Introduction
A colorectal polyp is a circumscribed mass of tissue
that projects above the surface of the bowel mucosa (1). Colorectal polyps are histologically
classified as neoplastic (adenomas) or non-neoplastic (2). Adenomatous polyps exhibit a malignant
potential and are classified as tubular, tubulovillous or villous
adenomas, depending on the presence and volume of villous tissue.
Adenoma detection rate (ADR) is defined as the number of patients
with adenomas identified per 100 patients screened and is
considered as an important predictor of the risk of interval
colorectal cancer (CRC) following a screening colonoscopy (3). Several studies on ADR have been
previously conducted. A nationwide survey of 3,266 patients in
France reported an ADR of 17.7% (578/3,266), whereas the ADR of
male patients was significantly higher compared with that of female
patients (21.2 vs. 14.5%, respectively; P<0.0001); furthermore,
the ADR of patients aged >50 years was significantly higher
compared with that of patients aged <50 years (20.6 vs. 8.5%,
respectively; P<0.0001) (4).
Another French study reported an ADR of 31% (5). In addition, a nationwide free
colonoscopy screening program was conducted in Germany for the
general population aged ≥55 years. That study included 2,821,392
screening colonoscopies performed between January, 2003 and
December, 2008 and reported an ADR of 19.4%, with a higher rate in
men compared with that in women (25.8 vs. 16.7%, respectively)
(6).
CRC is a high-morbidity malignant tumor with a high
incidence worldwide, accounting for 8% of new cancer cases in the
USA in 2014 (7). The majority of CRCs
arise from pre-existing adenomatous polyps. The causal association
between polypoid adenomas and colonic carcinomas has been
demonstrated on a pathological basis (8,9).
Currently, the key to controlling CRC is reliably detecting and
resecting adenomas prior to them becoming malignant. Colonoscopy is
the most common and efficient method for the detection and removal
of colorectal adenomas. ADR is considered to be an important factor
affecting the risk of CRC. A higher ADR may reduce the incidence of
CRC, thereby protecting asymptomatic average-risk individuals
against CRC. A retrospective review of all the patients who
underwent colonoscopy screening between 2003 and 2012 in the First
Affiliated Hospital of Guangxi Medical University (Nanning, China)
was conducted, with the aim of estimating the total ADR, comparing
the ADR among the different gender and age groups and calculating
the proportion of adenomatous polyps in each section of the large
bowel.
Patients and methods
Patients and data collection
All the patients who underwent colonoscopy at the
First Affiliated Hospital of Guangxi Medical University between
January, 2003 and December, 2012 were recruited in the present
study. All the procedures were performed by endoscopists
experienced in operational colonoscopy. All the patients underwent
sufficient bowel preparations and all the removed specimens were
pathologically examined. Data collected from the colonoscopy and
pathology reports included demographic information, timing of the
colonoscopy, number of polyps removed, polyp location and the
pathological types of the polyps. The patients were grouped
according to the colonoscopy date (2003–2012), gender (male and
female), age (<30, 30–39, 40–49, 50–59, 60–69, 70–79 and ≥80
years) and polyp location (ileocecum, ascending, transverse,
descending and sigmoid colon and rectum).
The exclusion criteria were as follows: A history of
colonoscopy and diagnosis of colorectal pathology; a history of
CRC; a history of colorectal surgery; suboptimal bowel preparation;
pathology report not available; and non-Chinese patients. The
results were collected and reviewed. Patients with multiple
adenomas were recorded only once when calculating the ADR. However,
when calculating the proportion of adenomatous polyps in each
location, for multiple polyps occurring in more than one location,
each location was recorded once. For example, if adenomatous polyps
were identified in the sigmoid colon and rectum of a patient, this
patient was added to the sigmoid colon as well as the rectum
groups.
Statistical analysis
The patient characteristics are described as
frequencies and percentages for the categorical variables. The
χ2 test was used to compare the ADR between different
age and gender groups. The statistical significance of the trend of
ADR across different age groups was calculated using the
Cochran-Armitage trend test. A two-tailed P-value of <0.05 was
considered to indicate statistically significant differences. All
the analyses were performed using SPSS 17.0 software (SPSS Inc.,
Chicago, IL, USA).
Results
ADR
Between 2003 and 2012, a total of 41,010 patients
(20,210 men and 20,800 women) underwent electronic colonoscopy in
our hospital and 7,219 (4,504 men and 2,715 women) were diagnosed
with at least one adenoma on pathological examination. Therefore,
the ADR of the patients screened in the present study was 17.6%.
The patient demographics per year are summarized in Table I. The ADR ranged between 12.7 and
20.1% within these 10 years, whereas no significant difference in
ADR was observed after 10 years. The ADR in male and female
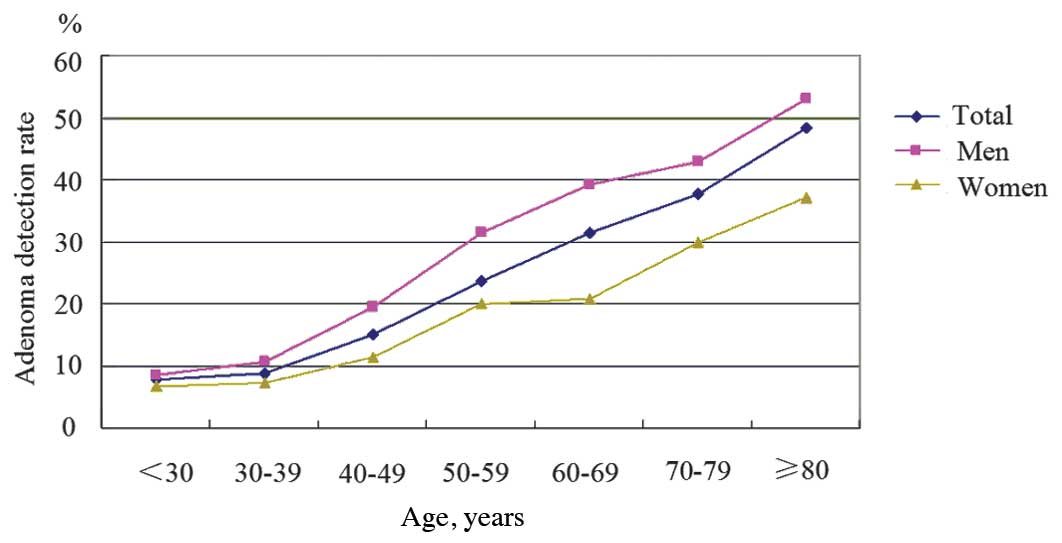
patients per age group is presented in Table II. The ADR increased from 7.8% in
patients aged <30 years to 48.4% in those aged >80 years.
There was a statistically significant trend of increasing ADR with
each decade of age in both genders (P<0.05) (Fig. 1). Furthermore, the ADR of patients
aged >50 years was significantly higher compared with that of
patients aged <50 years (28.8 vs. 11.0%, respectively;
P<0.05). In addition, in all the age groups, the ADR of male
patients was significantly higher compared with that of female
patients (all P<0.05), with a total ADR of 22.3% in men and
13.1% in women. For patients aged >50 years, the ADR was 37.0%
in men and 20.6% in women.
 | Table I.Patient demographics per year. |
Table I.
Patient demographics per year.
|
| Screened cases,
no. | Adenoma cases,
no. |
|
|---|
|
|
|---|
| Year | Men | Women | Men | Women | ADR (%) |
|---|
| 2003 | 989 | 1,075 | 170 | 93 | 12.7 |
| 2004 | 1,135 | 1,280 | 215 | 157 | 15.4 |
| 2005 | 1,422 | 1,505 | 303 | 189 | 16.8 |
| 2006 | 1,967 | 2,112 | 437 | 256 | 17.0 |
| 2007 | 1,876 | 1,926 | 498 | 266 | 20.1 |
| 2008 | 2,267 | 2,389 | 474 | 276 | 16.1 |
| 2009 | 2,321 | 2,304 | 506 | 329 | 18.1 |
| 2010 | 2,607 | 2,489 | 604 | 343 | 18.6 |
| 2011 | 2,647 | 2,751 | 679 | 382 | 19.7 |
| 2012 | 2,979 | 2,969 | 618 | 424 | 17.5 |
 | Table II.ADR for male and female patients in
each age group. |
Table II.
ADR for male and female patients in
each age group.
|
| Screened cases,
no. | Adenoma cases, no.
(ADR, %) |
|
|---|
|
|
|---|
| Age grouping
(years) | Total | Men | Women | Total | Men | Women | P-value |
|---|
| By decade |
|
|
|
|
|
|
|
|
<30 | 6,338 | 3,428 | 2,910 | 496 (7.8) | 299 (8.7) | 197 (6.8) | <0.001 |
|
30–39 | 9,402 | 4,465 | 4,937 | 834 (8.9) | 473 (10.6) | 361 (7.3) | <0.001 |
|
40–49 | 10,116 | 4,654 | 5,462 | 1,525 (15.1) | 910 (19.6) | 615 (11.3) | <0.001 |
|
50–59 | 7,785 | 3,566 | 4,219 | 1,834 (23.6) | 1,122 (31.5) | 712 (20.0) | <0.001 |
|
60–69 | 4,628 | 2,414 | 2,214 | 1,451 (31.4) | 946 (39.2) | 505 (20.9) | <0.001 |
|
70–79 | 2,293 | 1,364 | 929 | 862 (37.6) | 585 (42.9) | 277 (29.8) | <0.001 |
|
≥80 | 448 | 319 | 129 | 217 (48.4) | 169 (53.0) | 48 (37.2) | 0.003 |
| By the cut-off of
50 years |
|
|
|
|
|
|
|
|
<50 | 25,856 | 12,547 | 13,309 | 2,855 (11.0) | 1,682 (13.4) | 1,173 (9.3) | <0.001 |
|
≥50 | 15,154 | 7,663 | 7,491 | 4,364 (28.8) | 2,822 (37.0) | 1,542 (20.6) | <0.001 |
| Total | 41,010 | 20,210 | 20,800 | 7,219 (17.6) | 4,504 (22.3) | 2,715 (13.1) | <0.001 |
Adenomas
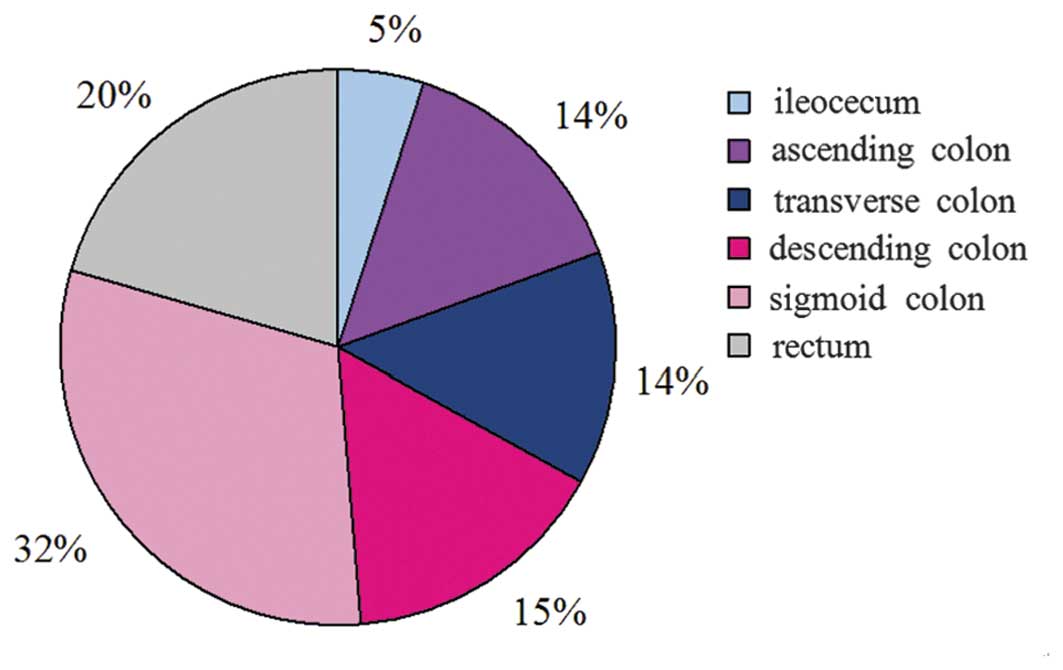
Of the 7,219 patients diagnosed with at least one
adenoma, 830 had adenomas in more than one location. In accordance
with the aforementioned statistical method, there were a total of
8,551 cases of adenomas in the present study. The statistical
analysis revealed that 433 cases of adenomas were detected in the
ileocecum (5.1%), 1,236 in the ascending colon (14.5%), 1,155 in
the transverse colon (13.5%), 1,321 in the descending colon
(15.4%), 2,654 in the sigmoid colon (31.0%) and 1,752 in the rectum
(20.5%). The proportion of adenomatous polyps in each section is
depicted in Fig. 2, with 20% of the
cases of adenomas occurring in the rectum, 47% in the left colon
(descending and sigmoid colon) and 33% in the right colon
(ileocecum, ascending and transverse colon).
Discussion
CRC is the third most common cause of cancer-related
mortality in the USA (7). From a
histological and endoscopic standpoint, CRC begins as a small
neoplastic polyp, which progressively enlarges and transforms
through a dysplastic stage into invasive cancer (10). The multistep progression from adenoma
to cancer requires almost a decade or more and is accompanied by a
number of characterized genetic alterations, involving mutation of
the adenomatous polyposis coli (APC), deleted in colorectal
carcinoma (DCC), Kirsten rat sarcoma viral oncogene homolog (K-Ras)
and p53 genes, loss of heterozygosity (LOH) and DNA
methylation.
APC gene mutation plays a significant role in the
development of colorectal adenoma and CRC (11). APC is a tumor suppressor gene located
on chromosome 5q. Germline mutation of the APC gene and subsequent
somatic mutation of the second APC allele are responsible for the
inherited familial adenomatous polyposis syndrome (12). There is evidence that the majority of
CRCs and adenomas contain a mutated APC gene and mutations of the
APC gene play a major role in the early development of colorectal
neoplasms (13). It has been
suggested that the APC protein may serve as a molecular
‘gatekeeper’ for the development of adenomas (14). A mutation of the ‘gatekeeper’ leads to
a permanent imbalance of cell division over cell death and disrupts
the cell-cell and cell-matrix interactions, leading to
inappropriate cell proliferation.
K-Ras gene activation is also considered to be an
early event in colorectal carcinogenesis. It was previously
reported that K-Ras mutations were detected in 58% of aberrant
crypt foci (ACF) (15). Furthermore,
ACF occured with a high frequency in the colons of animals treated
with colon carcinogens and in the grossly normal mucosas of
patients with colon cancer (16). In
addition, the abnormal proteins produced by the K-Ras oncogene were
frequently detected in colorectal neoplasms. These abnormal
proteins may continually stimulate downstream signal transduction
pathways involved in cell proliferation and malignant
transformation.
LOH on the long arm of chromosome 18 (18q) is
frequently observed in CRCs and advanced adenomas, but only
occasionally in earlier-stage adenomas (17), indicating that LOH on 18q occurs later
during the development from adenoma to carcinoma and may predict a
poor prognosis. LOH on 18q was found to be closely associated with
the inactivation of the DCC tumor suppressor gene. The DCC gene is
located on chromosome 18q21.2 and contained within the common
region LOH on 18q. DCC expression was found to be markedly
decreased or absent in the majority of CRCs and cell lines. The DCC
gene is one of the most commonly mutated tumor suppressor genes in
CRC and occurs mainly in the late phase of carcinogenesis (18). The DCC protein is a transmembrane
glycoprotein similar to the neural cell adhesion molecules
(19). LOH on DCC may result in
impaired contact between cells, thereby contributing to tumor
growth and invasion (20). More
recent evidence suggests that other tumor suppressor genes, namely
deleted in pancreatic carcinoma 4 and Mad-related 2, may also be
inactivated by allelic loss on chromosome 18q (21). In addition, mutation of the p53 tumor
suppressor gene on chromosome 17p has been identified in
approximately half of all CRCs and also frequently emerges in the
later stages of colorectal carcinogenesis (22). This mutation may allow a growing tumor
with multiple genetic alterations to evade cell cycle arrest and
apoptosis.
In the present retrospective study, the ADR of the
total 41,010 patients was 17.6%. The ADR in patients aged >50
years was 28.8% (4,364/15,154), which was significantly higher
compared with that in patients aged <50 years (11.0%). These
results are in accordance with most national guidelines, which
recommend that screening programs for CRC be initiated by the age
of 50 years for men and women of average risk, as the risk of CRC
increases in the sixth decade of life (23). In addition, similar to the results of
previous studies, the total ADR in men was found to be
significantly higher compared with that in women (22.3 vs. 13.1%,
respectively) in the present study. In patients aged >50 years,
the ADR was 37.0% in men and 20.6% in women. Based on the
differences in ADR according to gender, it has been suggested that
the age for initiating screening colonoscopy should be
gender-specific. In the present study, the ADR of male patients was
19.6% in the 40–49 age group, whereas the ADR of female patients
was 20.0% in the 50–59 age group and 20.9% in the 60–69 age group.
This result was consistent with a previous study, in which
comparable ADRs were found in men aged 45–49 and women aged 55–59
years (24). Sung et al
(25) suggested in the Asia Pacific
consensus recommendations for CRC screening that female subjects
may start screening at a later age, due to the relatively low
incidence of CRC at ages 50–55 years. It was previously reported
that the higher incidence in men may be due to the longer female
life span (26). Other studies
suggested that the length of exposure to estrogen and/or
progesterone may play a role in the gender difference (27,28). In
addition, other factors may also affect adenoma prevalence,
including family history of colon cancer, tobacco use, obesity and
diabetes mellitus (29). In clinical
practice, the ADR was also found to be closely associated with the
quality of bowel preparation, the cecal intubation rate, the level
of operating techniques of the endoscopists and the quality of the
endoscopic devices (4). These factors
may lead to missed adenomatous polyps or incompletely resected
adenomas.
In the present study, 47% of the detected adenomas
occurred in the left and 33% in the right colon. This result was in
agreement with a previous study, in which left-sided colorectal
adenomas were more prevalent compared with right-sided ones (56 vs.
44%, respectively) (30). Another
previous study, including a large sample of 7,590 adenomatous
polyps, found that 76.5% of the adenomas were localized in the left
and 23.5% in the right colon (31).
The precise reason for this phenomenon is not fully understood.
Fundamentally, the colon develops from two different embryonic
areas of the primitive gut, namely the midgut, which gives rise to
the small intestine through to the proximal two-thirds of the
transverse colon, and the hindgut, which gives rise to the distal
third of the transverse colon through to the upper anal canal
(32). A previous study demonstrated
that polyps with advanced pathology are smaller and more easily
missed in the right compared with the left colon (33). In addition, the difficulty in
detecting right-sided polyps, including suboptimal bowel
preparation, flat polyps located behind mucosal folds and the shape
of the colonic folds, may lead to missed adenomas. Recent studies
have demonstrated that colonoscopy is significantly less effective
in preventing proximal colon cancer and related mortality compared
with distal colon cancer (3,5).
The present study had certain limitations that must
be acknowledged: i) The study was completed in a single academic
center, which may make the present results only representative of a
regional situation; ii) information regarding the size and type of
adenomas were not available; therefore, they could not be further
subdivided; iii) all the patients included were Chinese; therefore,
the present results had racial limitations; iv) although all the
colonoscopy examinations were conducted by experienced
endoscopists, certain adenomas were inevitably missed; and v)
traditionally, the left colon includes the ileocecum, ascending
colon and proximal transverse colon and the right colon includes
the distal transverse, descending and sigmoid colon. However, in
the present retrospective study, the exact locations of the
adenomas in the transverse colon could not be verified; therefore,
all the adenomas in the transverse colon were classified into the
left colon group. This means that the distribution ratio of
adenomas in the right colon may in fact be higher than what was
calculated in this study.
Despite the abovementioned limitations, the present
study also has several strengths: All the data included in this
study were rigorously reviewed. The study included a large sample
of 41,010 patients, so that each group contained a sufficient
number of samples for comparison.
In conclusion, in this retrospective study of 41,010
Chinese patients, an ADR of 17.6% was calculated. Adenomas were
found to be more prevalent in male compared with female patients.
In addition, regardless of gender, there was a statistically
significant trend of increasing ADR with increasing age in this
study.
References
|
1
|
Bond JH: Polyp guideline: diagnosis,
treatment and surveillance for patients with nonfamilial colorectal
polyps. The Practice Parameters Committee of the American College
of Gastroenterology. Ann Intern Med. 119:836–843. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fenoglio-Preiser CM and Hutter RV:
Colorectal polyps: pathologic diagnosis and clinical significance.
CA Cancer J Clin. 35:322–344. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaminski MF, Regula J, Kraszewska E, et
al: Quality indicators for colonoscopy and the risk of interval
cancer. N Engl J Med. 362:1795–1803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barret M, Boustiere C, Canard JM, et al:
Société Française d'Endoscopie Digestive: Factors associated with
adenoma detection rate and diagnosis of polyps and colorectal
cancer during colonoscopy in France: results of a prospective,
nationwide survey. PloS One. 8:e689472013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coriat R, Lecler A, Lamarque D, et al:
Quality indicators for colonoscopy procedures: a prospective
multicentre method for endoscopy units. PloS One. 7:e339572012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pox CP, Altenhofen L, Brenner H,
Theilmeier A, Von Stillfried D and Schmiegel W: Efficacy of a
nationwide screening colonoscopy program for colorectal cancer.
Gastroenterology. 142:1460–1467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morson B: President's address. The
polyp-cancer sequence in the large bowel. Proc R Soc Med.
67:451–457. 1974.PubMed/NCBI
|
|
9
|
Hill MJ, Morson BC and Bussey HJ:
Aetiology of adenoma-carcinoma sequence in large bowel. Lancet.
1:245–247. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allen JI: Molecular biology of colon
polyps and colon cancer. Semin Surg Oncol. 11:399–405. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura Y: The adenomatous polyposis coli
gene and human cancers. J Cancer Res Clin Oncol. 121:529–534. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gryfe R, Swallow C, Bapat B, Redston M,
Gallinger S and Couture J: Molecular biology of colorectal cancer.
Curr Probl Cancer. 21:233–300. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Powell SM, Zilz N, Beazer-Barclay Y, et
al: APC mutations occur early during colorectal tumorigenesis.
Nature. 359:235–237. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita N, Minamoto T, Ochiai A, Onda M
and Esumi H: Frequent and characteristic K-ras activation and
absence of p53 protein accumulation in aberrant crypt foci of the
colon. Gastroenterology. 108:434–440. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pretlow TP, Brasitus TA, Fulton NC, Cheyer
C and Kaplan EL: K-ras mutations in putative preneoplastic lesions
in human colon. J Nat Cancer Inst. 85:2004–2007. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogelstein B, Fearon ER, Hamilton SR, et
al: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho KR and Fearon ER: DCC: linking tumour
suppressor genes and altered cell surface interactions in cancer?
Eur J Cancer. 31A:1055–1060. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang SC, Lin JK, Lin TC and Liang WY:
Loss of heterozygosity: an independent prognostic factor of
colorectal cancer. World J Gastroenterol. 11:778–784. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Li YF, Sun XW, et al: Correlation
analysis between loss of heterozygosity at chromosome 18q and
prognosis in the stage-II colon cancer patients. Chin J Cancer.
29:761–767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baker SJ, Fearon ER, Nigro JM, et al:
Chromosome 17 deletions and p53 gene mutations in colorectal
carcinomas. Science. 244:217–221. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levin B, Lieberman DA, McFarland B, et al:
American College of Radiology Colon Cancer Committee: Screening and
surveillance for the early detection of colorectal cancer and
adenomatous polyps, 2008: a joint guideline from the American
Cancer Society, the US Multi-Society Task Force on Colorectal
Cancer and the American College of Radiology. Gastroenterology.
134:1570–1595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferlitsch M, Reinhart K, Pramhas S, et al:
Sex-specific prevalence of adenomas, advanced adenomas and
colorectal cancer in individuals undergoing screening colonoscopy.
JAMA. 306:1352–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sung JJ, Lau JY, Young GP, et al: Asia
Pacific Working Group on Colorectal Cancer: Asia Pacific consensus
recommendations for colorectal cancer screening. Gut. 57:1166–1176.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Regula J, Rupinski M, Kraszewska E, et al:
Colonoscopy in colorectal-cancer screening for detection of
advanced neoplasia. N Engl J Med. 355:1863–1872. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chlebowski RT, Wactawski-Wende J,
Ritenbaugh C, et al: Womens Health Initiative Investigators:
Estrogen plus progestin and colorectal cancer in postmenopausal
women. N Engl J Med. 350:991–1004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei EK, Colditz GA, Giovannucci EL, Fuchs
CS and Rosner BA: Cumulative risk of colon cancer up to age 70
years by risk factor status using data from the Nurses' Health
Study. Am J Epidemiol. 170:863–872. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dominic OG, McGarrity T, Dignan M and
Lengerich EJ: American College of Gastroenterology Guidelines for
Colorectal Cancer Screening 2008. Am J Gastroenterol.
104:2626–2627; author reply 2628–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khodadoostan M, Fatemi R, Maserat E, et
al: Clinical and pathological characteristics of colorectal polyps
in Iranian population. East Afr J Public Health. 7:157–159.
2010.PubMed/NCBI
|
|
31
|
Gschwantler M, Kriwanek S, Langner E, et
al: High-grade dysplasia and invasive carcinoma in colorectal
adenomas: a multivariate analysis of the impact of adenoma and
patient characteristics. Eur J Gastroenterol Hepatol. 14:183–188.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jacobs ET, Thompson PA and Martínez ME:
Diet, gender and colorectal neoplasia. J Clin Gastroenterol.
41:731–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta S, Balasubramanian BA, Fu T, Genta
RM, Rockey DC and Lash R: Polyps with advanced neoplasia are
smaller in the right than in the left colon: implications for
colorectal cancer screening. Clin Gastroenterol Hepatol.
10:1395–1401, e1392. 2012. View Article : Google Scholar : PubMed/NCBI
|