Introduction
According to global data from the World Health
Organization, ~320 million people succumb to diabetes each year and
~70–80% of type 2 diabetic mortalities are due to cardiovascular
complications (1). Furthermore, the
risk of coronary heart disease is two to three times higher in type
2 diabetes patients compared with normal subjects, with insulin
resistance and hyperglycemia identified as important mechanisms in
the development of cardiovascular complications in diabetes
(2).
Previous epidemiological studies have demonstrated
that ~2/3 of acute coronary artery disease or stroke patients
exhibit impaired glucose tolerance or diabetes, indicating that
abnormal glucose metabolism has a negative impact on endothelial
function. Insulin resistance is considered to be the common ground
of type 2 diabetes and atherosclerosis (3). In addition, insulin resistance
inhibits nitric oxide (NO) synthesis in vascular endothelial cells
(4), promotes the secretion of
inflammatory mediators, such as tumor necrosis factor, plasminogen
activator inhibitor-1 (PAI-1), interleukin-6 (IL-6), C-reactive
protein (CRP) and free fatty acid (5,6), and
ultimately results in an abnormal blood glucose concentration and
dyslipidemia. These pathophysiological conditions consequently lead
to atherosclerosis, thrombosis and plaque ruptures, which may cause
acute life-threatening cardiovascular and cerebrovascular
complications (7).
Metformin is a first-line oral anti-diabetic agent
that reduces blood glucose concentration, blood lipid levels, blood
pressure and body weight (8). The
United Kingdom Prospective Diabetes Study (9) demonstrated that metformin
significantly reduced the risk of myocardial infarction by 39% in
patients with type 2 diabetes. In addition, it was demonstrated
that metformin markedly improved endothelial-dependent
vasodilation, whilst reducing the expression levels of
dysfunctional biomarkers, such as endothelin (ET)-1, PAI-1, IL-6,
and CRP in inflammatory and endothelial cells (10). Furthermore, metformin exhibited a
protective effect on blood vessels by ameliorating specific risk
factors for cardiovascular disease, and may improve endothelial
function by reducing oxidative stress and vascular inflammation,
stabilizing atherosclerotic plaques, inhibiting the proliferation
of smooth muscle cells and correcting insulin resistance (11). Thus, metformin is widely used in the
management of atherosclerosis, in stroke prevention and for
inhibiting restenosis following percutaneous transluminal coronary
angioplasty.
The formation of atherosclerosis involves
endothelial dysfunction, cellular proliferation, the migration of
smooth muscle cells, mononuclear phagocytic macrophage
differentiation and the formation of foam cells. Among these,
vascular endothelial dysfunction is the initiating factor. Current
clinical management for atherosclerosis patients includes stents,
bypass surgery and stem cell therapy, however, these methods cannot
be administrated proactively as vascular stenosis and visceral
damage have already caused damage at the time of diagnosis
(12). Therefore, correcting
insulin resistance and protecting against endothelial dysfunction
are current topics of interest (13,14).
In the present study, an in vitro insulin-resistant (IR)
endothelial cell model was successfully established and used to
assess the impact of metformin on the protection of endothelial
function.
Materials and methods
Materials and reagents
The human umbilical vein endothelial cell (HUVEC)
line was provided by Dr Ronggui Li of Jilin University (Changchun,
China). Trypsin, dimethyl sulfoxide (DMSO; Sigma-Aldrich, St.
Louis, MO, USA), fetal bovine serum (Gibco-BRL, Carlsbad, CA, USA)
and methyl thiazolyl tetrazolium blue (MTT; GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) were used in the present study.
Glucose, NO, and ET-1 assay kits were purchased from Nanjing
Jiancheng Biological Products Co., Ltd. (Nanjing, China).
Establishment of insulin resistance in
HUVECs
HUVECs were cultured in DMEM/low glucose (glucose,
5.5 mmol/l) and the third to fourth generations of cultured HUVECs
were harvested for use in the present study. To establish the in
vitro IR endothelial cell model, the cells were divided into
nine groups with six replicates per group: Negative control group,
the cells were cultured in 200 μl complete medium; insulin-treated
groups, the cells were administered with 30 mM glucose, 1 μM
dexamethasone and various concentrations of insulin
(10−2, 10−3, 10−4,
10−5, 10−6, 10−7, 10−8,
10−9 mmol/l). The cells were then cultured for 24, 48,
and 72 h. Following the defined culture periods, the glucose
concentration of the culture media was detected using the glucose
oxidase method, according to the manufacturer’s instructions
(Nanjing Jiancheng Biological Products Co., Ltd.).
Effects of metformin on IR HUVEC
cells
The present study investigated the effect of
metformin on the function of endothelial cells using the IR
endothelial cells established as above. The cells were divided into
nine groups, each with six replicates: The negative control group,
200 μl normal medium; the model group, IR cells; and, the metformin
groups, treated with 102, 101,
100, 10−1, 10−2, 10−3
and 10−4 mol/l metformin. After 48 h of culture, 2 μl
supernatant was collected from each sample. The glucose
concentration was detected using the glucose oxidase method, the NO
content was detected using a nitrate reduction assay and the ET-1
concentrations were detected using an enzyme-linked immunosorbent
assay kit, according to the manufacturer’s instructions (Nanjing
Jiancheng Biological Products Co., Ltd.).
SPSS statistical software (version 17.0; SPSS, Inc.,
Chicago, IL, USA) was used to process the data by performing an
analysis of variance, and a least significant differences test was
conducted for pairwise comparisons between the groups. The results
were expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Effect of metformin on the expression
level of endothelial nitric oxide synthase (eNOS) in IR HUVECs
Using the optimal concentration of metformin
obtained from the above experiments (10−3 mmol/l), the
present study investigated the effect of metformin on the
expression levels of eNOS, using western blotting as previously
described (14). BandScan software
(Informer Technologies, Inc., Los Angeles, CA, USA) was used to
analyze the grayscale, and the eNOS protein expression level was
defined as the grayscale ratio of the target protein (eNOS) to the
internal reference protein (β-actin). SPSS software (version 17.0;
SPSS, Inc.) was used to perform a t-test to compare the expression
level of eNOS between the IR + agent-treated group and the IR +
agent-free group (negative control group), as well as between the
IR + agent-free group and the non-IR group (blank group). P<0.05
was considered to indicate a statistically significant
difference.
Results
Establishment of the IR endothelial cell
model
The IR model was initially established using
endothelial cells. Insulin resistance was identified by determining
the glucose concentration in the culture media using the glucose
oxidase method. Compared with the negative control group, the
glucose concentration in the insulin-treated groups (insulin,
10−4 mmol/l; glucose, 30 mmol/l; dexamethasone, 1
μmol/l) was significantly increased at 24, 48 and 72 h (P<0.01;
Table I). The results of the
present study indicate that glucose consumption was reduced and,
thus, the IR model was successfully established.
 | Table IGlucose concentration in different
endothelial cell groups (n=6; mean ± standard deviation). |
Table I
Glucose concentration in different
endothelial cell groups (n=6; mean ± standard deviation).
| Group | 24 h | 48 h | 72 h |
|---|
| Negative control | 4.19±0.66 | 3.78±0.37 | 3.11±0.64 |
| 10−2
mmol/l insulin | 4.65±0.74b | 17.44±3.68 | 29.49±0.54b |
| 10−3
mmol/l insulin | 5.10±0.49b | 19.74±3.56b | 28.15±2.28b |
| 10−4
mmol/l insulin | 5.86±0.76b | 27.58±5.71b | 33.27±1.63b |
| 10−5
mmol/l insulin | 6.36±2.46b | 22.55±5.10b | 29.218±5.76b |
| 10−6
mmol/l insulin | 7.51±1.93b | 15.48±4.87b,c | 32.23±6.08b |
| 10−7
mmol/l insulin | 6.85±1.71b | 17.38±4.20b,c | 31.81±4.54b |
| 10−8
mmol/l insulin | 4.77±2.43b | 15.96±3.30b,d | 15.46±3.90b,d |
| 10−9
mmol/l insulin | 5.93±0.58b | 19.98±2.37b,d | 19.18±5.11b,d |
Protective effect of metformin against IR
HUVEC dysfucntion
To investigate the effect of metformin on HUVECs, NO
and ET-1 concentration was measured. Compared with the negative
control group, a significant difference was identified in the media
glucose concentration of cells treated with 10−3 and
10−4 mmol/l metformin (P<0.01; Table II). This indicates that metformin
affects the rate of glucose uptake in HUVECs by increasing their
insulin sensitivity. In addition, a significant increase in NO
content was identified in the groups treated with
10−1–10−3 mmol/l metformin (P<0.01;
Table II). However, no correlation
was identified between the NO and glucose concentration (P>0.05;
Table III), indicating that
metformin may improve endothelial function directly by increasing
the content of NO, independent of changes in the glucose
concentration. Furthermore, metformin reduced the ET-1
concentration (P<0.05; Table
II). No correlation was identified between the concentration of
ET-1 and glucose (P>0.05; Table
III), indicating that metformin reduced the endothelial cell
damage by decreasing the ET-1 concentration independent of the
glucose concentration. The optimal concentration of metformin for
improving insulin resistance was 10−3 mmol/l. The
concentration of NO and ET-1 were negatively correlated (P<0.05;
Table III), indicating that NO
and ET-1 were mutual restraint factors involved in endothelial cell
metabolic processes.
 | Table IIGlucose, NO and ET-1 concentration in
endothelial cells (n=6; mean ± standard deviation). |
Table II
Glucose, NO and ET-1 concentration in
endothelial cells (n=6; mean ± standard deviation).
| Group | Glucose, mmol/l | NO, μmol/l | ET-1, % |
|---|
| Negative control | 4.08±0.51 | 112.36±1.98 | 24.56±2.97 |
| Model control | 12.97±2.05b | 39.21±2.21b | 91.56±1.86b |
| 103 mmol/l
metformin | 5.59±3.11a | 35.24±3.01 | 87.31±4.215 |
| 102 mmol/l
metformin | 5.29±1.27a | 36.84±1.56 | 90.21±3.86 |
| 101 mmol/l
metformin | 6.46±2.09a | 38.01±2.99 | 88.14±2.74 |
| 100 mmol/l
metformin | 6.86±4.33a | 39.98±1.95 | 70.63±4.01 |
| 10−1
mmol/l metformin | 5.29±1.42a | 74.01±4.35a | 36.43±3.86a |
| 10−2
mmol/l metformin | 5.55±0.85a | 78.65±3.18a | 35.27±2.94a |
| 10−3
mmol/l metformin | 5.21±2.02a | 107.53±2.23a | 26.71±1.86a |
| 10−4
mmol/l metformin | 5.75±4.15a | 40.08±4.01 | 70.16±3.96 |
 | Table IIICorrelation between glucose, NO and
ET-1 concentration in endothelial cells. |
Table III
Correlation between glucose, NO and
ET-1 concentration in endothelial cells.
| Group | NO/glucose | ET-1/glucose | NO/ET-1 |
|---|
| Negative
control | No | No | N (r=−0.61) |
| Model control | No | No | N (r=−0.67) |
| 102
mmol/l metformin | No | No | N (r=−0.69) |
| 101
mmol/l metformin | No | No | N (r=−0.63) |
| 100
mmol/l metformin | No | No | N (r=−0.59) |
| 10−1
mmol/l metformin | No | No | N (r=−0.58) |
| 10−2
mmol/l metformin | No | No | N (r=−0.51) |
| 10−3
mmol/l metformin | No | No | N (r=−0.57) |
| 10−4
mmol/l metformin | No | No | N (r=−0.56) |
| 10−5
mmol/l metformin | No | No | N (r=−0.52) |
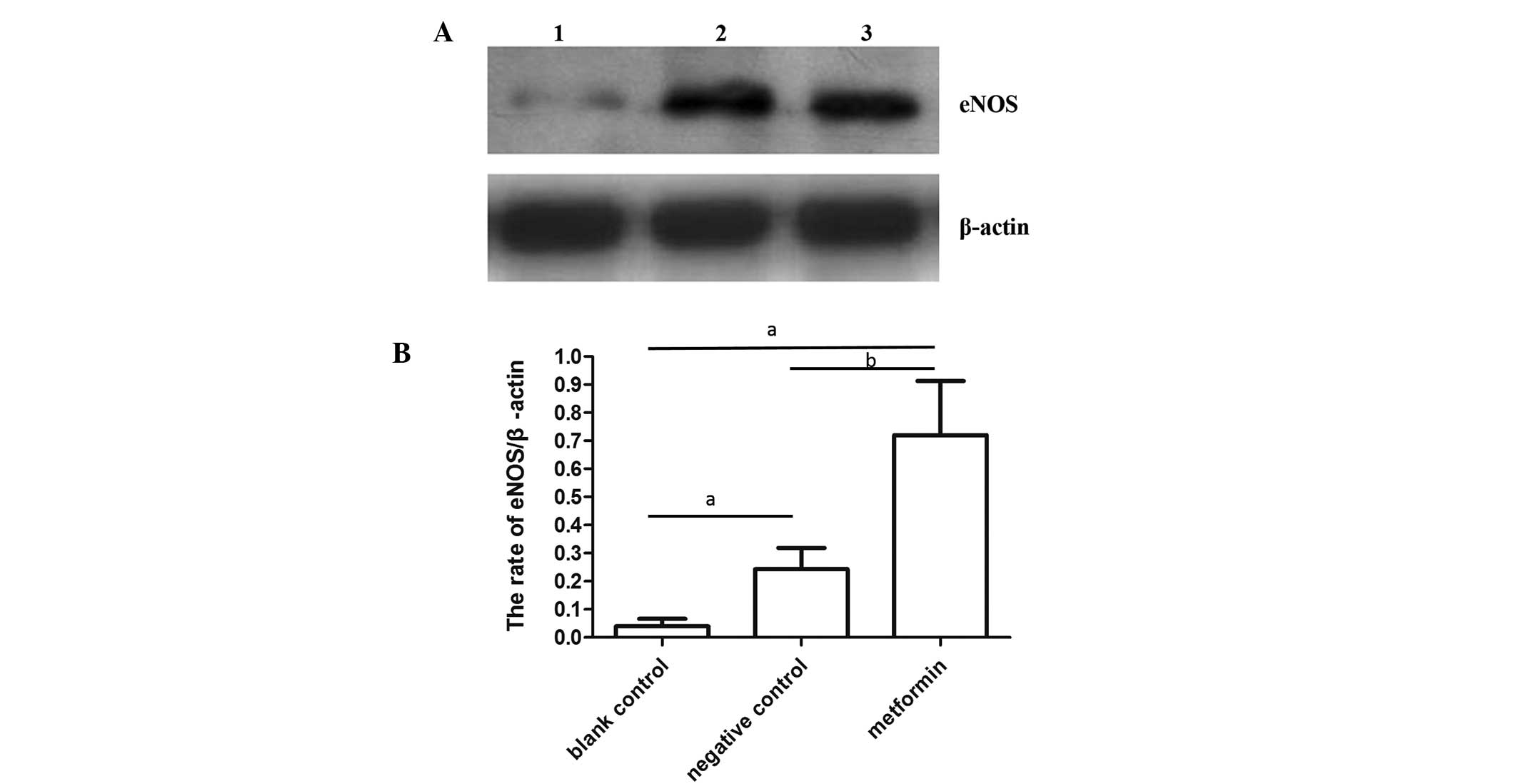
Effect of metformin on the eNOS protein
expression level of IR HUVECs
To investigate the effect of metformin on the
expression level of eNOS, western blot analyses were performed
(Fig. 1A). Administration of
metformin (10−3 mmol/l) significantly increased the
activity of eNOS when compared with the negative control (IR) and
blank control groups (P<0.01; Fig.
1B), indicating that metformin may improve endothelial function
by upregulating eNOS expression and, thus, increasing the NO
content.
Discussion
Insulin resistance is associated with endothelial
dysfunction and may be a predictor of early atherosclerosis.
However, the underlying mechanism by which insulin resistance
results in endothelial dysfunction remains controversial. The
administration of physiological concentrations of insulin has been
demonstrated to stimulate endothelial cells to produce NO, which
dilates the blood vessels and increases blood flow in IR patients.
However, the reduced insulin sensitivity results in decreased NO
production, therefore, weakening the vascular protection provided
by NO (15).
The present study established an in vitro IR
endothelial cell model and investigated the effect of metformin on
the protection of endothelial cell function. The results
demonstrated that metformin significantly improves glucose uptake
in IR endothelial cells, indicating that metformin improves
endothelial cell function. Furthermore, to investigate the impact
of metformin on endothelial cell function, NO and ET-1
concentration were determined. The optimal concentration at which
metformin protects endothelial cell function was 10−3
mmol/l. In the metformin-treated cells, the NO level was 2.74±0.42
times higher and the ET-1 concentration was 26.71±1.86% lower,
compared with the model (non-treated) group (Table II). In addition, metformin enhanced
the activity of eNOS up to 3.11±0.21 times compared with the
negative control group and 14.43±2.26 times compared with the blank
control group (Fig. 1B).
Metformin is commonly used in clinical practice as a
hypoglycemic agent, primarily for the treatment of type 2 diabetes.
Various studies have demonstrated that metformin improves the
endothelial dysfunction caused by high cholesterol. For example, in
one study, long-term use of metformin significantly reduced the
risk of stroke and myocardial infarction in patients exhibiting
hypercholesterolemia and atherosclerosis (16). Furthermore, Meaney et al
(17) reported that metformin
produced beneficial effects on nitroxidation and endothelial
function. Metformin enhanced NO metabolism, reduced CRP and caused
various protective endothelial function indices to increase, such
as advanced oxidation protein products. Furthermore, O’Hora et
al (18) demonstrated that
metformin directly stimulated NO production by the endothelium.
Endothelial cells regulate vascular tone by
releasing vasodilatory factors, such as bradykinin and NO, and
vasoconstrictor substances, such as thromboxane A2 and ET (19). Physiologically, the secretion of NO
and ET from endothelial cells remains in relatively dynamic
equilibrium. However, once endothelial cells are damaged, this
equilibrium is disrupted, resulting in various pathophysiological
consequences. Metformin may improve endothelial cell function by
increasing NO and eNOS levels and reducing the concentration of
ET-1, to further protect the endothelial cells against
atherosclerosis. However, other studies have demonstrated that
metformin improves endothelial cell function by inhibiting the
expression of endothelial cell angiotensin II type 1 receptor
(17) protecting vascular
endothelial function and reducing cardiovascular events in patients
with diabetes (20).
In conclusion, the present study demonstrated that
metformin enhances endothelial function. Thus, enhancing
endothelial function may be one of the mechanisms responsible for
the protective effect of metformin in reducing cardiovascular
complications in type 2 diabetes. Future in vitro studies
are required to investigate the specific pathway that is activated
by metformin.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar
|
|
2
|
Caprio S: 8th annual world congress on
insulin resistance, diabetes, and cardiovascular disease, November
4–6, 2010. Pediatr Endocrinol Rev. 8:400–402. 2011.PubMed/NCBI
|
|
3
|
Eyre H, Kahn R, Robertson RM, et al:
American Cancer Society, the American Diabetes Association, and the
American Heart Association Collaborative Writing Committee:
Preventing cancer, cardiovascular disease, and diabetes: a common
agenda for the American Cancer Society, the American Diabetes
Association, and the American Heart Association. Diabetes Care.
27:1812–1824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rizza S, Muniyappa R, Iantorno M, et al:
Citrus polyphenol hesperidin stimulates production of nitric oxide
in endothelial cells while improving endothelial function and
reducing inflammatory markers in patients with metabolic syndrome.
J Clin Endocrinol Metab. 96:E782–E792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brunner EJ, Kivimäki M, Witte DR, et al:
Inflammation, insulin resistance, and diabetes - Mendelian
randomization using CRP haplotypes points upstream. PLoS Med.
5:e1552008. View Article : Google Scholar
|
|
6
|
Siednienko J, Nowak J, Moynagh PN and
Gorczyca WA: Nitric oxide affects IL-6 expression in human
peripheral blood mononuclear cells involving cGMP-dependent
modulation of NF-KB activity. Cytokine. 54:282–288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kataoka Y, Shao M, Wolski K, et al:
Multiple risk factor intervention and progression of coronary
atherosclerosis in patients with type 2 diabetes mellitus. Eur J
Prev Cardiol. 20:209–217. 2013. View Article : Google Scholar
|
|
8
|
Li XM, Li Y, Zhang NN, Xie YH and Shi YQ:
Combination therapy with metformin and fenofibrate for insulin
resistance in obesity. J Int Med Res. 39:1876–1882. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed. Preliminary criteria
for the classification of systemic sclerosis (scleroderma).
Subcommittee for scleroderma criteria of the American Rheumatism
Association Diagnostic and Therapeutic Criteria Committee.
Arthritis Rheum. 23:581–590. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kadoglou NP, Tsanikidis H, Kapelouzou A,
et al: Effects of rosiglitazone and metformin treatment on apelin,
visfatin, and ghrelin levels in patients with type 2 diabetes
mellitus. Metabolism. 59:373–379. 2010. View Article : Google Scholar
|
|
11
|
Pansuria M, Xi H, Li L, Yang XF and Wang
H: Insulin resistance, metabolic stress, and atherosclerosis. Front
Biosci (Schol Ed). 4:916–931. 2012. View
Article : Google Scholar
|
|
12
|
Versari D, Daghini E, Virdis A, et al:
Endothelial dysfunction as a target for prevention of
cardiovascular disease. Diabetes Care. 32(Suppl 2): S314–S321.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto T, Noguchi E, Ishida K,
Kobayashi T, Yamada N and Kamata K: Metformin normalizes
endothelial function by suppressing vasoconstrictor prostanoids in
mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am
J Physiol Heart Circ Physiol. 295:H1165–H1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng G, Nystrom FH, Ravichandran LV, et
al: Roles for insulin receptor, PI3-kinase, and Akt in
insulin-signaling pathways related to production of nitric oxide in
human vascular endothelial cells. Circulation. 101:1539–1545. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li JL, Yang Z, Wu S and Kong J:
Relationship between endothelial nitric oxide synthase, insulin
resistance and macrovascular disease in patients with acute
myocardial infarction. J Int Med Res. 40:687–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tousková V and Haluzík M: Insulin
resistance and nitric oxide: molecular mechanisms and
pathophysiological associations. Cesk Fysiol. 60:40–47. 2011.(In
Czech).
|
|
17
|
Meaney E, Vela A, Samaniego V, et al:
Metformin, arterial function, intima-media thickness and
nitroxidation in metabolic syndrome: the mefisto study. Clin Exp
Pharmacol Physiol. 35:895–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O’Hora TR, Markos F, Wiernsperger NF and
Noble MI: Metformin causes nitric oxide-mediated dilatation in a
shorter time than insulin in the iliac artery of the anesthetized
pig. J Cardiovasc Pharmacol. 59:182–187. 2012. View Article : Google Scholar
|
|
19
|
Kolovou G and Giannakopoulou V:
Endothelial nitric oxide synthase gene variants and coronary heart
disease. Angiology. 63:84–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malin SK, Nightingale J, Choi SE, Chipkin
SR and Braun B: Metformin modifies the exercise training effects on
risk factors for cardiovascular disease in impaired glucose
tolerant adults. Obesity (Silver Spring). 21:93–100. 2013.
View Article : Google Scholar
|