Introduction
Nasopharyngeal carcinoma (NPC) is a highly invasive
malignant tumor, which occurs in the nasopharynx. Although it is
rare in the majority of countries (1), the incidence and mortality rates of
NPC are markedly high among Southern Chinese populations. The
five-year survival rate following combined treatment with
radiotherapy and adjuvant cisplatin chemotherapy is 50–60% and the
rates of five-year cumulative local relapse and distant metastasis
are 20–30 and 20–25%, respectively (2). Therefore, investigating distant
metastasis in NPC, and minimizing the occurrence of distant
metastasis have become key to further improve the efficacy of NPC
treatment and, thus, have important practical significance. The
KAI1/CD82 gene, which belongs to the transmembrane 4 superfamily
(TM4SF), was identified by Dong et al (3) in 1995. The inhibitory effects of the
TM4SF protein on tumor metastasis have been demonstrated (4); its cell-membrane location and
extensive glycosylation leads to cell-cell and cell-extracellular
matrix interactions, which subsequently affect tumor metastasis.
These interactions are extremely important with regard to the
invasion and metastasis of tumors. In this study,
immunohistochemistry and western blot analysis were used to detect
the levels of KAI1/CD82 protein expression in five different human
NPC cell lines, CNE-1, CNE-2Z, SUNE-1, SUNE-1-5-8F and SUNE
-1-6-10B, and immunohistochemistry was also performed to detect the
KAI1/CD82 protein expression in NPC and non-neoplastic
nasopharyngeal tissues. The association between abnormal KAI1/CD82
gene expression in NPC and patient age, gender, histological type,
T staging and lymph node staging were analyzed. The aim of this
study was to investigate novel methods, which may improve the
treatment efficacy and the prognosis, as well as reduce the
occurrence of metastasis.
Materials and methods
Specimens
The human NPC cell lines, CNE-1, CNE-2Z, SUNE-1,
SUNE-1-5-8F and SUNE -1-6-10B, with various metastatic
characteristics were purchased from Hefei Shengmai Reagent Co., Ltd
(Hefei, China). The detailed metastatic characteristics and levels
of differentiation are shown in Table
I.
 | Table IDifferentiation status and metastatic
characteristics of the five cell lines used in this study. |
Table I
Differentiation status and metastatic
characteristics of the five cell lines used in this study.
| Cell line | Level of
differentiation | Metastatic
characteristics |
|---|
| SUNE-1 | Poorly
differentiated | SqCa high |
| CEN-2Z | Poorly
differentiated | SqCa high |
| SUNE-1-5-8F | Poorly
differentiated | SqCa extremely
high |
| SUNE-1-6-10B | Poorly
differentiated | SqCa low |
| CEN-1 | Highly
differentiated | SqCa middle |
A total of 70 archived paraffin-embedded NPC
specimens were obtained from the Department of Pathology. The First
Affiliated Hospital of Bengbu Medical College (Bengbu, China)
between February 2007 and August 2010. The clinical data of all
patients were complete and no patients had received radiotherapy or
chemotherapy prior to biopsy. In addition, 30 archived
paraffin-embedded non-neoplastic nasopharyngeal tissue specimens
served as the control group, which were all samples of
nasopharyngeal chronic mucosal inflammation, with or without the
lymphoid hyperplasia. This study was conducted in accordance with
the Declaration of Helsinki and with approval from the ethics
committee of The First Affiliated Hospital of Bengbu Medical
College. Written informed consent was obtained from all
participants.
Immunohistochemical detection of
KAI1/CD82 expression in human NPC cell lines
After anabiosis, medium-changing and three passages,
the five NPC cell lines were seeded in the
pre-sterile-coverslip-paved six-well plates at a density of
~5×104 cells/ml. Following incubation at 37°C in an
atmosphere of 5% CO2 for 48 h, the coverslips were
removed and immunohistochemical staining with Histostain™-Plus kits
(Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China) was
performed at room temperature. The appearance of brown granules on
the cell surface and inside the cytoplasm was considered to
indicate positive KAI1/CD82 expression. A total of four
cell-attached coverslips of the KAI1/CD82 protein from each cell
line were randomly selected, and 500 cells in each cell-attached
coverslip were randomly selected under a microscope (BX50; Olympus,
Tokyo, Japan; magnification, ×400); the total number of cells in
the four cell-attached coverslips was 2,000. The number of positive
cells among the 500 randomly selected cells in each of the above
four cell-attached coverslips was counted to calculate the positive
KAI1/CD82 expression rate, with the following formula: Positive
rate of KAI1/CD82 expression (%) = number of positive cells / total
number of cells counted. According to the number of cells and the
percentage of positive cells, the results were classified into four
grades: No expression (−), no positive cells in the cell-attached
coverslip; low expression (+), >0% and <24% positive cells;
moderate expression (++), 25–50% positive cells; and high
expression (+++), >50% positive cells.
The χ2 test was used to compare the
positive rates of KAI1/CD82 protein among the cell line with the
lowest metastatic potential (SUNE-1-6-10B) and the other cell lines
with higher metastatic potentials.
Western blot analysis
A total of 1×107 cells of each of the
five NPC cell lines, which were wall-adherent, were collected.
Next, 200 μl cell lysate was added for the 30 min lysis on ice,
followed by centrifugation at 2,862 × g for 30 min at 4°C (5810R,
Eppendorf, Hamburg, Germany). The supernatant was obtained, and the
protein concentration was determined using the Coomassie Brilliant
Blue assay (G-250, Beijing Zhongshan Biotechnology Co., Ltd.). The
protein concentration was then adjusted to 50 μg/μl. Next, 10%
SDS-PAGE electrophoresis was performed for 3 h to separate the
proteins, which were then transferred to nitrocellulose membranes.
After three washes with phosphate buffered-saline (PBS; Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China) for 15
min each, l% bovine serum albumin (Fuzhou Maixin Biotechnology
Development Co., Ltd.) was used to block non-specific antigen
activity for 2 h. After blocking, the membranes were incubated with
mouse anti-human KAI1/CD82 monoclonal primary antibody (l:250;
sc-17752; Santa Cruz Biotechnology, Inc., CA, USA) overnight at
4°C, followed by washing with PBS. The alkaline goat anti-rat
polyclonal phosphatase-labeled secondary antibody (l:200; PV6002,
Beijing Zhongshan Biotechnology Co., Ltd.; general-type kit; Fuzhou
Maixin Biotechnology Development Co., Ltd.) was added, followed by
incubation at room temperature for 2 h. NBT/BCIP coloration was
conducted for 15 min (kits were provided by Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China). When clear
brown bands, which indicate positive staining, were observed on the
membranes, the coloration was terminated, followed by rinsing,
drying and preservation of the membranes.
Immunohistochemical detection of
KAI1/CD82 protein expression
All NPC and non-neoplastic nasopharyngeal tissue
specimens were fixed in 10% formalin, embedded in paraffin and cut
into 4 μm-thick serial sections. After dewaxing and hydration,
antigen retrieval in potassium citrate buffer was performed on the
sections using a microwave. Horseradish peroxidase (Fuzhou Maxim
Bioengineering Co. Ltd., Fuzhou, China) was then added to label the
avidin, followed by staining with 3,3′-Dimethylbenzidine (Fuzhou
Maixin Biotechnology Development Co., Ltd.). After rinsing with
water, the specimens were re-stained with hematoxylin (Fuzhou
Maixin Biotechnology Development Co., Ltd.), followed by an
additional rinse with water. The specimens were then dehydrated in
ethanol and cleared in xylene (Wuxi Jingke Chemical Co., Ltd.,
Wuxi, China), followed by mounting with neutral gum (Fuzhou Maixin
Biotechnology Development Co., Ltd.). PBS was used to replace the
primary antibody for the blank control group. The nasopharyngeal
tissues were observed under a microscope (BX50; Olympus), and the
appearance of brown particles in the cytoplasm was considered to
indicate positive expression. A total of four different views at
high magnification were performed to count 100 cells in each view,
which were divided into (+) to (+++) according to the percentage of
positive cells, which was scored as follows: no positive cells (−);
1–9% positive cells (+); 10–50% positive cells (++); >50%
positive cells (+++). The (+) to (+++) scores were classified as
positive expression, while (−) score was considered as negative
expression.
Statistical analysis
The western blotting data were analyzed using
one-factor analysis of variance and the immunohistochemistry data
were analyzed by the χ2 test. All data were analyzed
using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA)
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Immunohistochemical detection of
KAI1/CD82 protein levels in the five NPC cell lines
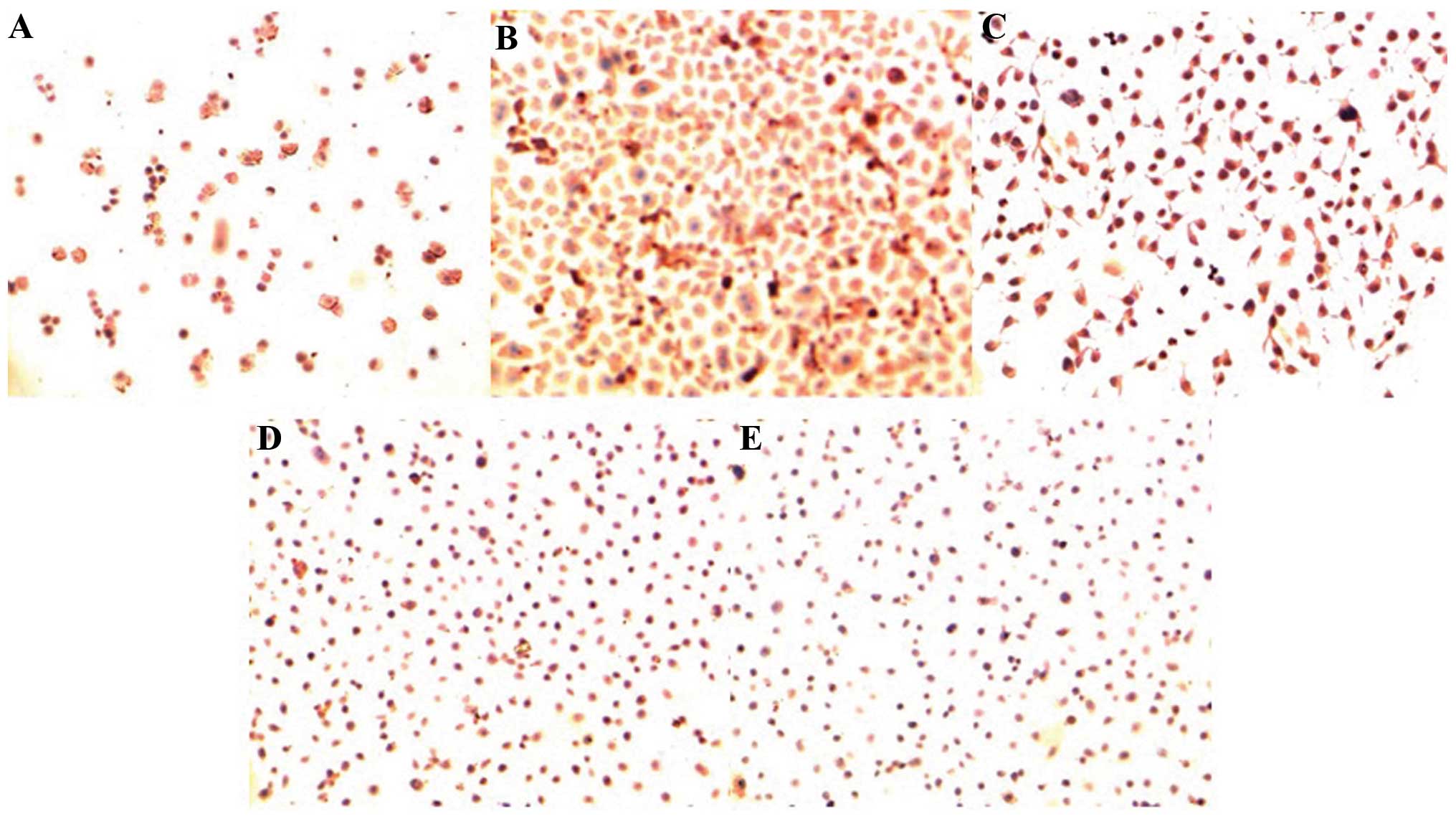
The immunohistochemical results showed that the
KAI1/CD82 protein was significantly expressed in the membrane
and/or cytoplasm of all five NPC cell lines, with the positive
signal of brown particles. The results revealed that the KAI1/CD82
protein was located in the membrane and/or cytoplasm in all five
cell lines. KAI1/CD82 was highly expressed in the cytoplasm and
membrane of the SUNE-1-6-10B cell line (tumorigenesis and low
metastatic potential), while low expression was exhibited in the
cytoplasm and membrane of the SUNE-1-5-8F cell line (high
tumorigenesis and metastatic potential). In the remaining cell
lines, the expression varied (Fig.
1). The positive rate of KAI1/CD82 protein expression in each
cell line was calculated and the χ2 test was performed,
which revealed that the positive expression rate of KAI1/CD82
protein in the SUNE-1-6-10B cells (tumorigenesis and low metastatic
potential) was significantly higher than that in the cell lines
with a higher metastatic potential (P<0.01).
SPSS 17.0 software was used to perform the
completely randomized-design χ2 test, which revealed
that the positive expression rate in the SUNE-1-6-10B cells was
significantly different when compared with the other groups
(P<0.05) (Table II).
Furthermore a significant difference in expression rate was
identified between the CNE-1 and SUNE-1 cell lines, and between the
CNE-2Z and SUNE-1-5-8F cell lines (P<0.05), while no significant
difference was identified between the SUNE-1 and CNE-2Z cell lines
(P>0.05).
 | Table IIComparison of KAI1/CD82 protein
positive expression rates in five cell lines. |
Table II
Comparison of KAI1/CD82 protein
positive expression rates in five cell lines.
| Cell line | Cells, n | Positive cells,
n | Positive rate
(%)a | χ2 | P-value |
|---|
| SUNE-1-6-10B | 2000 | 1410 | 70.5 | - | - |
| CNE-1a | 2000 | 814 | 40.7 | 459 |
4.89×10−83 |
| CNE-2Za | 2000 | 803 | 40.2 | 467 |
1.29×10−101 |
| SUNE-1a | 2000 | 736 | 36.8 | 526 |
6.77×10−100 |
| SUNE-1-5-8Fa | 2000 | 394 | 19.7 | 1196 |
6.41×10−248 |
Western blot analysis of KAI1/CD82
protein levels in the five NPC cell lines
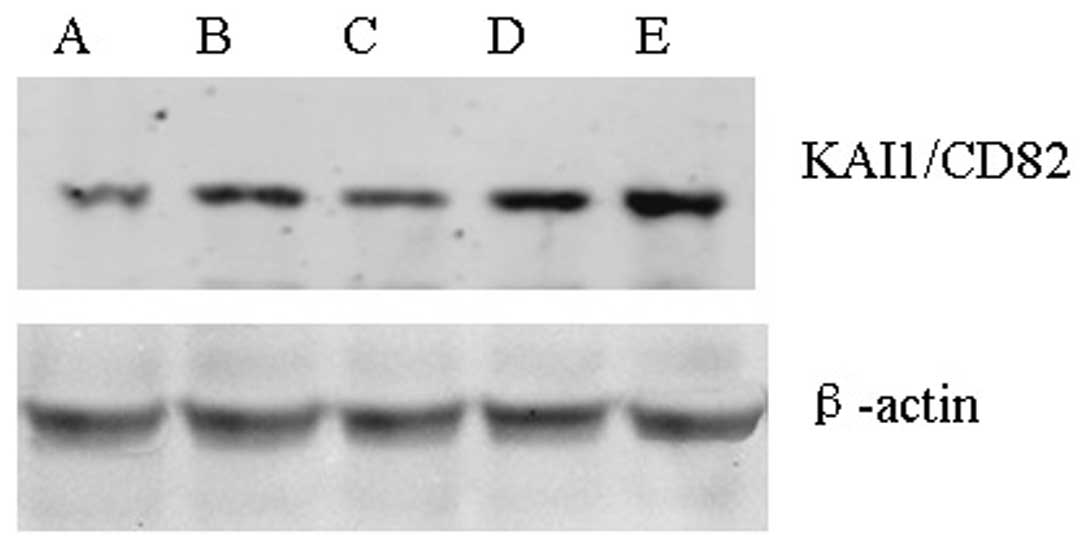
Western blot analysis revealed that the KAI1/CD82
protein expression levels differed in the five NPC cell lines
(Fig. 2). β-actin was used as the
internal reference. The results revealed that compared with the
SUNE-1-5-8F cell line, the protein expression of KAI1/CD82 in the
SUNE-1-6-10B and CNE-1 cell lines was significantly increased
(P<0.05) (Table III).
 | Table IIIExpression level changes of KAI1/CD82
gene in different human nasopharyngeal carcinoma cell lines. |
Table III
Expression level changes of KAI1/CD82
gene in different human nasopharyngeal carcinoma cell lines.
| Group | n | ODa |
|---|
| SUNE-1-6-10B | 3 | 1594+13.95 |
| CNE-1 | 3 | 1453+11.34 |
| CNE-2Z | 3 | 1326+11.78 |
| SUNE-1 | 3 | 1314+9.09 |
| SUNE-1-5-8F | 3 | 1245+10.42 |
SPSS 17.0 software was used to perform the LEVENE
homogeneity of variance test, the results of which were F=0.485 and
P=0.747 (P>0.05), indicating that the data of the five groups
exhibited homogeneity of variance. Furthermore, the completely
randomized-design analysis of variance results were P=0.000
(P<0.05) and F=315.775; therefore, it was considered in general
that the data obtained from the five groups were different. The
pairwise comparison with least significant difference and
Student-Newman-Keuls methods identified no significant difference
in the protein expression between the CNE-2Z and SUNE-1 cell lines
(P=0.195 and P>0.05, respectively); however, the pairwise
comparisons between the other groups identified a significant
difference (P<0.05).
Expression of the KAI1/CD82 protein in
NPC and non-neoplastic nasopharyngeal tissues
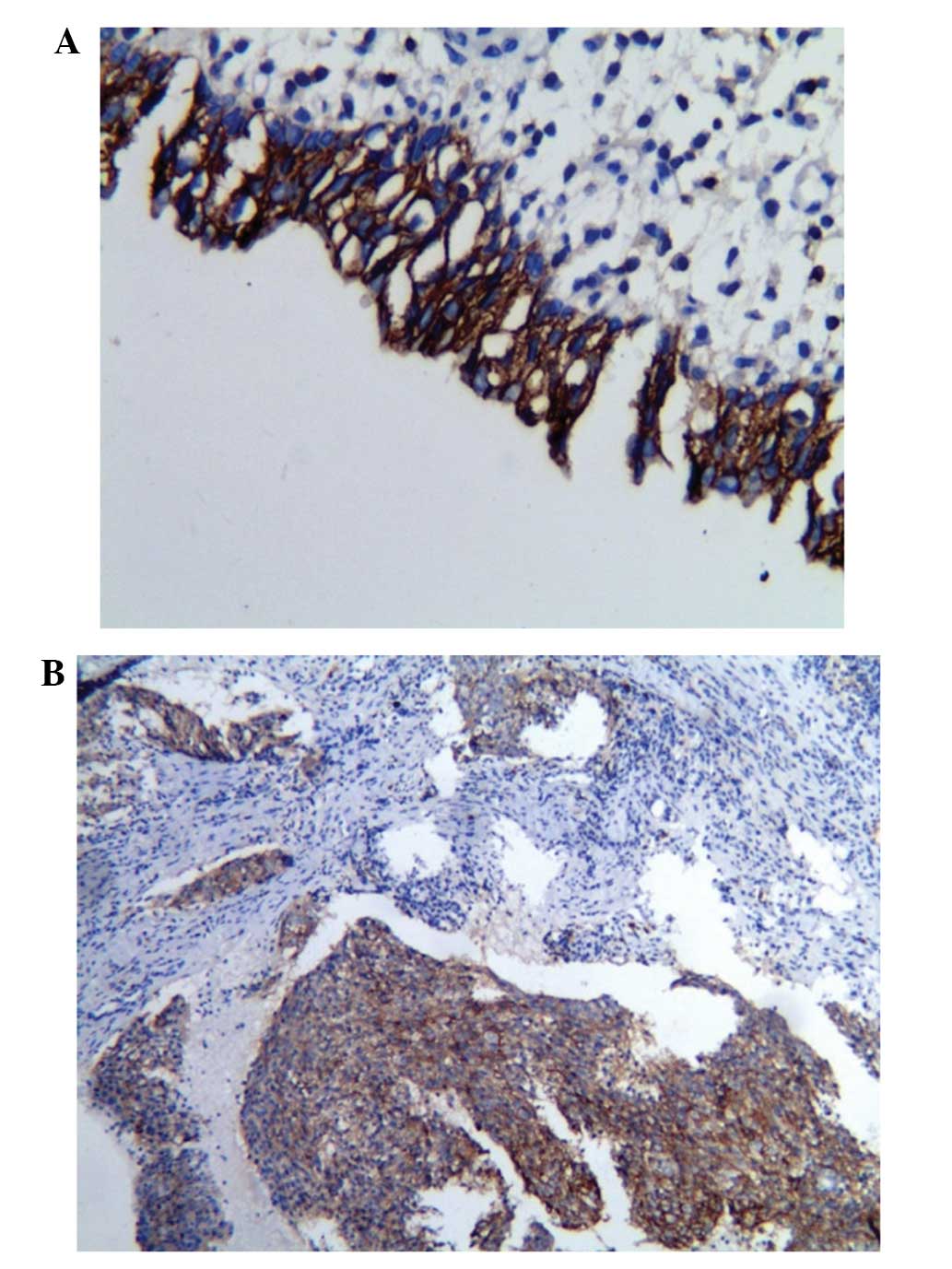
The immunohistochemical detection of the KAI1/CD82
protein expression in the two groups revealed that the positive
expression rate of the KAI1/CD82 protein in the non-neoplastic
nasopharyngeal tissues (21/30; 70.0%) was significantly higher than
that of NPC group (31/70; 44.3%), and the χ2 test
revealed a statistically significant difference in KAI1/CD82
protein expression between the two groups (Fig. 3) (P<0.05).
Association between KAI1/CD82 protein
expression and clinical factors
The expression of KAI1/CD82 protein in NPC was not
found to correlate with the clinical characteristics of patients,
including age, histological type and T staging, which was
determined according to the International Union Against Cancer’s
(UICC) TNM staging system (5).
However, the expression of KAI1/CD82 protein expression was found
to correlate with lymph node metastasis. The positive expression
rate of KAI1/CD82 protein in patients without lymph node metastasis
(N0), according to the UICC TNM staging system (5), was 68.4% (13/19), which was higher
than that (35.3%, 18/51) in the patients with the cervical lymph
node metastasis (N1–3), and this difference was
statistically significant (P<0.05). With increased N staging,
the KAI1/CD82 protein expression decreased (N0, 68.4%;
N1, 43.8%; N2, 33.3%; and N3,
25.0%) (Table IV).
 | Table IVAssociation between KAI1/CD82 protein
expression and patient clinical parameters. |
Table IV
Association between KAI1/CD82 protein
expression and patient clinical parameters.
| | KAI-1/CD82 protein
expression | | |
|---|
| |
| | |
|---|
| Clinical
parameter | n (%) | + | − | χ2 | P-value |
|---|
| Gender | | | | 0.320 | 0.572 |
| Male | 32 (45.7) | 13 | 19 | | |
| Female | 38 (54.3) | 18 | 20 | | |
| Age, years | | | | 0.170 | 0.681 |
| ≤50 | 41 (58.6) | 19 | 22 | | |
| >50 | 29 (41.4) | 12 | 17 | | |
| Pathological
type | | | | 0.854 | 1.000 |
| Squamous cell
carcinoma | 69 (98.6) | 32 | 37 | | |
|
Adenocarcinoma | 1 (1.4) | 0 | 1 | | |
| T staging | | | | 0.797 | 0.372 |
| T1–T2 | 21 (30.0) | 11 | 10 | | |
| T3–T4 | 49 (70.0) | 20 | 29 | | |
| N staging | | | | 6.157 | 0.013 |
| N0 | 19 (27.1) | 13 | 6 | | |
| N1 | 16 (22.9) | 7 | 9 | | |
| N2 | 27 (38.6) | 9 | 18 | | |
| N3 | 8 (11.4) | 2 | 6 | | |
Discussion
Metastasis refers to the process by which malignant
cells detach from the primary tumor and migrate to a secondary
tissue or organ via a variety of mechanisms. The cells continue to
proliferate and grow, finally forming a secondary tumor that
exhibits the same features as the primary tumor. Metastasis is one
of the basic biological characteristics of malignant tumors, and
the reason for the failure of treatment in the majority of cancer
patients. Therefore, controlling metastasis is the key to improving
the prognosis of cancer patients (6).
The KAI1/CD82 gene is located on the human
chromosome 11p11.2 (3), which
consists of 10 exons and nine introns (~80 kb) (3). The structure of the product of the
KAI1 gene is the same as CD82 and, thus, KAI1 is a member of the
TM4SF family. The KAI1/CD82 gene exhibits an inhibitory function
with regard to tumor metastasis, and this inhibition has been
confirmed in a number of studies investigating malignant cancer
(7–10). However, this inhibitory mechanism
remains unclear, and previous studies have shown that multiple
factors are involved, which may exert effects on their own or in
combination, including the integrin family (11), epidermal growth factor receptor
(12), EW12/PGRL (13), KITENIN (14,15)
and protein kinase C (16).
An initial study indicated that the KAI1/CD82 gene
exhibited the inhibition specifically towards the metastasis of
prostate cancer (17); however,
later studies have revealed that the inactivation of this gene
occurs in a number of other malignant tumors, including thyroid
(14), breast (18–20),
endometrial (16), laryngeal
(21), colon (22), gastric (7,23),
gallbladder (24), liver (25), kidney (26), bladder (27) and prostate (28) cancer. In addition, these studies
also demonstrated that the inactivation of the KAI1/CD82 gene was
associated with tumor metastasis.
Although the KAI1/CD82 gene has been reported to be
involved in numerous other cancer types, its involvement in NPC is
largely unclear. In the present study, immunohistochemistry and
western blot analysis were performed to investigate the expression
levels of the KAI1/CD82 gene in five different NPC cell lines,
which exhibited different metastatic characteristics. The KAI1/CD82
protein levels were found to correlate with the metastatic
characteristics of the NPC cell lines. The positive expression rate
of KAI1/CD82 in NPC was lower than that in the normal
nasopharyngeal tissues, indicating that the KAI1/CD82 gene may be
involved in the occurrence and development of NPC. In NPC, the
underexpression of the KAI1/CD82 protein was found to correlate
with lymph node metastasis. Furthermore, with the progression of N
staging, the expression rate of KAI1/CD82 protein was found to
gradually decline, indicating that the underexpression of KAI1/CD82
protein was associated with NPC metastasis, and that KAI1/CD82 may
be involved in the metastasis of NPC.
Controlling tumor metastasis is a major focus of
cancer research; tumor metastasis is the main reason for treatment
failure and patient mortality, and there are currently difficulties
with regard to the treatment of malignant cancer. Therefore, future
studies investigating the tumor metastasis-related genes are
required to improve the efficacy of tumor treatment. Since the
follow-up period in the present study was short, the association
between KAI1/CD82 gene expression and the treatment and prognosis
of NPC was not analyzed. Further study is required to elucidate the
true association between KAI1/CD82 expression and the biological
behaviors of NPC.
References
|
1
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siddique MA, Sabur MA, Kundu SC, et al:
Difficulty in diagnosis of nasopharyngeal carcinoma. Mymensingh Med
J. 21:158–161. 2012.PubMed/NCBI
|
|
3
|
Dong JT, Lamb PW, Rinker-Schaefer CW, et
al: KAI-1, a metastasis suppressor gene for prostate cancer on
human chromosome 11p11.2. Science. 268:884–886. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takeda T, Hattori N, Tokuhara T, et al:
Adenoviral transduction of MRP-1/CD9 and KAI-1/CD82 inhibits lymph
node metastasis in orthotropic lung cancer model. Cancer Res.
67:1744–1749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teo PM, Leung SF, Yu P, Tsao SY, Foo W and
Shiu W: A comparison of Ho’s, International Union Against Cancer,
and American Joint Committee stage classifications for
nasopharyngeal carcinoma. Cancer. 67:434–439. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brierly JD, Catton PA, O’Sullivan B, et
al: Accuracy of recorded tumor, node, and metastasis stage in a
comprehensive cancer center. J Clin Oncol. 20:413–419. 2002.
View Article : Google Scholar
|
|
7
|
Tsutsumi S, Shimura T, Morinaga N, et al:
Loss of KAI-1 expression in gastric cancer. Hepatogastroenterology.
52:281–284. 2005.PubMed/NCBI
|
|
8
|
Yang X, Wei LL, Tang C, Slack R, Mueller S
and Lippman ME: Overexpression of KAI1 suppresses in vitro
invasiveness and in vivo metastasis in breast cancer cells. Cancer
Res. 61:5284–5288. 2001.PubMed/NCBI
|
|
9
|
Huang CI, Kohno N, Ogawa E, Adachi M, Taki
T and Miyake M: Correlation of reduction in MRP-l/CD9 and KAIl/CD82
expression with recurrences in breast cancer patients. Am J Pathol.
153:973–983. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takaoka A, Hinoda Y, Sato S, Itoh F,
Adachi M, Hareyama M and Imai K: Reduced invasive and metastatic
potentials of KAI-1 transfected melanoma cells. Jpn J Cancer Res.
89:397–404. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He B, Liu L, Cook GA, et al: Tetraspanin
CD82 attenuates cellular morphogenesis through down-regulating
integrin alpha6-mediated cell adhesion. J Biol Chem. 280:3346–3354.
2005. View Article : Google Scholar
|
|
12
|
Charrin S, Manie S, Oualid M, et al:
Differential stability of tetraspanin/tetraspanin interactions:
role of palmitoylation. FEBS Lett. 516:139–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou B, Liu L, Reddivari M and Zhang XA:
The palmitoylation of metastasis suppressor KAI-1/CD 82 is
important for its motility-and invasiveness-inhibitory activity.
Cancer Res. 64:7455–7463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito Y, Yoshida H, Uruno T, et al: KAI-1
expression in tlyroidneoplasmg: its linkage with clinic opathologic
features in papillary carcinoma. Pathol Res Pract. 199:79–83. 2003.
View Article : Google Scholar
|
|
15
|
Chen Z, Mustafa T, Trojanowicz B, et al:
CD82 and CD63 in thyroid cancer. Int J Mol Med. 14:517–527.
2004.PubMed/NCBI
|
|
16
|
Liu FS, Dong JT, Chen JT, et al: KAI1
metastasis suppressor protein is down-regulated during the
progression of human endometrial cancer. Clin Cancer Res.
9:1393–1398. 2003.PubMed/NCBI
|
|
17
|
Lijovic M, Somers G and Frauman AG:
KAIl/CD82 Protein expression in primary prostate cancer and in BPH
associated with cancer. Cancer Detect Prev. 26:69–77. 2002.
View Article : Google Scholar
|
|
18
|
Christgen M, Bruchhardt H, Ballmaier M, et
al: KAI-1/CD82 is a novel target of estrogen receptor-mediated gene
repression and down regulated in primary human breast cancer. Int J
Cancer. 123:2239–2246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Christgen M, Christgen H, Heil C, et al:
Expression of KAI1/CD82 in distant metastases from estrogen
receptor-negative breast cancer, Inhibitory effect of KAI-1 gene on
breast cancer cell growth in vitro. Cancer Sci. 100:1767–1771.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malik FA, Sanders AJ, Kayani MA and Jiang
WG: Effect of expressional alteration of KAI-1 on breast cancer
cell growth, adhesion, migration and invasion. Cancer Genomics
Proteomics. 6:205–213. 2009.PubMed/NCBI
|
|
21
|
Zhang BH, Liu W, Li L, et al: KAI1/CD82
and MRP1/CD9 serve as markers of infiltration, metastasis, and
prognosis in laryngeal squamous cell carcinomas. Asian Pac J Cancer
Prev. 14:3521–3526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma ZB, Li K, Wang J and Guo GH: Role of
KAI1/CD82 polymorphisms in colon cancer risk in Han Chinese
population. Med Oncol. 30:668–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knoener M, Krech T, Puls F, et al: Limited
value of KAI1/CD82 protein expression as a prognostic marker in
human gastric cancer. Dis Markers. 32:337–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang WX, Song BG and Wang PJ: Expression
of nm23, KAI1 and spiral computed tomography findings in primary
gallbladder carcinoma. Chin Med J. 122:2666–2668. 2009.PubMed/NCBI
|
|
25
|
Guo C, Liu QG, Zhang L, Song T and Yang X:
Expression and clinical significance of p53, JunB and KAI1/CD82 in
human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
8:389–396. 2009.PubMed/NCBI
|
|
26
|
Yusenko MV and Kovacs G: Identifying CD82
(KAI1) as a marker for human chromophobe renal cell carcinoma.
Histopathology. 55:687–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
You J, Madigan MC, Rowe A, et al: An
inverse relationship between KAI1 expression, invasive ability, and
MMP-2 expression and activity in bladder cancer cell lines. Urol
Oncol. 9:1324–1330. 2010.
|
|
28
|
Lee HA, Park I, Byun HJ, et al: Metastasis
suppressor KAI1/CD82 attenuates the matrix adhesion of human
prostate cancer cells by suppressing fibronectin expression and β1
integrin activation. Cell Physiol Biochem. 27:575–586. 2011.
View Article : Google Scholar
|