12 | 24 | 48 |
|---|
| HIF-1α | 0.36+0.06 | 0.42+0.05 | 0.72+0.03 | 0.87+0.03a | 1.23+0.05a | 1.18+0.02a |
| IGF-1R | 0.22±0.02 | 0.25±0.03 | 0.27±0.03 | 0.67±0.12a | 0.65±0.17a | 0.78±0.15a |
| VEGF | 0.43±0.05 | 0.48±0.07 | 0.55±0.08 | 1.85±0.75a | 2.39±0.98a | 2.56±0.89a |
Effect of CoCl2-induced
hypoxia on HIF-1α, IGF-1R and VEGF protein expression
Western blot analysis was performed to examine the
effect of CoCl2-induced hypoxia on HIF-1α, IGF-1R and
VEGF protein expression in human HepG2 cells. The cells were
treated with 0, 50, 100, 200, 400 or 800 μmol/l CoCl2
for 12 h. As shown in Fig. 3A, the
expression of the HIF-1α, IGF-1R and VEGF proteins was not
significantly different in the cells treated with 50, 100 and 800
μmol/l CoCl2 compared with the control group. However,
the differences between the cells treated with 200 and 400 μmol/l
and the control group were significantly different (Table III). In addition, it was also
found that, under hypoxic conditions, the expression of the HIF-1α
protein was positively correlated with the expression of the VEGF
protein (r=0.90, P<0. 05) in a dose-dependent manner.
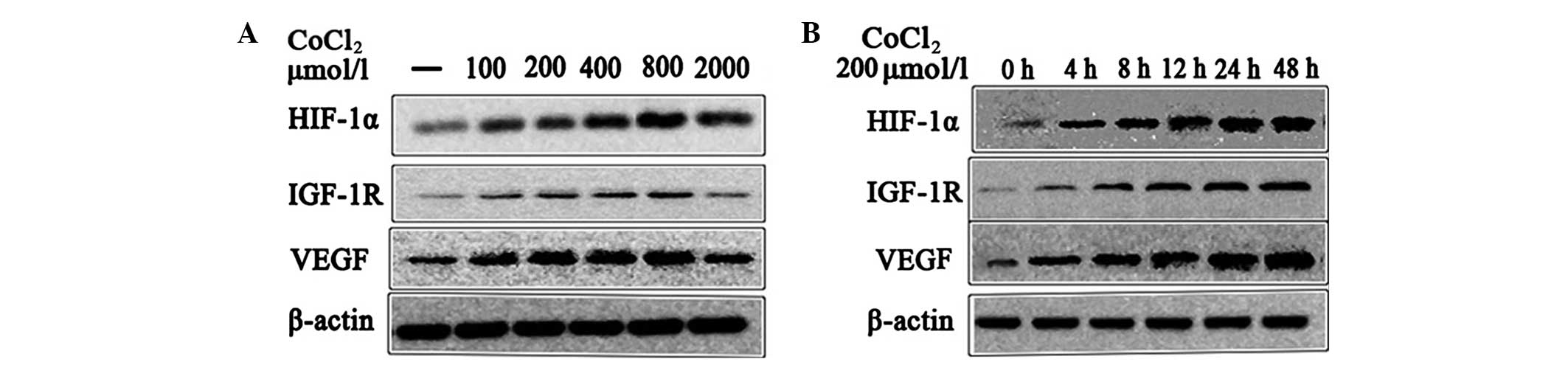 | Figure 3Effect of CoCl2-induced
hypoxia on HIF-1α, IGF-1R and VEGF protein expression. (A) The
HepG2 cells were treated with 0, 50, 100, 200, 400 or 800 μmol/l
CoCl2 for 12 h. (B) The HepG2 cells were treated for
0,4,8,12, 24 or 48 h with 200 μmol/l CoCl2. A
significant difference in HIF-1α, IGF-1R and VEGF mRNA expression
was observed when cells were treated with 200 and 400 μmol/l
CoCl2, and for 8, 12, 24 and 48 h, compared with the
control (P<0.05). CoCl2, cobalt chloride; HIF-1α,
hypoxia inducible factor-1α; ICF-1R, insulin-like growth factor-1
receptor; VEGF, vascular endothelial growth factor. |
 | Table IIIDose-dependent correlation between
hypoxia and gene expression. |
Table III
Dose-dependent correlation between
hypoxia and gene expression.
| CoCl2,
μmol/l |
|---|
|
|
|---|
| Gene | 0 | 50 | 100 | 200 | 400 | 800 |
|---|
| HIF-1α | 0.56±0.06 | 0.78±0.03 | 0.88±0.23 | 1.47±0.4a | 1.53±0.58a | 1.18±0.22 |
| IGF-1R | 0.64±0.07 | 0.81±0.11 | 1.01±0.43 | 1.46±0.3a | 1.51±0.67a | 0.87±0.08 |
| VEGF | 0.71±0.08 | 1.03±0.15 | 1.14±0.16 | 1.62±0.7a | 1.66±0.83a | 0.97±0.09 |
The cells were incubated for 0, 4, 8, 12, 24 or 48 h
with 800 μmol/l CoCl2. As shown in Fig. 3B, there was no significant
difference between the cells incubated for 4 h and the control
group, whereas the cells incubated for 8, 12, 24 and 48 h were
significantly different (Table
IV). It was also found that, under hypoxic conditions, the
expression of the HIF-1α protein was positively correlated with
expression of the VEGF protein (r=0.78, P<0.05) in a
time-dependent manner.
 | Table IVTime-dependent correlation between
hypoxia and gene expression. |
Table IV
Time-dependent correlation between
hypoxia and gene expression.
| Time, h |
|---|
|
|
|---|
| Gene | 0 | 4 | 8 | 12 | 24 | 48 |
|---|
| HIF-1α | 0.45±0.07 | 0.55±0.08 | 1.30±0.12a | 1.63±0.1a | 1.73±0.11a | 2.14±0.2a |
| IGF-1R | 0.31±0.03 | 0.40±0.10 | 1.10±0.10a | 1.51±0.0a | 1.87±0.11a | 2.68±0.8a |
| VEGF | 0.65±0.05 | 0.85±0.02 | 2.14±0.58a | 2.37±0.6a | 2.53±0.58a | 3.10±0.6a |
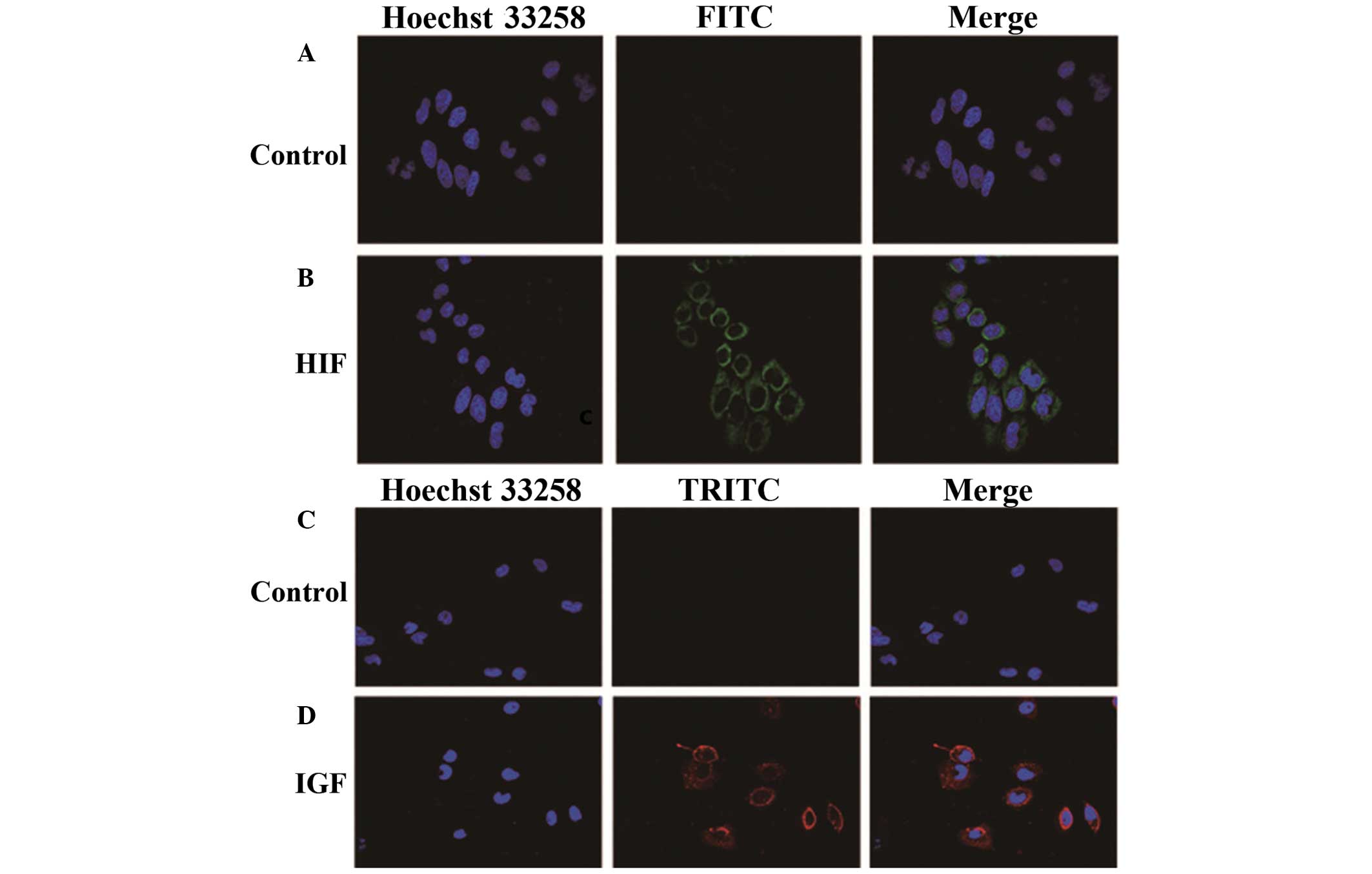
Effect of HIF-1α and IGF-1 expression in
hypoxic HepG2 cells
To detect the expression of HIF-1α or IGF-1 on the
membrane of hypoxic HepG2 cells, an immunofluorescence staining
assay was performed. The results revealed that HIF-1α and IGF-1
were highly expressed in hypoxic HepG2 cells compared with the
normoxia control group (Fig.
4).
Effect of CoCl2-induced
hypoxia on the production of VEGF
The effect of various concentrations and durations
of CoCl2 treatment on the production of VEGF in HepG2
cells was assessed using an ELISA assay. The results revealed that
the production of VEGF increased with increased concentration and
time, and it reached a peak at a concentration of 200 μmol/l
CoCl2 (Fig. 5A and
B).
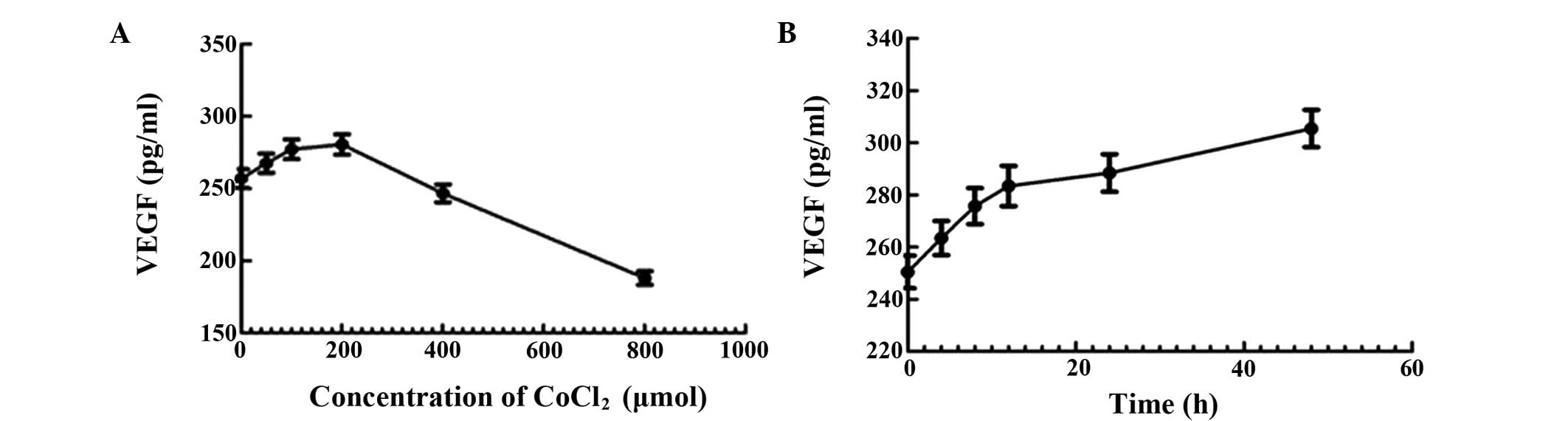 | Figure 5Effect of CoCl2-induced
hypoxia on the production of VEGF was determined by ELISA. (A)
HepG2 cells were treated with 0, 50, 100, 200, 400 or 800 μmol/l of
CoCl2 for 12 h. (B) HepG2 cells were treated for 0, 4,
8, 12, 24 or 48 h with 200 μmol/l CoCl2. VEGF, vascular
endothelial growth factor; CoCl2, cobalt chloride. |
Discussion
Hypoxic microenvironments and angiogenesis have been
a focus of tumor research over previous years. The expression of
numerous genes has been found to be regulated by hypoxic
microenvironments, which plays a crucial role in biological
characteristics. In the present study, CoCl2 was
utilized to mimic hypoxia. Ferrochelatase is inactived by a
substitution performed by cobalt ions under normoxic conditions,
which blocks oxygen absorptivity in cytoblasts. A hypoxic
microenvironment is subsequently formed (11).
Maintaining the oxygen balance is key for cell
survival. A series of abilities have evolved that adapt to the
changing oxygen concentrations over the process of development. If
the proliferation of tumor cells reaches or exceeds the speed of
angiogenesis, a hypoxic microenvironment is formed. HIF-1 is a
transcription factor found in mammalian cells cultured under
reduced oxygen tension, and plays an essential role in cellular and
systemic homeostatic responses to hypoxic microenvironments,
including the regulation of genes involved in energy metabolism,
angiogenesis and apoptosis. HIF-1α is rapidly degraded by the
proteasome under normal conditions but is stabilized by hypoxic
conditions (12). The process of
angiogenesis possesses multiple steps, which is fundamental for
tumor growth, invasion and metastasis. Growth factors are
over-expressed and released, resulting in tumor over-growth. This
promotes the vascular endothelial cells to migrate from the host
vessel to tumor tissue. HIF-1α and IGF-1 stimulate vascular
endothelial cell migration, proliferation, differentiation and
angiogenesis (13).
The present study identified that the cell viability
worsened with increasing concentrations of CoCl2, which
may be correlated with the cytotoxicity of CoCl2.
Furthermore, it was found that the HIF-1α, IGF-1R and VEGF proteins
were positively correlated with CoCl2, and therefore
hypoxia, in a dose- and time-dependent manner, demonstrated by the
various concentrations and incubation times. This aided
investigation into the best hypoxic conditions for solid tumor
studies.
Previous studies have revealed that cell death,
protein oxygenization and lipid preoxidation decrease when the
expression of the HIF-1α and IGF-1 proteins is normal, which
promotes the formation of novel blood vessels and the proliferation
of cells, inhibiting apoptosis. If not, the levels of lactate
dehydrogenase increase and the level of glutathione S-transferases
decreases, resulting in the inhibition of the formation of novel
blood vessels, which reduces the function of the cell membrane
(14,15). This is one possible explanation for
the small change or decrease in mRNA and protein levels compared
with the control group when the concentration reached 800 μmol/l or
the incubation time was ≥24 h. VEGF production declined in HepG2
cells once the concentration of CoCl2 was >200
μmol/l. In addition, the side-effects of the increased drug
concentration on the cells must be considered.
IGF-1 is a mitogenic and anti-apoptotic growth
factor that regulates cellular proliferation, differentiation and
cell death. The effects of IGF-1 are mediated through growth
hormones, oncogenes, anti-oncogenes and hypoxic microenvironments
(16). Previous studies have found
that abnormal levels of IGF-1 in malignant tumors, including
breast, lung, prostate and liver cancers, may be correlated with
autocrine IGF-1 expression (17–20).
In the present study, it was found that HepG2 cells in hypoxic
conditions can secrete IGF-1, which was positively correlated with
CoCl2, and therefore hypoxia, in a dose- and
time-dependent manner. The biological activities of IGF-1 are
mediated via the IGF-1 receptor (IGF-1R), which belongs to the
receptor tyrosine kinase family of membrane receptors. The effects
of IGF-1R on cell growth and apoptosis are mediated through binding
to IGF-binding proteins (IGFBPs) in the circulation. Following
receptor and IGFBP activation, two canonical signaling cascades are
activated, the phospho-inositide-3-kinase (PI3K) and
mitogen-activated protein kinase (MAPK) pathways. Ultimately, the
activation of the PI3K and MAPK pathways governs their specific
effects on cellular behavior. When adaptor molecules, including
insulin receptor substrates, are recruited and undergo tyrosine
phosphorylation, the PI3K pathway is activated. Subsequently, there
is an increase in the levels of the membrane-bound phospholipid
phosphatidyl inositol-3,4,5-triphosphate and the recruitment of Akt
to the membrane. Akt is a central mediator of the intracellular
effects of IGF-1R and is involved in metabolism, cell survival,
cell migration and proliferation through various mechanisms.
Activation of the MAPK pathway by IGF-1 also occurs downstream of
adaptor proteins, including MAPK kinase kinase, Raf, MAPK kinase,
MEK and MAPK. Extracellular-signal-regulated kinase 1/2 then exerts
mitogenic and inflammatory effects (21,22).
Previous studies have found that activation of the PI3K and MAPK
pathway can induce the activation of the VEGF receptor pathway that
is mediated by IGF-1, through the regulation of HIF-1α (23–26).
It has also been reported that the IGF system suppresses
angiogenesis through the PI3K/HIF-1α/VEGF signaling pathways in a
hypoxic microenvironment in ovarian cancer (27). This is consistent with the findings
of the present study, but additional investigation is required to
determine whether the decrease in VEGF is due to the PI3K/HIF-1α
signaling pathway.
In summary, the present study has demonstrated that
CoCl2 can induce HepG2 cells under hypoxic conditions at
a moderate concentration and appropriate incubation time. The
results presented in the current study indicated that IGF-1, which
is secreted by hypoxic HepG2 cells, can promote the accumulation of
HIF-1α mRNA and protein. Subsequently, HIF-1α regulated the
expression of VEGF mRNA and protein in hypoxic HepG2 cells. VEGF is
closely associated with the development and metastasis of HCC, and
inhibition of IGF-1 or HIF-1α may be an promising target for
hepatocellular carcinoma. However, whether certain other underlying
molecular mechanisms promote the accumulation of HIF-1α to regulate
VEGF expression remains poorly understood.
Acknowledgements
This study was supported by grants from the Simcere
anti-tumor therapy of China International Medical Foundation (grant
no., CIMF-F-H001-172), the Medical Department of Science and
Technology of Military (grant no., 11MA082), and the Department of
Science and Technology of Zhangzhou (grant no., ZZ2013J26). This
study was completed in the Cancer Research Center of Medical
College of Xiamen University. The authors would like to thank the
staff of the lab for their excellent technical assistance.
References
|
1
|
Thun MJ, DeLancey JO, Center MM, et al:
The global burden of cancer: priorities for prevention.
Carcinogenesis. 31:100–110. 2010. View Article : Google Scholar :
|
|
2
|
Jemal AB, Bray F, Center M, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma-epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar
|
|
4
|
Sherman M: Hepatocellular carcinoma.
epidemiology, risk factors and screening. Semin Liver Dis.
25:143–154. 2005. View Article : Google Scholar
|
|
5
|
Hiratsuka S: Vasculogenensis, angiogenesis
and special features of tumor blood vessels. Front Biosci (Landmark
Ed). 16:1413–1427. 2010. View
Article : Google Scholar
|
|
6
|
Lim CS, Kiriakidis S, Sandison A, et al:
Hypoxia-inducible factor pathway and diseases of the vascular wall.
J Vasc Surg. 58:219–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang F, Tang YT, Guo L and Jiao XY: The
role of insulin-like growth factor I and hypoxia inducible factor
1α in vascular endothelial growth factor expression in type 2
diabetes. Ann Clin Lab Sci. 43:37–44. 2013.PubMed/NCBI
|
|
8
|
Hervouet E, Pecina P, Demont J, et al:
Inhibition of cytochrome c oxidase subunit 4 precursor processing
by the hypoxia mimic cobalt chloride. Biochem Biophys Res Commun.
344:1086–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeon ES, Shin JH, Hwang SJ, et al: Cobalt
chloride induces neuronal differentiation of human mesenchymal stem
cells through upregulation of microRNA-124a. Biochem Biophys Res
Commun. 444:581–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Wang C, Wang Y, et al: Cobalt
chloride decreases fibroblast growth factor-21 expression dependent
on oxidative stress but not hypoxia-inducible factor in Caco-2
cells. Toxic Appl Pharmacol. 264:212–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wülfing P, Götte M, Sonntag B, et al:
Overexpression of Endothelin-A-receptor in breast cancer:
regulation by estradiol and cobalt-chloride induced hypoxia. Int J
Oncol. 26:951–960. 2005.PubMed/NCBI
|
|
12
|
Rosmorduc O and Housset C: Hypoxia: a link
between fibrogenesis, angiogenesis, and carcinogenesis in liver
disease. Semin Liver Dis. 30:258–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shojaei F: Anti-angiogenesis therapy in
cancer: current challenges and future perspectives. Cancer Lett.
320:130–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomita S, Kihira Y and Tamaki T:
Pathophysiological responses to hypoxia in vascular remodeling by
hypoxia-inducible factor-1. Seikagaku. 85:265–268. 2013.PubMed/NCBI
|
|
15
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seccareccia E and Brodt P: The role of the
insulin-like growth factor-I receptor in malignancy: An update.
Growth Horm IGF Res. 22:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y and Yee D: Targeting insulin and
insulin-like growth factor signaling in breast cancer. J Mammary
Gland Biol Neoplasia. 17:251–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dziadziuszko R, Camidge DR and Hirsch FR:
The insulin-like growth factor pathway in lung cancer. J Thorac
Oncol. 3:815–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozkan EE: Plasma and tissue insulin-like
growth factor-I receptor (IGF-IR) as a prognostic marker for
prostate cancer and anti-IGF-IR agents as novel therapeutic
strategy for refractory cases: a review. Mol Cell Endocrinol.
344:1–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu J and Zhu AX: Targeting insulin-like
growth factor axis in hepatocellular carcinoma. J Hematol Oncol.
4:302011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calvisi DF, Frau M, Tomasi ML, Feo F and
Pascale RM: Deregulation of signalling pathways in prognostic
subtypes of hepatocellular carcinoma: Novel insights from
interspecies comparison. Biochim Biophys Acta. 1826:215–237.
2012.
|
|
22
|
Morrione A, Neill T and Iozzo RV:
Dichotomy of decorin activity on the insulin-like growth factor-I
system. FEBS J. 280:2138–2149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferguson RD, Alikhani N, Vijayakumar A,
Fierz Y, Cannata D and Yakar S: IGF-1 cellular action and its
relationship to cancer: evidence from in vitro and in vivo studies.
Insulin-like Growth Factors and Cancer. 12. LeRoith D: Springer;
New York, NY: pp. 105–146. 2012, View Article : Google Scholar
|
|
24
|
Huang X, Zhang QY, Jiang QY, Kang XM and
Zhao L: Polysaccharides derived from Lycium barbarum suppress
IGF-1-induced angiogenesis via PI3K/HIF-1α/VEGF signalling pathways
in MCF-7 cells. Food Chem. 131:1479–1484. 2012. View Article : Google Scholar
|
|
25
|
Slomianya MG, Black LA, Kibbey MM, Day TA
and Rosenzweig SA: IGF-1 induced vascular endothelial growth factor
secretion in head and neck squamous cell carcinoma. Biochem Biophys
Res Commun. 342:851–858. 2006. View Article : Google Scholar
|
|
26
|
Wang F, Liu S, Xi S, Yan L, Wang H, Song Y
and Sun G: Arsenic induces the expressions of angiogenesis-related
factors through PI3K and MAPK pathways in SV-HUC-1 human
uroepithelial cells. Toxicol Lett. 222:303–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
King SM, Modi DA, Eddie SL and Burdette
JE: Insulin and insulin-like growth factor signaling increases
proliferation and hyperplasia of the ovarian surface epithelium and
decreases follicular integrity through upregulation of the
PI3-kinase pathway. J Ovarian Res. 6:122013. View Article : Google Scholar : PubMed/NCBI
|