Introduction
Choriocarcinoma is a malignant disease characterized
by abnormal trophoblastic hyperplasia and anaplasia, absence of
chorionic villi, hemorrhage, and necrosis. Approximately 25% of
choriocarcinoma cases follow abortion or tubal pregnancy, 25% are
associated with term or preterm gestation, and the remaining 50%
arise from hydatidiform moles, however, few hydatidiform moles are
estimated to progress to choriocarcinoma. Primary gestational
choriocarcinoma is identified in the uterus in cases of atypical
genital bleeding; however, gestational choriocarcinoma that
primarily occurs outside the uterus is rare. Primary lesions have
previously been detected in the ovary, fallopian tube, omentum,
lung and liver (1–5). Choriocarcinoma may present with various
symptoms depending on the disease site. For example, pulmonary
lesions may induce cough and hemoptysis, whereas brain lesions may
induce headaches and visual impairment. Clinical symptoms similar
to those of an ectopic pregnancy, including abdominal pain and
intra-abdominal bleeding, may develop when a primary lesion is
present in the abdominal cavity outside the uterus (1,2,4,5).
Conversely, the lung, liver, brain, vagina and digestive tract are
frequently metastasized by gestational choriocarcinoma that
primarily developed in the uterus (6). In the current patient, the primary
lesion was present in the uterus and metastasized to the uterine
serosa, which is a very rare metastatic site. To the best of our
knowledge, this is the first study to describe gestational
choriocarcinoma with uterine serosal metastasis. Written informed
consent was obtained from the patient.
Case report
The patient was a 30-year-old female, gravida 2 and
para 1. Her previous and familial medical histories were
unremarkable. The patient was diagnosed with missed abortion and
treated with intra-uterine curettage at 10 weeks of gestation by a
physician. Fetal components were macroscopically observed in the
uterine content, and no hydatidiform mole was present. A small
volume of genital bleeding had persisted intermittently.
The patient presented to the Department of
Obstetrics and Gynecology at Jichi Medical University, Japan for
the first time three months after intra-uterine curettage with a
positive result on a commercial pregnancy test and abdominal pain.
Based on a pelvic examination and transvaginal ultrasonography,
missed abortion in early pregnancy was considered, as a gestational
sac and fetus were not detected. Since her abdominal pain was very
mild, a closer observation was selected. No ultrasound findings
suggestive of gestational trophoblastic neoplasia (GTN) were noted
at that time. The patient re-visited our department two days later
as her abdominal pain and bleeding had exacerbated. Transvaginal
ultrasonography revealed that no gestational sac was present in the
uterus, and blood clots were retained in the vesico-uterine and the
pouch of Douglas, for which emergency surgery was performed on the
same day due to a suspected ruptured ectopic pregnancy. The
patient's serum human chorionic gonadotropin (hCG) level was 12,000
mIU/ml. Laparotomy revealed a large blood clot in the pouch of
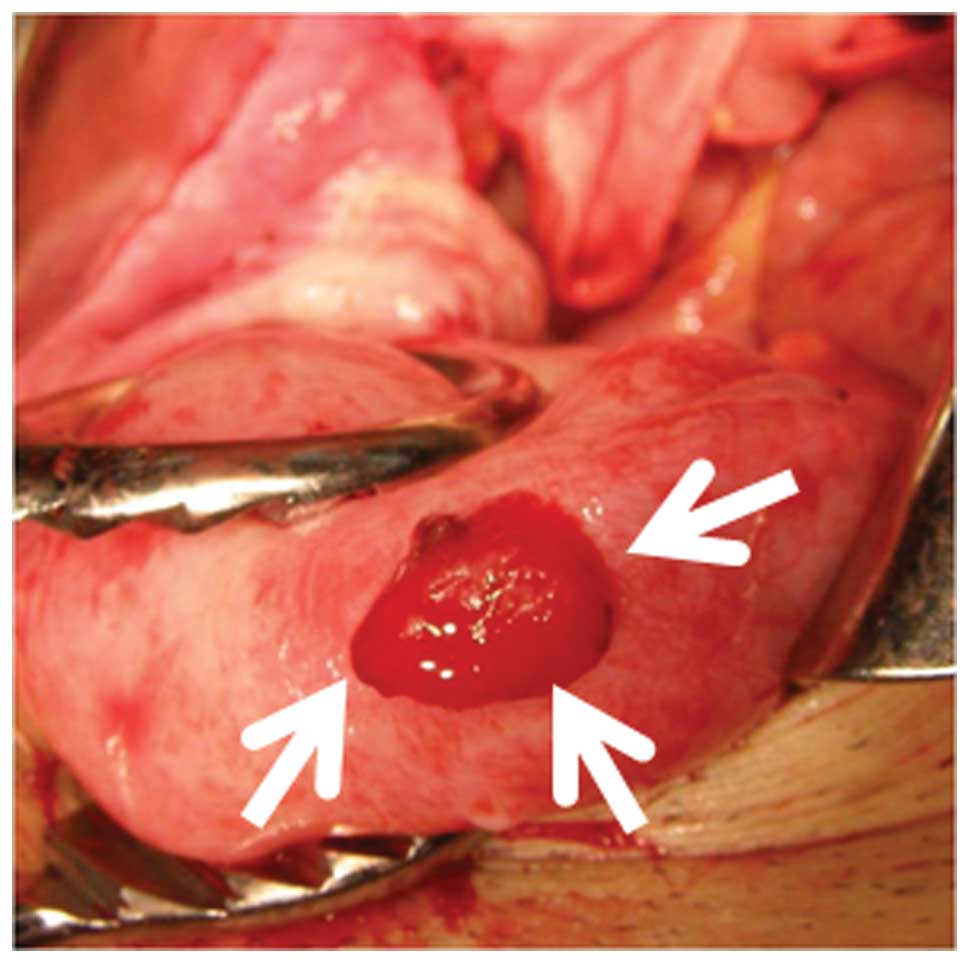
Douglas. Intra-abdominal blood loss was 550 ml. A 20×10-mm mass
with oozing blood protruded from the surface in the uterine fundus
(Fig. 1), and its mass was judged to
be the site of the peritoneal pregnancy. The mass and adjacent
myometrium were resected en bloc. The patient's bilateral
fallopian tubes and ovaries were normal, and her postoperative
course was favorable.
However, on postoperative day 6 the patient's serum
hCG level was 10,000 mIU/ml, demonstrating unfavorable reduction,
and no villous architecture or fetal component was noted in the
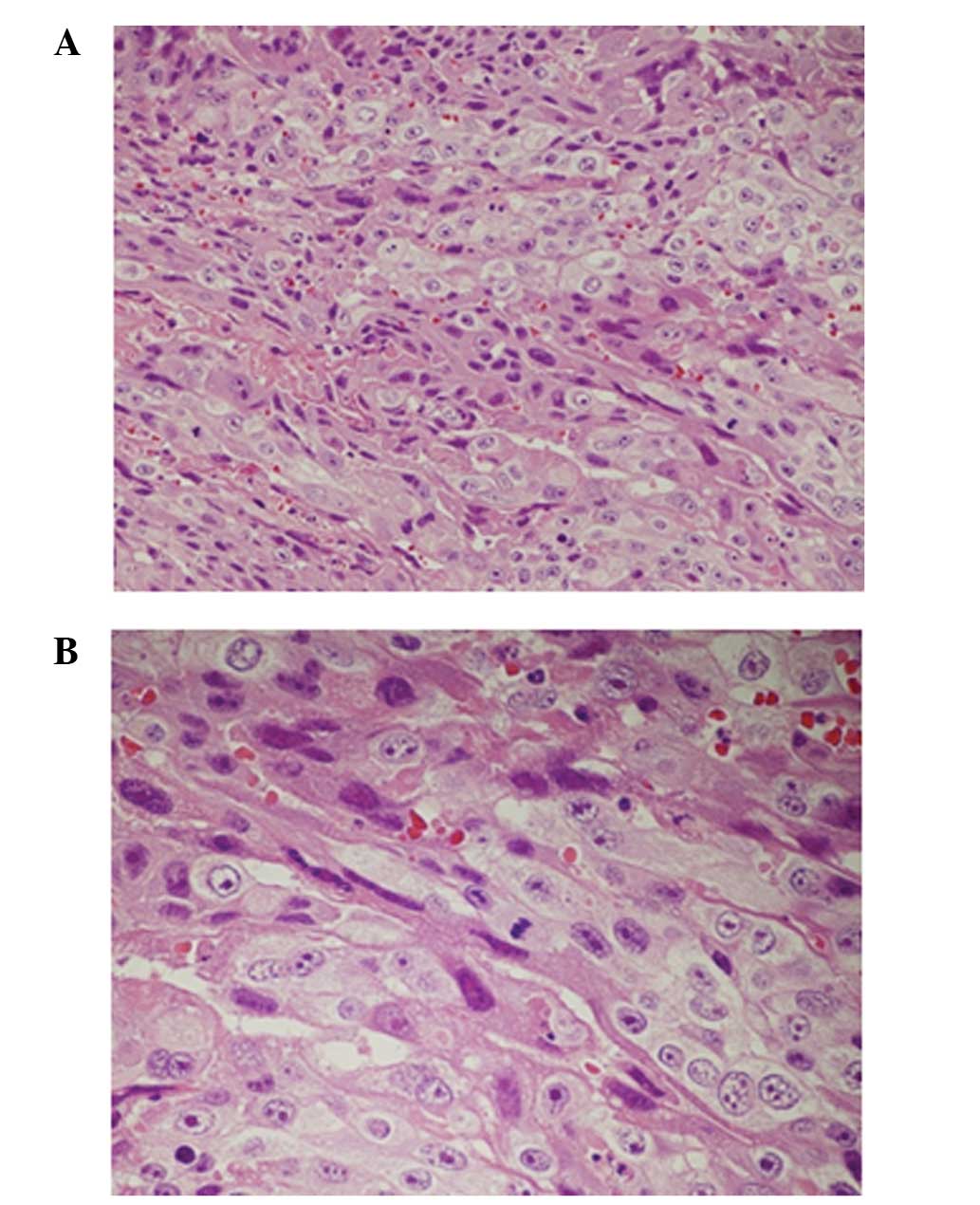
excised specimen, whereas trophoblast proliferation was observed in
the uterine tissue on histopathological examination (Fig. 2). A trophoblastic disease, such as
choriocarcinoma and hydatidiform mole, was suspected; therefore,
intra-uterine curettage, head and thoracoabdominal computed
tomography (CT), and pelvic magnetic resonance imaging (MRI) were
performed. A histopathological examination of the endometrial
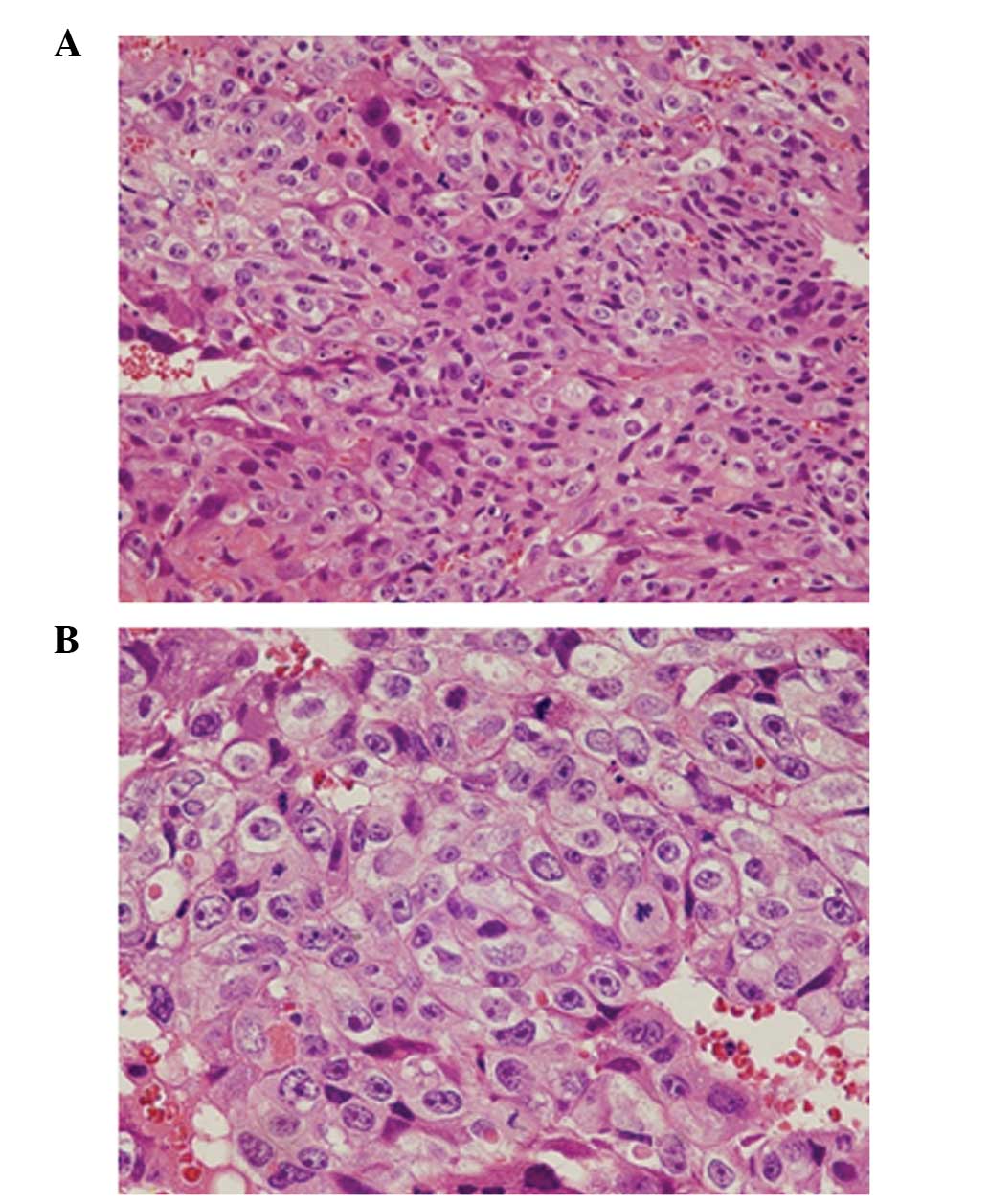
curettage sample revealed the absence of villous tissue, whereas
the proliferation of prominently atypical syncytiotrophoblasts and
cytotrophoblasts was noted, and a number of cells were mitotic
(Fig. 3). The patient was diagnosed
with choriocarcinoma based on these findings. The histopathological
results of the resected uterine lesion and endometrial curettage
sample revealed that the primary lesion was present in the uterus
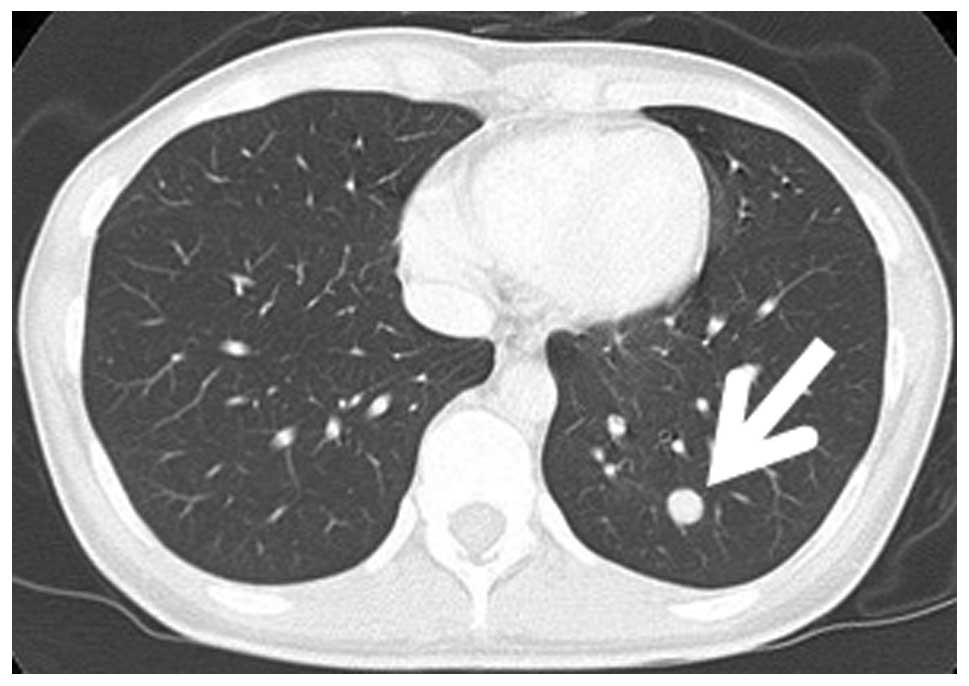
and metastasized to the uterine serosa. A 9-mm round nodule was
present in the left lower lung field on thoracoabdominal CT
(Fig. 4), and a total of six small
nodules were scattered in the bilateral lung fields. No
abnormalities were observed in the abdominal region. Head CT and
pelvic MRI also revealed no abnormalities.
For this patient, the FIGO score was 5 [age, 30
years; antecedent pregnancy, missed abortion; interval from index
pregnancy, <4 months; pre-treatment serum hCG, 12,000 IU/l;
largest tumor size, 2 cm; site of metastasis, lung and uterine
serosa; number of metastases, 8 (7 in the lung and 1 in the uterine
serosa); previously failed chemotherapy, none], and the FIGO stage
was III. Although it was a low-risk GTN, choriocarcinoma was
diagnosed based on the histopathological findings. Accordingly, it
was considered that this case should be treated as high-risk GTN,
and five cycles of methotrexate+etoposide+actinomycin D (MEA)
therapy were administered.
Endometrial cytology using Softsite® (Softmedical,
Tokyo, Japan) became negative following two cycles of MEA therapy.
Although endometrial cytology is not a routine assessment for GTN
under treatment, we performed it to confirm the disappearance of
choriocarcinoma cells. The patient's serum hCG level decreased
below the detection limit after three cycles. The complete
disappearance of the metastatic lesion in the lung was confirmed by
CT after five cycles. No recurrence has occurred four years and 9
months after the completion of MEA therapy. In addition, the
patient became pregnant and gave birth one year and 3 months after
the completion of MEA therapy.
Discussion
The findings of this case indicated that gestational
choriocarcinoma may cause uterine serosal metastasis. Furthermore,
certain patients who undergo surgery for a suspected peritoneal
pregnancy may have gestational choriocarcinoma, similar to this
case.
Gestational choriocarcinoma may cause uterine
serosal metastasis. To the best of our knowledge, this is the first
case study in which the primary lesion was present in the uterus
and metastasized to the uterine serosa. A PubMed search was
conducted using two keywords, ‘choriocarcinoma’ and ‘serosal
metastasis’ or ‘peritoneal metastasis’. No case report was
identified with regard to metastatic choriocarcinoma from a primary
lesion in the uterus to the uterine serosa. A similar case report
describing gestational choriocarcinoma on the surface of subserosal
leiomyoma was identified (7).
However, this study was different from ours since the primary
lesion was present on the surface of subserosal leiomyoma. In our
case, a metastatic lesion, but not a primary lesion, was present in
the uterine serosa.
The lungs, liver, brain, vagina and digestive tract
are common sites of metastasis (6).
Three mechanisms have been proposed for uterine serosal metastasis.
Firstly, the lesion may have been hematogenously implanted on the
uterine serosa. Lung metastasis was also present in this patient,
which suggested hematogenous metastasis. It remains unclear whether
the lesion returned to the uterine serosa after entering the
systemic circulation or whether it directly reached the uterine
serosa through a myometrial blood vessel. Secondly, the
intra-uterine lesion may have passed through the fallopian tubes,
dropped into the abdominal cavity, and engrafted on the uterine
serosa. For example, in a peritoneal pregnancy, a fertilized egg
may fall out of the fallopian tube into the abdominal cavity and
engraft on the peritoneum. Thirdly, the intra-uterine lesion may
have penetrated the myometrium and reached the uterine serosa. The
possibility of intra-abdominal bleeding caused by penetration of
the myometrium by GTN and an actual case of myometrial penetration
by a lesion have been reported previously (8). However, this could not be pathologically
investigated in our patient since the uterus was not excised.
Therefore, we cannot confirm which hypothesis was the most
relevant. However, the first hypothesis appears to be the most
plausible. We suspect that the lesion directly reached the uterine
serosa through a myometrial blood vessel. The second and third
hypotheses are less plausible than the first for the following
reasons. According to the second hypothesis, an intra-uterine
lesion detaching from the fallopian tube is more likely to form a
lesion in the pouch of Douglas than on the surface of the uterine
serosa. This is due to the fact that a detached lesion is more
likely to go deeper into the abdomen. According to the third
hypothesis, a lesion penetrating the myometrium is more likely to
be detected in the myometrium by ultrasonography or MRI. However,
in the present case, no lesion was detected in the myometrium by
ultrasonography or MRI.
Certain patients who undergo surgery for suspected
peritoneal pregnancy may have gestational choriocarcinoma, similar
to this case. When a uterine serosal metastatic lesion bleeds,
symptoms similar to those of an ectopic pregnancy develop.
Gestational choriocarcinoma arising from the ovary, fallopian tube,
omentum, liver and the surface of the subserosal myoma have been
reported previously, and all of these were diagnosed as
choriocarcinoma by postoperative histopathological examinations
following surgery performed for a suspected ectopic pregnancy
(1,2,4,5,7). Symptoms
similar to those of an ectopic pregnancy develop when the lesion
bleeds, regardless of whether the intra-abdominal lesion is a
primary or metastatic lesion. When the lesion is surgically
resected, a macroscopic examination is required to determine
whether chorionic tissue is present in the lesion. In addition, it
is essential to ensure that the lesion is an ectopic pregnancy by
histopathological examination and to confirm postoperative
reduction in the serum hCG level to below the detection limit. If
these attempts fail, the diagnosis of choriocarcinoma may be
delayed. This case highlights the significance of these
confirmations.
The findings of the present case indicated that
gestational choriocarcinoma may cause uterine serosal metastasis.
Furthermore, certain patients who undergo surgery for suspected
peritoneal pregnancy may have gestational choriocarcinoma, similar
to this case. Therefore, it is essential to confirm the
postoperative histopathological diagnosis and reduction in serum
hCG level in patients who have undergone surgery for a peritoneal
pregnancy, such as this patient.
References
|
1
|
Wan X, Li J and Xie X: Extrauterine
choriocarcinoma of the greater omentum after tubal pregnancy: case
report. Int J Gynecol Cancer. 16:1476–1478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mittal S, Aird I and Haugk B: Gestational
choriocarcinoma in liver mimicking ruptured ectopic pregnancy. J
Obstet Gynaecol. 32:4992012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maestá I, Leite FV, Michelin OC and
Rogatto SR: Primary pulmonary choriocarcinoma after human chorionic
gonadotropin normalization following hydatidiform mole: a report of
two cases. J Reprod Med. 55:311–316. 2010.PubMed/NCBI
|
|
4
|
Sakumoto K, Nagai Y, Inamine M and
Kanazawa K: Primary omental gestational choriocarcinoma ascertained
by deoxyribonucleic acid polymorphism analysis. Gynecol Oncol.
97:243–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Küçüközkan T, Savan K, Aydin E, Sönmez S,
Duran B and Kaygi O: Choriocarcinoma associated with ectopic
pregnancy after tubal sterilisation. Acta Obstet Gynecol Scand.
71:636–638. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berkowitz RS and Goldstein DP: Chorionic
tumors. N Eng J Med. 335:1740–1748. 1996. View Article : Google Scholar
|
|
7
|
Chen MJ, Yang JH, Lin MC, Ho HN and Yang
YS: An unusual gestational choriocarcinoma occurring primarily on
the surface of a subserous leiomyoma. BJOG. 111:188–190. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashraf-Ganjooei T and Ghaemmaghami F:
Patients with presenting unusual manifestations with gestational
trophoblastic neoplasm: case series and review of literatures. Arch
Gynecol Obstet. 277:465–470. 2008. View Article : Google Scholar : PubMed/NCBI
|